Abstract
High pressure-high temperature techniques are used to synthesise new solid state compounds and materials that can be developed for technological applications. Laboratory and synchrotron x-ray diffraction and optical spectroscopy are combined with ab initio calculations to determine the structures and properties of new materials. We describe recent work on major classes of new materials including boron-rich solids, transition metal nitride superconductors, nitride spinels and light element solids based in the C–N–H system using examples from our own work.
Similar content being viewed by others
Keyword
- Solid state chemistry
- new materials
- boron-based solids
- nitride spinels
- nitride superconductors
- carbon nitride
Introduction
The pioneering research of P.W. Bridgman first led to major developments in high-pressure physics and solid state chemistry. He also investigated materials properties of metals, ceramics and other substances (Bridgman, 1931; McMillan, 2005). The field of high-pressure research made another leap forward with the invention of the diamond anvil cell that permitted direct observation of samples held under extreme pressures that now extend into the multimegabar range (Eremets, 1996; Holzapfel and Isaacs, 1997; Jamieson et al., 1959; Jayaraman, 1983; Piermarini, 2001; Van Valkenburg, 1962; Weir et al., 1959). Following the laboratory preparation of diamond (Bovenkerk et al., 1959; Bundy et al.,1995,1996; Hazen, 1999) and the synthesis of cubic boron nitride (c-BN, Wentorf, 1957) high-pressure–high-temperature research became an important tool for synthesis and exploration of new materials with potential technological applications (McMillan, 2002). Materials investigated include new superhard phases, materials with unusual electronic, magnetic and superconducting properties, structural ceramics and solid state compounds with wide-gap optoelectronic behaviour and catalytic properties. X-ray diffraction carried out in the laboratory on quenched samples or in situ at high pressures and high temperatures at synchrotron beamlines is a primary technique for determining the structure and compressibility properties of the new materials, as well as establishing their recovery to ambient conditions. This is complemented by optical techniques including Raman scattering and FTIR or UV-visible spectroscopy. The electrical conductivity and magnetic properties of new materials are investigated in situ in the DAC or large volume devices and upon recovery to ambient conditions.
New materials exploration: high-P,T strategies
Techniques introduced by Bridgman led to development of new high pressure apparatus generally termed “large volume” devices (Eremets, 1996; Holzapfel and Isaacs, 1997; Hazen, 1999; Liu and Bassett, 1986). In particular, the opposed anvil geometry gave rise to the “belt” press used in the synthesis of diamond and to “toroidal” anvil instruments developed in Russia (Hall, 1960; Khvostanstev et al., 2004). That work led to the “Paris–Edinburgh” press developed as a portable device for neutron scattering and other experiments at large facilities and in the laboratory (Besson et al., 1992). The piston cylinder apparatus envisaged by Kennedy for diamond synthesis was developed for geological studies and is now used routinely in solid state chemistry and materials investigations (Hazen, 1999; Boyd and England, 1960; Coes, 1955). Modern multi-anvil devices that reach 24 GPa or higher were developed from von Platen’s and T. Hall’s original designs for diamond production (Hazen, 1999; von Platen, 1962). These instruments are used in laboratories to investigate the synthesis and recovery to ambient conditions of new solid state compounds and technological materials. Because large press techniques can be scaled up to achieve industrial scale production of important materials following examples of diamond and c-BN, they set a benchmark for advanced materials research. First, new materials must be recoverable to ambient conditions in a useful form. That usually limits the materials applications to powdered polycrystalline samples obtained from high-P,T synthesis or single crystalline materials of high value (e.g., large gem-quality diamonds or substrates for optoelectronic materials; Sumiya et al., 2000; Krukowski et al., 2001). Developing new technologically relevant materials differs from exploratory solid state science in that commercial and environmental considerations must also be taken into account.
The DAC permits direct observation and in situ studies of materials under high pressure conditions extending into the multimegabar range. Laser-heating and resistive heating methods permit simultaneous studies under high-P,T conditions, as well as synthesis of new solid state compounds and potentially important technological materials. In situ studies during release of pressure allow us to evaluate the recovery of the new compounds to ambient conditions and the evolution of their properties during decompression. Useful strategies for exploration and development of new materials combine in situ and high-P,T synthesis studies using DAC and large volume technique with ab initio calculations to predict the stability and properties of new phases and interpret experimental results.
X-ray diffraction studies at high pressure lead to determination of the equation of state and the bulk or shear moduli, often used as a guide to identifying materials with high hardness (He et al., 2004; Brazhkin, et al., 2002; Haines et al., 2001; Teter, 1998; Veprek, 1999). Spectroscopy gives information on electronic and phonon structure and measurements of magnetism and electrical conductivity at high P guide formation and recovery of new phases with potentially useful superconducting or multiferroic behaviour by observing the evolution of these properties as a function of the density.
Boron-rich solids
Elemental boron exhibits structure-property relations and bonding unique within the periodic table. According to traditional valency rules it has three electrons that typically form three covalent bonds resulting in planar molecular units or clusters with pyramidal structures. However these are electron deficient relative to the 8-electron rule and they readily form new compounds or complexes with electron-donating species often resulting in increased coordination of the B atoms or incorporation of “impurity” atoms into boron-rich structures. Motifs based on dodecahedral or icosahedral clusters containing triangular units are common in molecular and solid state boron chemistry. These are generally incompatible with developing long-range translation symmetry and the result is a series of highly complex boron-based structures among the elemental forms and compounds with metals and non-metallic species. The element is available with varying degrees of purity in amorphous or α- or β-structured forms: the β-B105 structure is thought to be most thermodynamically stable. Superconductivity has been observed in boron at very high pressure and new high pressure forms have been recognised that have interesting and potentially useful electronic and magnetic properties (Oganov et al., 2009; Haüssermann et al., 2003; Papaconstantopoulos and Mehl, 2002; Eremets et al., 2001). Reports of superconductivity in B-doped diamond are now under investigation (Yokoya et al., 2005; Dubrovinskaia et al., 2006; Ekimov et al.,2006).
The c-BN structure was predicted and synthesised shortly after the first laboratory production of diamond to give rise to a new high-pressure material that has become technologically important (Wentorf, 1957). It is second hardest to diamond and is used for cutting and grinding applications: modern research continues to investigate high-P,T syntheses of this phase along with advanced diamond production (Sumiya et al., 2000; Demazeau et al.,1990; Solozhenko, 2002; Solozhenko et al.,2000,2002; Pal’yanov et al., 2002; Sokol et al., 2001; Sung and Tai, 1997). c-BN is a wide-gap semiconductor with potential applications in high-T electronics and optoelectronics and high-P,T research has succeeded in producing doped single crystals (Taniguchi et al., 2002), as well as of h-BN for UV laser applications (Watanabe et al., 2004). Exploratory high-P,T research to develop new superhard compounds in the C-B-N system continues and a new ordered cubic compound BC2N competitive in hardness with c-BN was discovered recently (Solozhenko et al., 2001a, b,2002a).
The α-B structure forms the basis for series of suboxides, subnitrides and other chalcogenide or pnictide compounds (Figure 1). Here electron-rich species such as O, N, P, As occupy trigonal sites between the B12 icosahedra. Materials such as B6O1−x provide high-hardness refractory ceramics (He et al., 2002,2004; Hubert et al., 1996,1998a, b; Lundstrom,1997; Lundstrom and Andreev,1996; Rizzo et al., 1962). B6O is also a wide-gap semiconductor that might be developed for high-T thermoelectric applications. During high-P,T synthesis experiments from B + B2O3 mixtures at 3–4 GPa it was discovered that the O content could be increased to near unity (B6O0.96) and the resulting materials formed macroscopic icosahedral particles up to ~40 γm in diameter (Figure 1) (Hubert et al., 1996,1998a, b; McMillan et al., 1999). These formed as a result of multiple twinning within the rhombohedral α-B structure. The growth can also be described in terms of Mackay packing, that constitutes a novel space-filling scheme ordering successive layers of B12 icosahedra and O atoms in a radial stacking pattern without translational symmetry (Hubert et al., 1998a, b; McMillan et al., 1999). Formation of analogous B6N was reported from high-P,T synthesis but the results have recently been questioned (Hubert et al., 1997; Solozhenko et al., 2006).
The α-B structure based on B12 icosahedra linked by electron-deficient 3-centre B-B bonding readily accepts electron rich species such as O, N, etc. to result in high-hardness semiconducting materials. The result is a rhombohedral (R3¯ m) structure (XRD shown at centre). High-P,T synthesis results in unusual macroscopic icosahedra formed multiple twinning or by Mackay packing (Hubert et al., 1997,1998a, b; McMillan et al., 1999).
Transition metal nitrides
Transition metal nitrides, carbides and borides form important refractory materials with high hardness and low compressibility used as abrasives and for structural applications including anvils for high P devices (Oyama,1996; Toth,1971; Kaner et al., 2005). They are generally metallic and members including cubic ε-NbN and hexagonal δ-MoN can achieve high superconducting Tc values (15–17 K; Chen et al., 2004; Shebanova et al., 2006). The hexagonal structures are based on metallic layers with interstitial trigonal prismatic or octahedral sites for the nonmetal species (Figure 2). δ-MoN synthesis from reactions such as MoCl5 + NH3 results in poorly crystalline material with disordered N atoms and Tc ~5 K (Lengauer,1988). Annealing at high P prevents N2 loss and leads to N ordering and Tc increases to 12–17 K, comparable with ε-NbN (Bezinge et al., 1987; Bull et al., 2004). The hypothetical cubic compound MoN has been predicted to achieve very high Tc values ranging up to 30 K. However the known cubic phase in the system is non-stoichiometric (γ-Mo2N) with a rocksalt structure containing 50% vacancies on the anion sites. High P,T experiments including laser-heated DAC and multi anvil syntheses have determined that cubic MoN is unlikely to exist, consistent with theoretical predictions (Hubert et al., 1997,1998a, b; McMillan et al., 1999; Machon et al., 2006). However, it appears that cubic solid solutions between NbN and MoNx can be prepared, although the superconducting Tc appears to vanish upon substitution of Mo for Nb. Hexagonal (Mo,Nb)N phases can also be prepared and their Tc values are being studied. This work has now revealed a new oxynitride Mo3O2N with Tc ~ 17 K (Bailey and McMillan,2010).
Left: the hexagonal MoN structure. The Mo layers define trigonal prismatic sites for N atoms that are 50% occupied in each layer. Centre: High P annealing results in N ordering and a doubling of the hexagonal unit cell along the c axis. SQUID magnetometry results shows the increase in Tc from disordered to ordered MoN (right) (Bull et al., 2004).
Spinel nitrides
The spinel structure is common among oxides, chalcogenides and halides with AB2X4 stoichiometry. The type mineral MgAl2O4 is used as a substrate for the semiconductor industry and in optically transparent and scratch resistant coatings. Transition metal oxide spinels MFe2O4 (M = Fe2+, Ni2+, Cu2+, Mn2+, Zn2+, Mg2+ etc.) are magnetic materials used for information storage and in transformer cores. Other important spinels include cation deficient Li1−x(Mn,Fe)2O4 used in battery technology and for fuel cells and in catalysis. However, until 1999, no spinels based on the N3- anion had been prepared. In that year, however, reports of spinel-structured γ-Si3N4 and γ-Ge3N4 synthesised at high-P,T and Sn3N4 prepared from SnI4 in liquid NH3 appeared (Leinenweber et al., 1999; Serghiou et al., 1999; Zerr et al., 1999; Scotti et al., 1999; Schnick,1999; Sekine et al., 2000). The new nitride spinels have high hardness and low compressibility and they could become useful refractory ceramics for structural and abrasive applications (Tanaka et al., 2002; Jiang et al., 2001,2002; Soignard et al., 2001). They are also predicted to have wide direct bandgaps that could give rise to novel optoelectronic materials operating in the UV range and high-T semiconductors (Dong et al., 2000; Leitch et al., 2004; Ching et al., 2001a). Now Ga and (Si,Al) oxynitride spinels are available and research is under way to realise the first transition metal containing examples of nitride and oxynitride spinels that are predicted to be stable (Schwarz et al., 2002; Sekine et al., 2001; Kinski et al., 2005; Soignard et al., 2005; Ching et al., 2001b; Hector et al., 2006; McMillan et al., 2007).
C–N–H solids
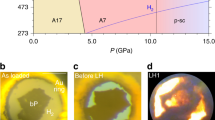
Since the prediction that dense phases of C3N4 might have extremely low compressibility and potentially high hardness (Cohen,1985; Liu and Cohen,1989; Teter and Hemley,1996) there has been intense interest in developing the high-P,T chemistry of carbon nitrides and other materials in the C–N–H system. The original study suggested the new C3N4 phase might be isostructural with β-Si3N4. Later calculations suggested that other dense phases could also be found in the system, with α- as well asβ-Si3N4 structures, or structures based on cubic spinel or wurtzite types. Many attempts at synthesis of high-density C3N4 compounds have been reported using high-P,T methods or by deposition from the vapour phase but to date the structures and chemical composition of the materials produced have not been fully characterised (Malkow,1996; Horvath-Bordon et al., 2006). In a collaboration with T.U. Darmstadt we used the laser heated DAC to synthesise a new defect-wurtzite (dwur) phase of C2N3H (C2N2(NH)) from dicyandiamide (DCDA) precursor at P>27 GPa (Horvath-Bordon et al., 2007). Dwur-C2N3H was recovered to ambient conditions and its structure and composition were determined by electron diffraction and EELS in the TEM along with nano-SIMS and ab initio calculations. Recently we obtained an x-ray diffraction pattern for dwur-C2N3H following high-P,T DAC synthesis and determined the bulk modulus (Ko~250 GPa) in agreement with ab initio calculations (Salamat et al., 2009). The x-ray diffraction results along with Raman scattering indicate that new C–N–H materials remain to be discovered following high-P,T synthesis. Dwur-C2N3H can provide a useful precursor to predicted high-density C3N4 phases (Figure 3).
DFT calculated (1) versus experimental (2) XRD pattern for dwur-C2N3H obtained using LiF as PTM (Salamat et al., 2009).
Summary
High-P,T research including synthesis, in situ studies of structure and properties and theoretical calculations is an important component of solid state science with the identification of new phases and compounds and P,T stability relations. The studies also reveal new materials with potentially important technologically relevant properties.
References
Bailey E., P. F. McMillan, J. Mater. Chem., submitted (2010).
Besson J.-M., G. Hamel, T. Grima, R. J. Nlmes, J. S. Loveday, S. Hull, D. Hausermann, High Pressure Res, 8, 625 (1992).
Bezinge A., K. Yvon, J. Muller, W. Lengauer, P. Ettmayer, Solid State Commun, 63, 141 (1987).
Bovenkerk H. P., F. P. Bundy, H. T. Hall, H. M. Strong, R. H. Wentorf, Nature, 184, 1094 (1959).
Boyd F. R., J. L. England, J Geophys Res, 65, 741 (1960).
Brazhkin V. V., A. G. Lyapin, R. J. Hemley, Phil Mag A, 82, 231 (2002).
Bridgman P. W., The Physics of High Pressure (G Bell and Sons, London, 1931).
Bull C. L., P. F. McMillan, E. Soignard, K. Leinenweber, J Solid State Chem, 177, (2004)
Bundy F. P., W. A. Bassett, M. S. Weathers, R. J. Hemley, H.-k. Mao, A. F. Goncharov, Carbon, 34, 141 (1996).
Bundy F. P., H. T. Hall, H. M. Strong, R. H. Wentorf, Nature, 176, 51 (1955).
Chen X.-J., V. V. Struzhkin, S. Kung, H.-k. Mao, R. J. Hemley, Phys Rev B, 70, (2004).
Ching W. Y., S.-D. Mo, L. Ouyang, Phys Rev B, 63, 245110 (2001a)
Ching W. Y., S.-D. Mo, I. Tanaka, M. Yoshiya, Phys Rev B, 63, 064102 (2001b).
Coes L., J Am Ceram Soc, 38, 333 (1955).
Cohen M. L., Phys Rev B, 32, 7988 (1985).
Dong J., O. F. Sankey, S. K. Deb, G. H. Wolf, P. F. McMillan, Phys Rev B, 61, 11979 (2000).
Dubrovinskaia N., G. Eska, G. A. Sheshin, H. Braun, J Appl Phys, 99, 033903 (2006).
Ekimov E. A., V. A. Sidorov, E. D. Bauer, N. N. Mel’nik, N. J. Curro, J. D. Thompson, S. M. Stishov, Nature, 428, (2004).
Eremets M., High Pressure Experimental Methods (Oxford University Press, Oxford, 1996).
Eremets M. I., V. V. Struzhkin, H. Mao, R. J. Hemley, Science, 293, 272 (2001).
Haines J., J. M. Léger, J. Bocqillon, Ann Rev Mater Sci, 31, 1 (2001).
Hall H. T., Rev Sci Instruments, 31, 125 (1960).
Haüssermann U., S. I. Simak, R. Ahuja, B. Johansson, Phys Rev Lett, 90, (2003).
Hazen R. M., The Diamond Makers (Cambridge Univ Press, Cambridge, UK, 1999).
He D. W., M. Akaishi, B. L. Scott, Y. Zhao, J Mater Res, 17, 284 (2002).
He D., S. Shieh, T. S. Duffy, Phys Rev B, 70, 184121 (2004).
Hector A. L., A. W. Jackson, P. F. McMillan, O. Shebanova, J Solid State Chem, 179, 1383 (2006).
Holzapfel W. D., N. S. Isaacs, High-Pressure Techniques in Chemistry and Physics: A Practical Approach (Oxford University Press, Oxford, 1997).
Horvath-Bordon E., R. Riedel, A. Zerr, P. F. McMillan, G. Auffermann, Y. Prots, R. Kniep, P. Kroll, Chem Soc Rev, 35, 987 (2006).
Horvath-Bordon E., R. Riedel, P. F. McMillan, P. Kroll, G. Miehe, P. van Aken, A. Zerr, P. Hoppe, O. Shebanova, I. McLaren, S. Lauterbach, E. Kroke, R. Boehler, Angew Chem Int Ed, 46, 1476 (2007).
Hubert H., L. A. J. Garvie, K. Leinenweber, P. Buseck, W. T. Petuskey, P. F. McMillan, 410, 191 (1996).
Hubert H., L. A. J. Garvie, P. R. Buseck, W. T. Petuskey, P. F. McMillan, J Solid State Chem, 133, 356 (1997).
Hubert H., B. Devouard, L. A. J. Garvie, M. O’Keeffe, P. R. Buseck, W. T. Petuskey, P. F. McMillan, Nature, 391, 376 (1998a).
Hubert H., L. A. J. Garvie, B. Devouard, P. R. Buseck, W. T. Petuskey, P. F. McMillan, Chem Mater, 10, 1530 (1998b).
Jamieson J. C., A. W. Lawson, N. D. Nachtrieb, Rev Sci Instrum, 30, (1959).
Jayaraman A., Rev Mod Phys, 55, 65 (1983).
Jiang J. Z., F. Kragh, D. J. Frost, K. Stahl, H. Lindelov, J Phys Cond Matter, 13, L515, (2001).
Jiang J. Z., H. Lindelov, L. Gerward, K. Stahl, J. M. Recio, P. Mori-Sanchez, S. SCarlson, M. Mezouar, E. Dooryhee, A. Fitch, D. J. Frost, Phys Rev B, 65, 161202 (2002).
Kaner R. B., J. J. Gilman, S. H. Tolbert, Science, 308, 1268 (2005).
Khvostanstev L. G., V. N. Slesarev, V. V. Brazhkin, High Press Res,24, 371 (2004).
Kinski I., G. Miehe, G. Heymann, R. Theissmann, R. Riedel, H. Huppertz, Z Naturforsch, 60b, 832 (2005).
Krukowski S., M. Bockowski, B. Lucznik, I. Grzegory, S. Porowski, T. Suski, Z. Romanowski, J Phys Cond Matter, 13, 8881 (2001).
Leinenweber K., M. O’Keeffe, M. Somayazulu, H. Hubert, P. F. McMillan, G. H. Wolf, Chem Eur J, 5, 3076 (1999).
Leitch S., A. Moewes, L. Ouyang, W. Y. Ching, T. Sekine, J Phys Cond Matter, 16, 6469 (2004).
Lengauer W., J Cryst Growth, 87, 295 (1988).
Liu L.-G., W. Bassett, Elements, Oxides, Silicates: High-Pressure Phases with Implications for the Earth’s Interior (Oxford University Press, New York, 1986).
Liu A. L., M. L. Cohen, Science, 245, 841 (1989).
Lundstrom T., J Solid State Chem, 133, 88 (1997).
Lundstrom T., Y. G. Andreev, Mater Sci Eng A, 209, (1996).
Machon D., D. Daisenberger, E. Soignard, G. Shen, T. Kawashima, P. F. McMillan, Phys Stat Solidi (a), 203, 831 (2006).
Malkow T., Mater Sci Eng A, 302, 309 (1996).
McMillan P. F., Nature Mater, 1, (2002).
McMillan P. F., Nature Mater, 4, (2005).
McMillan P. F., H. Hubert, A. Chizmeshya, L. A. J. Garvie, W. T. Petuskey, B. Devouard, J Solid State Chem, 147, 281 (1999).
McMillan P. F., O. Shebanova, D. Daisenberger, R. Quesada Cabrera, E. Bailey, A. L. Hector, V. Lees, D. Machon, A. Sella, M. Wilson, Phase Trans, 80, 1003 (2007).
Oganov A. R., J. Chen, G. C, M. Y, C. W. Glass, Z. Liu, T. Yu, O. O. Kurakevych, V. L. Solozhenko, Nature, 457, (2009).
Oyama S. T., The Chemistry of Transition Metal Carbides and Nitrides (Blackie Academic, Glasgow, 1996).
Pal’yanov Y. N., A. G. Sokol, Y. M. Borzdov, A. F. Khokhryakov, N. V. Sobolev, Am Mineral, 87, 1009 (2002).
Papaconstantopoulos D. A., M. K. Mehl, Phys Rev B, 65, 172510 (2002).
Piermarini G. J., J Res Nat Inst Standards Technol, 106, 889 (2001).
Rizzo H. F., W. C. Simmons, H. O. Bielstein, J Electrochem Soc, 109, 1079 (1962).
Salamat A., K. Woodhead, P. F. McMillan, R. Quesada Cabrera, J.-P. Perrillat, A. Rahman, D. Adriens, F. Cora, Phys Rev B, 80, 104106 (2009)
Scotti N., W. Kockelmann, J. Senker, S. Traβel, H. Jacobs, Z Anorg Allg Chem, 625, 1435 (1999).
Schnick W., Angew Chem Int Ed, 38, 3309 (1999).
Schwarz M., A. Zerr, E. Kroke, G. Miehe, I.-W. Chen, M. Heck, B. Thybusch, B. T. Poe, R. Riedel, Angew Chem Int Ed, 41, 788 (2002).
Sekine T., H. He, T. Kobayashi, M. Tansho, K. Kimotot, Chem Phys Lett, 344, 395 (2001).
Sekine T., H. He, T. Kobayashi, M. Zhang, F. Xu, Appl Phys Lett, 76 (2000).
Serghiou G., G. Miehe, O. Tschauner, A. Zerr, R. Boehler, J Chem Phys, 111 (1999).
Shebanova O., P. F. McMillan, E. Soignard, High Pressure Res, 26, 87 (2006).
Soignard E., M. Somayazulu, J. Dong, O. F. Sankey, P. F. McMillan, J Phys Cond Matter, 13, 557 (2001).
Soignard E., D. Machon, P. F. McMillan, J. Dong, B. Xu, K. Leinenweber, Chem Mater, 17, 5465 (2005).
Sokol A. G., Y. N. Pal’yanov, G. A. Pal’yanova, A. F. Khokhryakov, N. V. Sobolev, Diamond Related Mater, 10, 2131 (2001).
Solozhenko V. L., High Pressure Res, 22, 519 (2002a).
Solozhenko V. L., Phys Chem Chem Phys, 4, 1033 (2002b).
Solozhenko V. L., D. Andrault, G. Fiquet, M. Mezouar, D. C. Rubie, Appl Phys Lett, 78, 1385 (2001a).
Solozhenko V. L., S. N. Dub, N. Novikov, Diamond Related Mater, 10, 2228 (2001b).
Solozhenko V. L., Y. Le Godec, S. Klotz, M. Mezouar, V. Z. Turkevich, J.-M. Besson, Phys Chem Chem Phys, 4, 5386 (2002a).
Solozhenko V. L., V. Z. Turkevich, O. O. Kuryakevych, W. A. Crichton, M. Mezouar, J Phys Chem B, 106, 6634 (2002b).
Solozhenko V. L., Y. Le Godec, O. O. Kurakevych, C R Chimie, 9, 1472 (2006).
Sumiya H., N. Toda, S. Satoh, New Diamond Frontier Carbon Technol, 10, 233 (2000).
Sung C.-M., M.-F. Tai, Int J Refractory Metals Hard Mater, 15, 237 (1997).
Tanaka I., F. Oba, T. Sekine, E. Ito, A. Kubo, K. Tatsumi, H. Adachi, T. Yamamoto, J Mater Res, 17, 731 (2002).
Taniguchi T., T. Teraji, S. Koizumi, K. Watanabe, S. Yamaoka, Jpn J Appl Phys, 41, L109 (2002).
Teter D., Mater Res Soc Bull, 23, 22 (1998).
Teter D., R. J. Hemley, Science, 271 (1996).
Toth L. E., Transition Metal Carbides and Nitrides (Academic, New York, 1971).
Van Valkenburg A., Rev Sci Instrum, 33 (1962).
Veprek S., J Vac Sci Technol A, 17, 2401 (1999).
von Platen B., in: Modern Very High Pressure Techniques, ed. R. H. Wentorf (Butterworth, Washington DC, 1962), p. 118.
Watanabe K., T. Taniguchi, H. Kanda, Nature Materials, 3 (2004).
Weir C. E., E. R. Lippincott, A. Van Valkenburg, E. N. Bunting, J Res Nat Bur Standards, 63A, 55 (1959).
Wentorf R. H., J Chem Phys, 26, 956 (1957).
Yokoya T., T. Nakamura, T. Matsushita, T. Muro, Y. Takano, M. Nagao, T. Takenouchi, H. Kawarada, T. Oguchi, Nature, 438, 647 (2005).
Zerr A., G. Miehe, G. Serghiou, M. Schwarz, E. Kroke, R. Riedel, H. Fueβ, P. Kroll, R. Boehler, Nature, 400 (1999).
Acknowledgments
PFM acknowledges support from EPSRC SRF EP/D07357X and Portfolio grant EP/D504782 (held jointly with C.R.A. Catlow and P. Barnes). The work reported for dwur-C2N3H forms part of the Ph.D. research of A. Salamat and K. Woodhead. New data on superconducting MoN and related oxynitride phases were obtained by Dr. E. Bailey at UCL.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this paper
Cite this paper
McMillan, P.F. (2010). High-Pressure Synthesis of Materials. In: Boldyreva, E., Dera, P. (eds) High-Pressure Crystallography. NATO Science for Peace and Security Series B: Physics and Biophysics. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9258-8_30
Download citation
DOI: https://doi.org/10.1007/978-90-481-9258-8_30
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9257-1
Online ISBN: 978-90-481-9258-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)