Abstract
The Tasmanian devil (Sarcophilus harrisii), so named for its blood-curdling, nocturnal shrieks and snarls, is the largest of the carnivorous marsupials. Although once widely persecuted, concerted efforts are now being made to save the devil from extinction following the emergence of a fatal transmissible malignancy known as devil facial tumour disease (DFTD). DFTD is unusual in that the infectious agent is the cancer cell itself. This chapter discusses the aetiology and pathogenesis of DFTD as well as the profound impact the spread of DFTD has had on the devil’s conservation status. Strategies for managing DFTD and conserving the devil will be explored and the contribution of new sequencing technology to the field of conservation genetics and genomics will be examined with regard to the Tasmanian devil and DFTD.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 The Tasmanian Devil and the Emergence of DFTD
The Tasmanian devil (Sarcophilus harrisii), is the largest of the Dasyuridae, a speciose and morphologically diverse family of carnivorous marsupials that are represented in most terrestrial habitats in Australia and New Guinea (Crowther and Blacket, 2003). S. harrisii diverged in the mid Miocene, approximately 12.4 million years ago, and originally thrived on the Australian mainland (Krajewski et al., 2000). Tasmanian populations were separated from their mainland conspecifics after the formation of the Bass Strait 14,000 years ago (Lambeck and Chappell, 2001). Now the devil is confined to the island state; their extinction on mainland Australia approximately 400 years ago was likely associated with the introduction and establishment of the dingo (Jones et al., 2003). Since the extinction of the devil’s larger, sympatric relative, the thylacine (Thylacinus cynocephalus), the Tasmanian devil has held the mantle of World’s Largest Remaining Marsupial Carnivore.
Tasmanian devils are non-territorial, nocturnal, hunter-scavengers. Adults typically inhabit wide ranges throughout open sclerophyll forest and coastal scrub and are found at low densities in alpine areas, extensively cleared agricultural land and dense forests (Guiler, 1970; Jones et al., 2004). Although occasionally predatory, devils are largely specialised scavengers and opportunistic feeders (Owen and Pemberton, 2005). Devils are a relatively short-lived species, living for 5–6 years in the wild. Mature devils are sexually dimorphic; males weigh up to 13 kg and females are considerably smaller at around 9 kg. Female devils are monoestrus with mating usually occurring in March. Four pouch young can be accommodated in the female’s rear-facing pouch and weaning success is high in the wild. Joeys leave the pouch at about 105 days, are independent at 9 months and reach sexual maturity themselves at 2 years of age (Guiler, 1970).
There is anecdotal evidence that devil populations have suffered from several fluctuations in the 200 years since European settlement (Guiler, 1970). No reliable data exist to verify the extent or cause of these population fluxes, however infectious disease, the extinction of the thylacine and anthropogenic factors, such as habitat destruction and culling, have all been implicated (Jones et al., 2004; Bradshaw and Brook, 2005). Certainly both the devil and the thylacine were singled out as “stock-destroying vermin” and were the subject of intense persecution by graziers for over 100 years (Owen and Pemberton, 2005). Molecular marker profiling of devils across Tasmania has revealed poor population structuring and a distinct lack of genetic diversity at microsatellite and MHC loci, consistent with periods of low population density (Jones et al., 2004; Siddle et al., 2007). Prior to the emergence of DFTD, however, devil populations were robust and widespread across Tasmania with the exception of the southwest where population density was and remains low (Jones et al., 2004).
In 1996 a devil with a large facial lesion was photographed at Mt William in the state’s northeast (Hawkins et al., 2006). Although striking, the tumour was not thought to be particularly unusual as devils seem to be particularly susceptible to neoplasia (Griner, 1979; Canfield et al., 1990). In subsequent years, however, an increasing number of devils from northeast Tasmania presented to the Department of Primary Industries and Water’s Animal Health Laboratories with grossly similar facial tumours. As tumour cases appeared further afield it became apparent that the developing tumour epidemic was due to an infectious process.
2 DFTD Pathogenesis
DFTD is an aggressive, debilitating malignancy. Tumorigenesis is characterised by the proliferation of round to spindle-shaped cells in the dermis of the head and neck or the submucosal tissue of the oral cavity. Small nodules rapidly progress to become large, multicentric masses with ulcerating, exudative surfaces (Fig. 23.1a, b). DFTD is locally invasive and highly metastatic with regional lymph node involvement and metastasis to thoracic and abdominal viscera occurring in 65% of cases (Loh et al., 2006a) (Fig. 23.1c). Disease progression is rapid and the mortality rate is 100%. Affected animals may die as little as 3 months after the first appearance of lesions (Hawkins et al., 2006). Mortality results when large primary tumours obstruct feeding, leading to starvation, or from complications associated with metastasis (Pyecroft et al., 2007). The typical histological appearance of DFTD, characterised by multinodular aggregates of undifferentiated round cells, prompted early suggestions that DFTD was a lymphosarcoma, possibly of viral aetiology (Ladds et al., 2003). Immunostaining subsequently revealed a neuroectodermal origin for the tumour and it was noted that DFTD shares gross pathological and immunophenotypic features with Merkel cell carcinoma of humans (Loh et al., 2006b). Sophisticated sequencing approaches to investigating DFTD recently elucidated the cell lineage of the tumour which was shown to express genes characteristic of Schwann cells or Schwann cell precursors (Murchison et al., 2010).
Gross pathology – DFTD lesions initially appear as small, well-circumscribed nodules arising in the dermis of the head and neck, or the submucosa of the oral cavity (a) and rapidly become locally invasive, often preventing affected animals from feeding (b). Metastasis to thoracic viscera is common. Image c depicts a shower of small, nodular metastases on the serosal surface of the lungs
3 DFTD Cytogenetics: Evidence for Cellular Transmission
The genetic basis of neoplasia has been recognised for over 100 years, with cytogenetic observations providing the first insights into the clinical significance of chromosomal anomalies. In 1914 Theodor Boveri first suggested that an excessive number of chromosomes might be a cause of malignancy. Aneuploidy is now known to be one of the most common features of cancer, found in approximately 90% of solid tumours and 75% of haematological tumours in humans (Weaver and Cleveland, 2006). It remains unclear whether aneuploidy, when present, is a driver of carcinogenesis or whether it is simply an incidental finding, a background against which other processes initiate and steer carcinogenesis. Evidence from naturally occurring tumours suggests that aneuploidy represents one of several types of genetic instability which renders cells susceptible to the accelerated acquisition of mutations, eventually leading to cancer development (Lengauer et al., 1997). The underlying cause of aneuploidy is also unclear although several causative gene mutations have been determined, many involving the mitotic spindle checkpoint (Rajagopalan and Lengauer, 2004). In addition to aberrations in chromosome number, cancer cells are often characterised by structural chromosome defects such as translocations, inversions, duplications or deletions. Recurrent chromosome rearrangements may even be pathognomonic for specific cancer types and in some cases cytogenetic techniques are crucial prognostic tools (Mitelman et al., 1997). One of the best characterised chromosome rearrangements is the Philadelphia (Ph) chromosome, formed by the reciprocal translocation of the q arms of chromosomes 9 and 22, found in 90% of cases of human chronic myelogenous leukaemia (CML) (Nowell and Hungerford, 1960; Deininger and Druker, 2003). The Ph chromosome was the first recurring chromosome rearrangement to be associated with a specific cancer type. Since this breakthrough additional haematological malignancies, as well as some solid tumours, have been shown to be caused by recurring chromosome rearrangements (Mitelman, 2000).
Tumour development is now known to be a dynamic process akin to Darwinian evolution. Cancer cells progressively acquire somatic mutations and those that afford a growth advantage are selected for and propagated in waves of clonal expansion (Nowell, 1976). This is evident in the heterogeneity of tumour cell populations, each neoplastic clone reflecting a new step in the progression from benign to malignant. Favourable mutations confer on cancer cells a proliferative advantage and are manifest as the physiological traits that define malignancy; namely acquired growth signal autonomy, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative lifespan, angiogenesis and metastatic capability (Hanahan and Weinberg, 2000).
The discipline of cancer cytogenetics has been crucial to our understanding of DFTD transmission and development. In early investigations of DFTD transmission, electron microscopy failed to detect evidence of viral involvement in DFTD carcinogenesis (Loh et al., 2006b). Not until cytogenetic investigations of DFTD were undertaken was it suggested that DFTD tumour cells were themselves the causative agent (Pearse and Swift, 2006). Diploid S. harrisii cells contain 12 autosomes and a pair of sex chromosomes; XX in female devils and XY in males (Fig. 23.2a). G-banding of metaphase spreads revealed DFTD cells to be aneuploid and marked by extensive chromosome rearrangements. Moreover, these chromosome rearrangements were identical in every animal studied. The DFTD karyotype is distinguished by the loss of both copies of chromosome 2, one copy of chromosome 6 and both sex chromosomes, and the addition of four unidentified marker chromosomes (Fig. 23.2b). This signature karyotype is found in all tumours regardless of the sex of the affected animal or the stage of DFTD progression. It is highly improbable that the same complex tumour karyotype independently arises from a recurring rearrangement in every affected animal. Even if it were possible, the stepwise accumulation of progressive chromosome aberrations should be evident within individual tumours and between animals. However, no intermediate stages of karyotypic evolution have been observed; all affected animals studied so far have a homogeneous tumour cell population that is more or less identical to the original G-banded tumour. Additional subtle chromosome rearrangements have been recognised in some animals and also appear to be stable and conserved (Department of Primary Industries, 2009). The clinical significance of these karyotypic “strains” is not known, nor whether they represent selection for mutations that confer favourable tumour traits. Nevertheless, the basic clonality of DFTD strongly suggests that tumours are not endogenously derived but are transplanted between animals, much like a tissue allograft. Pearse and Swift’s serendipitous discovery of one animal with a pericentric inversion of chromosome 5 confirmed that DFTD could not be host derived, as the inversion was present only in the devil’s lymphocyte preparations and not his tumour (Pearse and Swift, 2006). Genotyping of host and tumour tissue at microsatellite and MHC loci has verified that DFTD is a clonal cell line that is genetically different from the animals it is grafted to (Siddle et al., 2007). The infectious nature of DFTD has been confirmed by transmission trials, in which DFTD pathogenesis was reproduced after subcutaneous inoculation of naïve animals with culture-derived tumour cells (Pyecroft et al., 2007). The manner in which cellular transmission occurs is yet to be definitively identified, however, the devil’s tendency to bite during mating and communal feeding (Hamede et al., 2008), and the friable consistency of the facial tumours suggests that DFTD is transplanted by biting (Pearse and Swift, 2006).
Tumour cytogenetics – (a) The diploid Tasmanian devil karyotype consists of 14 chromosomes, including two sex chromosomes (X and Y in this male devil). (b) The DFTD karyotype (strain 1) is aneuploid and distinguished by stable chromosome rearrangements. Several chromosomes are rearranged, identifiable only as four marker chromosomes
Although it is tempting to speculate about the possible causes of DFTD, it is unlikely that we will ever know whether the initiating event was exogenously induced or a spontaneously arising somatic mutation. By the time DFTD was discovered in 1996, the tumour was already a transmissible cell line; a thriving clone that out-competed its precursors to become the sole cell type in a highly malignant tumour.
4 Immunogenetics of Transmissible Tumours
Naturally occurring cellular transmission of an infectious tumour has been reported in only one other disease. Canine transmissible venereal tumour (CTVT) is a sexually transmitted histiocytic tumour of domestic dogs (Cohen, 1985; Mozos et al., 1996). The tumour has a global distribution with a higher prevalence in countries with large populations of free-roaming dogs. Transplantation of tumour cells occurs during coitus when primary tumours of the external genitalia make direct contact with vaginal or penile mucosa. Metastasis is uncommon (<5%) and tumours are responsive to vincristine chemotherapy. There are variable reports of naturally occurring remission in immunocompetent dogs; these animals develop a robust antibody response that is protective upon subsequent re-exposure (Das and Das, 2000). Like DFTD, CTVT is characterised by a conserved, highly rearranged karyotype that is found in tumours worldwide (Fujinaga et al., 1989). In addition to its characteristic cytogenetic aberrations, CTVT cells can be distinguished from host cells by the insertion of a LINE (long interspersed nuclear element) transposable element upstream of the c-myc oncogene (Katzir et al., 1987). CTVT is a much older cell line than DFTD, having arisen up to 15,000 years ago in a wolf or early breed of dog (Rebbeck et al., 2009). The common ancestor of existing CTVT clones existed long after the tumour’s origin, until about 200 years ago, indicating that today’s cell line is a particularly successful clone that has overtaken the original cell line. Indeed, CTVT has evolved an effective means of escaping the host immune response, modifying cell surface expression of major histocompatibility complex (MHC) molecules in order to invade by stealth. The MHC locus is comprised of a highly polymorphic set of genes that encode two classes of antigen-presenting molecules found on the surface of cells. Class I and II MHC molecules bind the targets of antibody and cytotoxic T cell responses and are important in the adaptive immune response against pathogens, tumours and foreign grafts (Parham et al., 1989). Class I molecules are expressed on the surface of all cells and are responsible for recognition of endogenous antigens, whereas class II molecules recognise exogenous antigens and are only found on the surface of antigen presenting cells. Structurally, MHC molecules consist of conserved lengths of amino acids that stabilise the protein, and a rapidly evolving, highly polymorphic peptide binding region (PBR) that enables recognition of a diverse range of antigens (Bjorkman et al., 1987; Hughes and Nei, 1988). Modulation of MHC expression is commonly employed by tumour cells to overcome host immunity and achieve uninhibited growth (Garcia-Lora et al., 2003). CTVT cells downregulate MHC (or DLA; dog leukocyte antigen) class I and II antigen expression during transplantation and progression so that tumour growth can occur in the absence of a host immune response (Murgia et al., 2006; Hsiao et al., 2008). Those tumours that spontaneously regress do so because MHC expression is reinstated, provoking infiltration by lymphocytes and macrophages, which aid in graft rejection through cytokine secretion (Cohen, 1985; Hsiao et al., 2008).
How then has DFTD managed to out-manoeuvre the devil’s immune system in order to become a thriving somatic cell parasite? Unlike CTVT, DFTD does not appear to manipulate its MHC expression to escape the host immune response, as it has been shown to express both class I and II MHC genes (Siddle et al., 2007). Nevertheless, DFTD transplants do not activate host T cells as inflammatory cell infiltration of tumours is limited (Loh et al., 2006a). This aspect of DFTD prompted a number of studies of the basic functionality of the Tasmanian devil immune system.
Devils have a full complement of lymphoid organs and a typical range of circulating white blood cells (Woods et al., 2007). They are able to produce a robust antibody response and their neutrophils effectively phagocytose and digest bacteria (Kreiss et al., 2008; Kreiss et al., 2009). Lymphocytes isolated from the peripheral blood of healthy devils proliferate when stimulated by T-cell mitogens (PHA and Con A), however, pooling of lymphocytes from different devils fails to induce a lymphocyte response (Siddle et al., 2007; Woods et al., 2007). The devil, therefore, is immunologically competent, except when challenged with allogeneic tissue. In an outbred population, polymorphism of MHC class I genes enables recognition and rejection of tissue transplants from unrelated individuals (Hughes and Nei, 1988). Conversely, poor MHC diversity impedes recognition of non-self and has been shown to facilitate allograft acceptance in inbred wild populations of cheetah (Acinonyx jubatus) (Yuhki and O’Brien 1990) and pocket gopher (Thomomys bottae) (Sanjayan et al., 1996). Genotyping of MHC genes from 21 Tasmanian devils demonstrated extremely low diversity, particularly at class I loci (Siddle et al., 2007). Low MHC polymorphism and failure of mixed lymphocyte responses strongly suggest that DFTD is transmitted due to histocompatibility between tumour and host devils. CTVT may have similarly emerged when the domestication process caused a genetic bottleneck, facilitating allograft acceptance due to host-graft compatibility. In the following 15,000 years the canine tumour has evolved strategies that enable it to affect outbred hosts (Murgia et al., 2006). Another transmissible tumour, which has arisen in inbred laboratory populations of the Syrian hamster is also thought to be due to a paucity of MHC diversity (Banfield et al., 1965; McGuire et al., 1985). DFTD, however, is the first transmissible tumour to threaten a wild species with extinction and provides a conclusive link between MHC polymorphism and population health.
5 DFTD Epidemiology and Impact
Wildlife extinction due solely to infectious disease is considered a rare event. In fact, species extirpation due to disease outbreaks has been conclusively shown in very few vertebrate species: Taudactylus acutirostris, an Australian frog (Schloegel et al., 2006); and the Christmas Island rats, Rattus macleari and Rattus nativitatis (Wyatt et al., 2008). The causative agents in these cases, respectively, Batrachochytrium dendrobatidis and Trypanosoma lewisi, are both able to affect more than one species or persist in an unaffected reservoir host, an important factor in their success as pathogens. According to standard epidemiological models, it is unlikely for a host-specific pathogen to cause the extinction of a wild species as transmission rates tend to decrease as host density decreases (McCallum and Dobson, 1995). Yet, the emergence of DFTD, a single-host pathogen, has profoundly affected the conservation status of the Tasmanian devil. This is due in large part to the manner in which the disease is transmitted. DFTD transmission is frequency-dependent, meaning the rate of transmission depends on the frequency of host contact, not on host density (McCallum, 2008; Lachish et al., 2009). This important epidemiological detail was determined from mark recapture data in northeast Tasmania. In the Mt William area where DFTD was first reported, tumour prevalence remains as high as 33% despite devil population declines of 90% (Lachish et al., 2007). This model of disease transmission is consistent with our understanding of devil behaviour and DFTD dissemination. The tumour is transmitted by direct contact, most likely via bite wounds. Mating interactions are associated with a higher incidence of penetrating facial bites (Hamede et al., 2008) and DFTD may therefore be considered a sexually transmitted disease, most of which, in humans, are transmitted in a frequency-dependent manner.
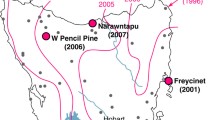
One of the major implications of frequency-dependent transmission is that DFTD is propagated even when devil populations are low. Density-dependent diseases (infectious disease such as influenza viruses are modelled in this manner) require host population density to remain above a certain threshold for disease maintenance. When host density decreases to a critical level disease transmission is no longer possible. It is therefore considered improbable for density-dependent disease to cause extinction, except in the presence of other contributing factors (Hilker et al., 2009). By contrast, DFTD is unlikely to peter out even when devil densities are extremely low. It is this aspect of DFTD transmission that has had such a strong impact on devil numbers. In 1996, when the first case of DFTD was observed, the devil was at a low risk of extinction (Maxwell et al., 1996). Since then, the state-wide devil population is thought to have diminished by more than 60%. The impact of DFTD has been modelled from data collected on the Freycinet Peninsula on Tasmania’s east coast where devil populations were closely monitored before and after DFTD’s appearance there in 2001. The arrival of DFTD on the peninsula coincided with a profound decline in devil numbers and caused acute changes to the life-history and age structure of the population. Because transmission of DFTD usually occurs during mating interactions, the devils most at risk are those of breeding age. Eight years after DFTD first swept through Freycinet National Park, the devil population is almost completely comprised of animals younger than 3 years of age, with adult devils accounting for a mere 20% of the population (Lachish et al., 2009). There has been an increase in precocial breeding throughout the peninsula, with females producing their first litter at 1 year of age instead of 2 (Jones et al., 2008). The compensatory value of this reproductive response is limited however; in affected populations the prevalence of disease among 2 year olds exceeds 50% and many females will not survive to wean their only litter (McCallum, 2008). In little more than 10 years, DFTD has swept westwards throughout Tasmania and now threatens the last remaining disease-free populations in Tasmania’s far northwest (Fig. 23.3). Given the rapid spread of DFTD, it is likely that in 5–10 years time the disease will occur across the entire range of the devil, with wild Tasmanian devil extinction projected to occur within 25–35 years (McCallum et al., 2007). Given this alarming prognosis, the Tasmanian devil was listed as an endangered species by the International Union for the Conservation of Nature and Natural Resources (IUCN) in May 2008 (IUCN, 2009).
Spread of DFTD throughout Tasmania, adapted from (McCallum, 2008) – the logarithmic scale on the right of the map depicts a timeline from Tasmanian devil divergence to the formation of the Bass Strait and the devil’s extirpation from mainland Australia following the introduction of the dingo. The final segment on the scale correlates with the spread of DFTD since 1996 when the disease was first reported. It is predicted that DFTD will be present throughout the devil’s range by 2020
An additional complicating factor in the emergence of DFTD relates to the most recent introduction of foxes to Tasmania (Saunders et al., 2006). It is thought that the existence of devils in Tasmania has prevented foxes from becoming established following previous incursions over the last 100 years. The loss of an apex predator can precipitate widespread ecosystem disturbances with effects on mesopredator abundance being of particular concern (Ritchie and Johnson, 2009). The wild extinction of Tasmanian devils would leave their predatory niche vulnerable to appropriation by foxes or feral cats. Foxes have been implicated in the widespread extinction of small mammals on the Australian mainland, a catastrophe that Tasmania’s biodiversity has hitherto been free from (Kinnear et al., 2002).
6 Disease Management
The emergence of DFTD has highlighted the importance of early, decisive responses in the management of wildlife disease outbreaks. Humane destruction of infected animals may have limited transmission sufficiently to achieve disease eradication when the tumour first emerged, however, DFTD was not immediately suspected to be an infectious disease and culling of infected animals remained contentious even after overwhelming evidence indicated that the tumours were transmissible. As a single-host pathogen transmitted by direct contact in a population contained on a relatively small island, DFTD constituted a comparably manageable pathogen (Cousins and Roberts, 2001). Nevertheless, field monitoring was prioritised and by the time it was agreed that intervention was urgently required, DFTD was too widespread to eradicate by culling. Studies on the Forestier Peninsula on the east coast of Tasmania indicate that some disease suppression can be achieved by systematically removing infected animals from the population (Jones et al., 2007). Although widespread euthanasia of infected devils is not a pragmatic management solution at this stage, strategic culling may still be employed in high priority devil populations, such as those near the advancing disease front, or in infected populations thought to be genetically valuable.
Management options are now focused on conserving “insurance” stocks of disease-free animals for re-release in the event of wild extinction. The insurance strategy aims to conserve as much genetic diversity as possible in a DFTD-free population that will be physically and behaviourally fit for eventual release (DPIW and ARAZPA, 2007). The strategy recommends sourcing founder devils from DFTD-free wild populations according to a strict protocol that minimises the risk of introducing an infected devil into the insurance population. Founder devils are quarantined to verify their DFTD-free status before being transferred to mainland zoos for intensively managed breeding. Based on conservative modelling, a founder base of 150 individuals is predicted to conserve 95% of wild source genetic diversity (Jones et al., 2007). The successful rehabilitation of species such as the black-footed ferret have demonstrated that wild species may be rescued from extinction by breeding from much smaller founding populations (Grenier et al., 2009).
If devils were to become extinct in the wild, the ecosystem-wide impacts of their absence could be catastrophic. Even the transient loss of devils from their predatory niche may facilitate mesopredator release with dire consequences for Tasmania’s biodiversity. The maintenance of free-ranging devils in fenced-off, disease-free exclosures or off-shore Tasmanian islands has been discussed as a management strategy to ensure that insurance devils remain an “ecologically functional” species (Jones et al., 2007). Neither strategy has as yet been employed; a fencing strategy is likely to be expensive and difficult to engineer, and the release of devils onto an island ecosystem that has not previously supported them remains contentious.
Vaccine production remains a long-term solution for DFTD control. Vaccination is a cornerstone of disease prevention in veterinary and human medicine, and is playing an increasingly important role in wildlife disease control (Plumb et al., 2007). The age of targeted cancer therapy was heralded by the development of imatinib, a tyrosine kinase inhibitor that acts directly on the fusion gene product created by the Ph chromosome rearrangement in CML patients (Deininger and Druker, 2003). Like CML, DFTD is associated with a pathognomonic karyotype, however, with little known about the clinical significance of DFTD-associated chromosome rearrangements, no targets have been identified for treatment or prevention. One of the difficulties in developing cancer vaccines lies in the antigenicity of tumour cells. Unlike virus-associated cancers, spontaneously arising tumour cells may be only weakly immunogenic and unless a tumour-specific antigen can be identified there is a risk of inducing autoimmunity (Rice et al., 2008). At this stage DFTD has not been characterised sufficiently to identify tumour-specific therapeutic targets. Should this challenge be negotiated, the stability of the DFTD genome may lend itself to vaccine development. Chromosomally unstable tumours present a therapeutic challenge as CIN provides an adaptive mechanism by which tumours are able to evolve drug resistance (Rajagopalan et al., 2003). Cytogenetic observations of DFTD indicate that the karyotype is stable, suggesting that a targeted therapy might be successful in treating disease. The failure of DFTD to elicit any immune response in the devil, however, may limit attempts to induce immunity through vaccination (Woods et al., 2007). Additionally, the development of a novel vaccine that is safe and efficacious is a lengthy and expensive process, and the successful delivery of vaccines to free-ranging wildlife presents yet another challenge (Haydon et al., 2006). At this stage it is unlikely that a vaccine will be produced in time to prevent DFTD from spreading throughout the range of devil. Nevertheless, even 10 years from now, a DFTD vaccine would be a valuable management resource for demographically strategic use in habitat corridors and along the frontline of the disease, as well as protection of insurance devils prior to release. Further research is required in order to determine whether vaccination remains a viable option in the management of DFTD.
One possible, though improbable, outcome of DFTD emergence is natural selection for resistance to infection (McCallum, 2008). It is not known whether the faint genetic variation between east and west coast devils correlates with sufficient MHC diversity to enable antigen recognition and antibody production in western Tasmanian devils. This seems unlikely as no resistant devils have been observed in any of the widespread mark-recapture programs. The rate at which DFTD continues to sweep through Tasmania leaves little time for the evolution of resistance and the likelihood of adaptive variation occurring in an endangered species with poor genetic diversity is low (Kohn et al., 2006).
7 A Role for Genomics in Tasmanian Devil Conservation
Advances in the development of genetic markers have been of great value to conservation programs and are now essential tools in the management of endangered populations. Traditionally these markers have been used to gauge genetic variation and population structure, estimate effective population size, resolve phylogenetic and phylogeographic queries and guide captive breeding programs (Primmer, 2009). Rapid developments in sophisticated new genome sequencing technology and the explosion of genomic resources available to non-model organisms have already had a significant impact on the management of some endangered species. The endangered California condor (Gymnogyps californianus), largest of the North American land birds, suffered an acute population bottleneck associated with severe habitat loss and lead poisoning. Genetic variation within the tiny founder population was assessed from restriction fragment length polymorphism (RFLP), mitochondrial DNA and microsatellite studies and was used to advise a successful captive breeding program. A high prevalence of autosomal recessive chondrodystrophy complicated conservation efforts by causing an unacceptable incidence of embryonic mortality. In the absence of an assay to identify carrier birds it was impossible to eliminate the condition without compromising genetic diversity in the breeding population. Cross-species fluorescent in situ hybridisation (FISH) of condor metaphase spreads with chicken chromosome paints indicated strong homology between the two species (Raudsepp et al., 2002). Subsequently, a comparative chicken-condor map was generated by screening a condor BAC library using overgo probes obtained from the zebra finch and chicken genome projects. In total 93 loci were mapped, including several candidates for inherited chondrodystrophy (Romanov et al., 2006). A candidate gene approach to mining the BAC library is being complemented by single nucleotide polymorphism (SNP) screening of the condor transcriptome, generated by 454 sequencing of fibroblast cDNA libraries (Romanov et al., 2009). The California condor conservation program has demonstrated how sophisticated genomic resources, and in particular new sequencing technology, are enabling rapid advances in threatened species conservation. Even those species where the resources are not available for large scale sequencing projects may eventually be “genome-enabled”; nearly 1,000 IUCN red-listed mammals are within the same order or group of a sequenced species (Kohn et al., 2006). Therefore the genome of the domestic cat (Felis catus) will not only contribute to the health and well-being of companion animals but will also benefit the management of endangered wild felids (O’Brien et al., 2008).
Like the condor, the Tasmanian devil will benefit from the application of new genomic resources to disease management. Genetic and genomic approaches have already been instrumental in demonstrating the aetiology and pathogenesis of DFTD. The disease was thought to be virally or environmentally induced until cytogenetic and genotyping studies convincingly showed that DFTD was a clonal cell line. The genomic toolkit is gradually being expanded to include genome sequence and gene expression data that will contribute greatly to management of insurance populations and laboratory investigations of DFTD carcinogenesis.
Next generation sequencing technologies have revolutionised cancer genomics. Shotgun sequencing provides an unbiased approach to rapidly discovering pathologically significant mutations. Whereas candidate gene approaches have proved laborious and protracted, tumour genome sequencing has dramatically increased the catalogue of cancer-associated somatic mutations, even for those malignancies that have been rigorously studied for decades (Ding et al., 2008; Ley et al., 2008). Deep sequencing of the DFTD transcriptome sequence revealed a pattern of gene expression consistent with that of myelinating cells (Murchison et al., 2010). It was subsequently determined that the tumour expresses the Schwann cell-specific myelin protein periaxin (PRX), suggesting that DFTD arose from a Schwann cell or a Schwann cell precursor. Using new sequencing technology to refine DFTD cell classification beyond its broad immunostaining characteristics has therefore generated a new, specific diagnostic marker (PRX) and will provide crucial insights into the molecular pathways involved in carcinogenesis, possibly revealing therapeutic targets. The catalogue of expressed genes yielded by the devil transcriptome provides a framework with which to investigate gene expression across a large number of tumours at different stages of progression and from various locations in Tasmania. The significance of the various cytogenetic strains of DFTD may be clarified and surveillance for tumour evolution that is not cytogenetically evident will be possible. Sequencing projects evidently make critical contributions to endangered species management programs and should continue to be a priority in the conservation of the Tasmanian devil.
8 Implications of DFTD for Conservation Biology and Future Directions
Emerging diseases of wildlife are increasingly underlining the relationship between environmental, human and wildlife health. The emergence of zoonotic diseases such as severe acute respiratory syndrome (SARS) and Hendra virus have highlighted the importance of conservation medicine to global health (Daszak and Karesh). The resources pouring into wildlife disease are largely directed at those zoonotic pathogens that pose a spill-over risk to the public. Although cancer is an important cause of mortality in wild species, resources tend to be limited for investigating such diseases (McAloose and Newton, 2009). Regardless, considerable progress has been made in DFTD research. An integrated approach has elucidated the immunogenetic and epidemiological background against which such an unusual pathogen has been able to emerge and take hold. Continued efforts towards understanding DFTD tumorigenesis and conserving genetic diversity may yet rescue this iconic species from extinction.
Three particularly important lessons have emerged from the outbreak of DFTD. Firstly, DFTD has illustrated the vulnerability of inbred wild populations to the emergence of new pathogens and the importance of maintaining genetic diversity at functionally important loci such as the MHC. Genetic variation is crucial to population fitness in the event of emerging infectious disease (O’Brien and Evermann, 1988). The profound impact of DFTD on Tasmanian devil numbers provides an alarming example of the potential consequences of inbreeding and loss of genetic diversity in wild populations. Secondly, DFTD has challenged our understanding of the dynamics of infectious disease in free-living wildlife. It is clear now that, counter to prevailing theory, single-host pathogens can pose a major extinction risk when disease transmission is frequency-dependent. Finally and perhaps most importantly, the DFTD outbreak underscores the importance of rapid responses to wildlife disease, even when very little is known about the causative pathogen or mode of transmission. In the case of DFTD, considerable progress has been made in a short amount of time, however, the outlook for the devil is tenuous at best. New high-throughput sequencing platforms are proving essential to conservation genomics. In the case of the devil, a well-stocked genomic toolkit will equip conservationists with the means to judiciously manage insurance populations and will provide a gene-level insight into DFTD pathogenesis, bringing us closer to the development of preventative or curative therapy.
References
Banfield WG, Woke PA, Mackay CM, Cooper HL (1965) Mosquito transmission of a reticulum cell sarcoma of hamsters. Science 148:1239–1240.
Bjorkman PJ, Saper MA, Samraoui B, et al. (1987) Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329:506–512.
Bradshaw CJA, Brook BW (2005) Disease and the devil: density-dependent epidemiological processes explain historical population fluctuations in the Tasmanian devil. Ecogeography 28:181–190.
Canfield P, Hartley W, Reddacliff G (1990) Spontaneous proliferations in Australian marsupials – a survey and review. 2. Dasyurids and bandicoots. J Comp Pathol 103:149–158.
Cohen D (1985). The canine transmissible venereal tumor: a unique result of tumor progression. Adv Cancer Res 43:75–112.
Cousins DV, Roberts JL (2001) Australia’s campaign to eradicate bovine tuberculosis: the battle for freedom and beyond. Tuberculosis 81:5–15.
Crowther MS, Blacket MJ (2003) Biogeography and speciation in the dasyuridae: why are there so many kinds of dasyurids? In: Jones M, Dickman C, Archer M (eds) Predators with Pouches: The Biology of Carnivorous Marsupials. CSIRO, Collingwood.
Das U, Das AK (2000) Review of canine transmissible venereal sarcoma. Vet Res Commun 24:545–556.
Deininger MW, Druker BJ (2003) Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol Rev 55:401–423.
Department of Primary Industries and Water (2009) New Strains of DFTD Emerging. Retrieved 21 November 2009, from http://www.tassiedevil.com.au
Ding L, Getz G, Wheeler DA, et al. (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455:1069–1075.
DPIW and ARAZPA (2007) Save the Tasmanian Devil Program – Insurance Population Strategy. Retrieved 30th August 2009, from http://www.dpiw.tas.gov.au/inter.nsf/Attachments/EKOE-75M8ET/$FILE/STTD_IPS_v1_290707.pdf
Fujinaga T, Yamashita M, Yoshida MC, et al. (1989) Chromosome analysis of canine transmissible sarcoma cells. Zentralbl Veterinarmed A 36:481–489.
Garcia-Lora A, Algarra I, Collado A, Garrido F (2003) Tumour immunology, vaccination and escape strategies. Eur J Immunogenet 30:177–183.
Grenier MB, McDonald DB, Buskirk SW (2009) Rapid population growth of a critically endangered carnivore. Science 317:779.
Griner LA (1979) Neoplasms in Tasmanian devils (Sarcophilus harrisii). J Natl Cancer Inst 62:589–595.
Guiler ER (1970) Observations of the Tasmanian devil, Sarcophilus harrisii (Marsupialia: Dasyuridae) I. Numbers, home range, movements and food in two populations. Aust J Zool 18:49–62.
Hamede RK, McCallum H, Jones M (2008) Seasonal, demographic and density-related patterns of contact between Tasmanian devils (Sarcophilus harrisii): implications for transmission of devil facial tumour disease. Austral Ecol 33:614–622.
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70.
Hawkins CE, Baars C, Hesterman H, et al. (2006) Emerging disease and population decline of an island endemics, the Tasmanian devil Sarcophilus harrisii. Biol Conserv 131:307–324.
Haydon DT, Randall DA, Matthews L, et al. (2006) Low-coverage vaccination strategies for the conservation of endangered species. Nature 443:692–695.
Hilker FM, Langlais M, Malchow H (2009) The Allee effect and infectious diseases: extinction, multistability, and the (dis-)appearance of oscillations. Am Nat 173:72–88.
Hsiao YW, Liao KW, Chung TF, et al. (2008) Interactions of host IL-6 and IFN-gamma and cancer-derived TGF-beta1 on MHC molecule expression during tumor spontaneous regression. Cancer Immunol Immunother 57:1091–1104.
Hughes AL, Nei M (1988) Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335:167–170.
IUCN (2009) IUCN Red List of Threatened Species. Version 2009.1. Retrieved 25 August 2009, from http://www.iucnredlist.org
Jones ME, Cockburn A, Hamede R, et al. (2008) Life-history change in disease-ravaged Tasmanian devil populations. Proc Natl Acad Sci USA 105:10023–10027.
Jones ME, Jarman PJ, Lees CM, et al. (2007) Conservation management of Tasmanian devils in the context of an emerging, extinction-threatening disease: devil facial tumor disease. EcoHealth 4:326–337.
Jones M, Oakwood M, Belcher CA, et al. (2003). Carnivore concerns: problems, issues and solutions for conserving Australia’s marsupial carnivores. In: Jones M, Dickman C, Archer M (eds) Predators with Pouches: The Biology of Carnivorous Marsupials. CSIRO, Collingwood.
Jones ME, Paetkau D, Geffen E, Moritz C (2004) Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Mol Ecol 13:2197–2209.
Katzir N, Arman E, Cohen D, Givol D, Rechavi G (1987) Common origin of transmissible venereal tumors (TVT) in dogs. Oncogene 1:445–448.
Kinnear JE, Sumner NR, Onus ML (2002) The red fox in Australia – an exotic predator turned biocontrol agent. Biol Conserv 108:335–359.
Kohn MH, Murphy WJ, Ostrander EA, Wayne RK (2006) Genomics and conservation genetics. Trends Ecol Evol 21:629–637.
Krajewski C, Wroe S, Westerman M (2000) Molecular evidence for the pattern and timing of cladogenesis in dasyurid marsupials. Zool J Linn Soc 130:375–404.
Kreiss A, Fox N, Bergfeld J, et al. (2008) Assessment of cellular immune responses of healthy and diseased Tasmanian devils (Sarcophilus harrisii). Dev Comp Immunol 32:544–553.
Kreiss A, Wells B, Woods GM (2009) The humoral immune response of the Tasmanian devil (Sarcophilus harrisii) against horse red blood cells. Vet Immunol Immunopathol 130:135–137.
Lachish S, Jones M, McCallum H (2007) The impact of disease on the survival and population growth rate of the Tasmanian devil. J Anim Ecol 76:926–936.
Lachish S, McCallum H, Jones M (2009) Demography, disease and the devil: life-history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii). J Anim Ecol 78:427–436.
Ladds P, Loh R, Jones M, Tucker P (2003). Probable Lymphosarcoma in the Tasmanian devil (Sarcophilus harrisii). Proceedings of the Australian Society for Veterinary Pathology Annual Conference, Elizabeth Macarthur Agricultural Institute, Menangle, New South Wales, Australia.
Lambeck K, Chappell J (2001) Sea level change through the last glacial cycle. Science 292:679–686.
Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386:623–627.
Ley TJ, Mardis ER, Ding L, et al. (2008) DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456:66–72.
Loh R, Bergfeld J, Hayes D, et al. (2006a) The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). Vet Pathol 43:890–895.
Loh R, Hayes D, Mahjoor A, et al. (2006b) The immunohistochemical characterization of devil facial tumor disease (DFTD) in the Tasmanian devil (Sarcophilus harrisii). Vet Pathol 43:896–903.
Maxwell S, Burbridge AA, Morris K (1996). The 1996 Action Plan for Australian Marsupials and Monotremes. Environment Australia, Canberra.
McAloose D, Newton AL (2009) Wildlife cancer: a conservation perspective. Nat Rev Cancer 9:517–526.
McCallum H (2008) Tasmanian devil facial tumour disease: lessons for conservation biology. Trends Ecol Evol 23:631–637.
McCallum H, Dobson A (1995) Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol Evol 10:190–194.
McCallum H, Tompkins DM, Jones M, et al. (2007) Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth 4:318–325.
McGuire KL, Duncan WR, Tucker PW (1985) Syrian hamster DNA shows limited polymorphism at class I-like loci. Immunogenetics 22:257–268.
Mitelman F (2000) Recurrent chromosome aberrations in cancer. Mutat Res 462:247–253.
Mitelman F, Johansson B, Mandahl N, Mertens F (1997) Clinical significance of cytogenetic findings in solid tumors. Cancer Genet Cytogenet 95:1–8.
Mozos E, Mendez A, Gomez-Villamandos JC, Martin De Las Mulas J, Perez J (1996) Immunohistochemical characterization of canine transmissible venereal tumor. Vet Pathol 3:257–263.
Murchison E, Tovar C, Hsu A, et al. (2010) The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science 327:84–87.
Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA (2006) Clonal origin and evolution of a transmissible cancer. Cell 126:477–487.
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194:23–28.
Nowell PC, Hungerford DA (1960) A minute chromosome in human chronic granulocytic leukemia. Science 132:1497.
Owen D, Pemberton D (2005). Tasmanian Devil: A Unique and Threatened Animal. Allen & Unwin, Crows Nest.
O’Brien SJ, Evermann JF (1988) Interactive influences of infectious disease and genetic diversity in natural populations. Trends Ecol Evol 3:254–259.
O’Brien SJ, Johnson W, Driscoll C, et al. (2008) State of cat genomics. Trends Genet 24:268–279.
Parham P, Lawlor DA, Lomen CE, Ennis PD (1989) Diversity and diversification of HLA-A,B,C alleles. J Immunol 142:3937–3950.
Pearse AM, Swift K (2006) Allograft theory: transmission of devil facial-tumour disease. Nature 439:549.
Plumb G, Babiuk L, Mazet J, et al. (2007) Vaccination in conservation medicine. Rev Sci Tech 26:229–241.
Primmer CR (2009) From conservation genetics to conservation genomics. Ann NY Acad Sci 1162:357–368.
Pyecroft SB, Pearse A-M, Loh R, et al. (2007) Towards a case definition for devil facial tumour disease: what is it? EcoHealth 4:336–351.
Rajagopalan H, Lengauer C (2004) Aneuploidy and cancer. Nature 432:338–341.
Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C (2003) The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer 3:695–701.
Raudsepp T, Houck ML, O’Brien PC, et al. (2002) Cytogenetic analysis of California condor (Gymnogyps californianus) chromosomes: comparison with chicken (Gallus gallus) macrochromosomes. Cytogenet Genome Res 98:54–60.
Rebbeck CA, Thomas R, Breen M, Leroi AM, Burt A (2009) Origins and evolution of a transmissible cancer. Evolution 63:2340–2349
Rice J, Ottensmeier CH, Stevenson FK (2008) DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer 8:108–120.
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Romanov MN, Koriabine M, Nefedov M, de Jong PJ, Ryder OA (2006) Construction of a California condor BAC library and first-generation chicken-condor comparative physical map as an endangered species conservation genomics resource. Genomics 88:711–718.
Romanov MN, Tuttle EM, Houck ML, et al. (2009) The value of avian genomics to the conservation of wildlife. BMC Genomics 10(Suppl 2):S10.
Sanjayan MA, Crooks K, Zegers G, Foran D (1996) Genetic variation and the immune response in natural populations of pocket gophers. Conserv Biol 10:1519–1527.
Saunders G, Lane C, Harris S, Dickman C (2006). Foxes in Tasmania. A Report on an Incursion of an Invasive Species, Invasive Animals Cooperative Research Centre, Canberra.
Schloegel L, Hero J-M, Berger L, et al. (2006) The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3:35–40.
Siddle HV, Kreiss A, Eldridge MD, et al. (2007) Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc Natl Acad Sci USA 104:16221–16226.
Weaver BA, Cleveland DW (2006) Does aneuploidy cause cancer? Curr Opin Cell Biol 18:658–667.
Woods GM, Kreiss A, Belov K, et al. (2007) The immune response of the Tasmanian devil (Sarcophilus harrisii) and devil facial tumour disease. EcoHealth 4:338–345.
Wyatt KB, Campos PF, Gilbert MT, et al. (2008) Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One 3:e3602.
Yuhki N, O’Brien SJ (1990) DNA variation of the mammalian major histocompatibility complex reflects genomic diversity and population history. Proc Natl Acad Sci USA 87:836–840.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Bender, H.S. (2010). Devil Facial Tumour Disease (DFTD): Using Genetics and Genomics to Investigate Infectious Disease in an Endangered Marsupial. In: Deakin, J., Waters, P., Marshall Graves, J. (eds) Marsupial Genetics and Genomics. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9023-2_23
Download citation
DOI: https://doi.org/10.1007/978-90-481-9023-2_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9022-5
Online ISBN: 978-90-481-9023-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)