Abstract
The liver undergoes changes in structure and function in old age. There are age-related changes in liver mass, blood flow and hepatocyte and sinusoidal cell morphology. These changes are associated with significant impairment of many hepatic metabolic and detoxification activities. This has implications for systemic aging and age-related disease. For example the age-related impairment of the hepatic metabolism of lipoproteins predisposes to cardiovascular disease. The effects of caloric restriction in the liver are beneficial in terms of ameliorating many of deleterious phenotypic effects of ageing, but from the mechanistic point of view, caloric restriction does not just simply reverse or delay age-related cellular changes. Instead caloric restriction appears to act via discrete cellular mechanisms such as sirtuin pathways that impact cellular bioenergetics, apoptosis and other cellular functions. The liver has as a pivotal coordinating role when dietary intake is reduced, therefore many of the effects of caloric restriction are mediated by the liver.
Similar content being viewed by others
Keywords
1 Introduction
In the past it was proposed that the liver does not undergo significant aging changes because of its large functional reserve, regenerative capacity and dual blood supply (Popper 1986; Schmucker 1998). However, age-related changes in hepatic function are substantial and have potential impact on systemic exposure to xenobiotics (neurotoxins, carcinogens, pharmaceutics) and endogenous substrates associated with disease (lipoproteins, advanced glycation endproducts, immune complexes) (Table 11.1). Accordingly, age-related changes in the liver have implications for many diseases of aging and the aging process itself. Rather than the liver just being considered as a convenient source of tissue for general aging studies, the effects of aging on the liver per se have now been emphasized and considered an important focus for aging research (Boyer 2001; Geller and Zenick 2005; Schmucker 2005; Serste and Bourgeois 2006). The liver also has a pivotal role in coordinating metabolism in response to nutrition therefore many of the benefits of caloric restriction are likely to be mediated, at least in part, by the direct effects of diet on hepatic function. Indeed in 1985, Hans Popper proposed the concept (“thesis 1”) that “The liver exerts several effects on the aging of the whole organism. This may offer an opportunity to delay or minimize senescence of the organism by modulation of hepatic activity for instance by diets or even drugs” (Popper 1986).
2 Hepatic Mass
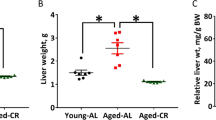
One of the earliest descriptions of the aging liver was “brown atrophy”, which is the reduction in liver mass associated with deposition of the aging pigment, lipofuscin (Popper 1986; Zeeh and Platt 1990; Porta 1991; Le Couteur and McLean 1998). The reduction in liver size as a fraction of body weight is usually in the order of 25–35%, although in some studies no major changes have been detected (Table 11.2) (Van Bezooijen 1984).
There is a parallel reduction in the number of hepatocytes (Tauchi and Sato 1975; 1978) that is likely to be secondary to the age-related increase in apoptosis of hepatocytes (James et al. 1998; Ando et al. 2002). Conversely, hepatocytes derived from long living Ames dwarf mice are even more likely to undergo apoptosis when exposed to oxidative stress in vitro (Kennedy et al. 2003) suggesting that increased hepatocyte apoptosis might be useful in delaying aging. Caloric restriction has usually been associated with a reduction in liver size even more marked than that seen in age-matched controls (Table 11.3). Conclusions about the effects of caloric restriction on hepatocyte apoptosis are varied with some showing that apoptosis is enhanced at least in pre-neoplastic liver cells (Grasl-Kraupp et al. 1994; Muskhelishvili et al. 1996) while others have shown a reduction in stress-induced apoptosis (Cohen et al. 2004; Pearson et al. 2008). In passing it should be noted that aging in humans is now a major risk factor for non alcoholic fatty liver disease (NASH), which is becoming more prevalent presumably as a result of the obesity epidemic (Clark 2006). Therefore in the future, old age in humans may no longer be associated with brown atrophy of the liver, but in fact the opposite.
3 Hepatic Blood Flow
Most studies in humans and animals have shown that total hepatic blood flow is reduced in the order of 30–50%, which equals or exceeds the degree of age-related reduction in liver mass (Wynne et al. 1989b; 1990b; Le Couteur and McLean 1998; Schmucker 1998; 2001; Schmucker 2005). However, the haemodynamic responsiveness of the perfused rat liver does not change (Le Couteur et al. 1992) nor is there any increase in portal pressure in old age (Le Couteur and McLean 1998). Liver perfusion, which is the flow per mass of liver, is also reduced in old age but to a lesser extent than total blood flow. Using the clearance of colloidal albumin, liver perfusion in rats was reduced about 15% by age 36 months (Brouwer et al. 1985). Recently Ito et al. (2007) used high resolution in vivo microscopy to investigate aging in mouse liver. There was a 14% reduction in the numbers of perfused sinusoids between 1 and 27 months of age and a 35% reduction in sinusoidal blood flow. This was associated with a marked increase in the perisinusoidal expression of ICAM-1 and a consequential increase in leukocyte adhesion and sinusoidal plugging (Ito et al. 2007). Another study reported no major aging changes in sinusoidal perfusion, leukocyte adhesion or sinusoidal diameter. Even so, in this study there was a reduction in sinusoidal flow of about 30% between 3 and 24 months (Vollmar et al. 2002). As yet, there have been few studies, if any, that have reported the effect of caloric restriction on hepatic perfusion and blood flow. In other tissues, caloric restriction increased blood flow and perfusion in the brain (Lynch et al. 1999) and decreased blood flow in fat tissue (Crandall et al. 1984).
4 Hepatocyte Morphology
Hepatocytes have been inconsistently reported to increase in size with aging in rodents and humans while most reports show increased numbers of polyploid and binucleate cells (Schmucker 1976; 1978; Tauchi and Sato 1978; Engelmann et al. 1981; Popper 1986; Schmucker 1998). Hepatocyte mitochondria become larger and fewer in number, sometimes forming mega-mitochondria (Tauchi and Sato 1978; Sastre et al. 1996). There is a marked increase in the dense components of the cytoplasm consisting of lysosomes and lipofuscin deposits (Porta 1991; Schmucker 1998) and this feature is preserved in isolated hepatocytes (Van Bezooijen et al. 1974). Various other age-related lesions and pathologies have been reported in rodent livers including biliary dilatation and hyperplasia, focal areas of inflammation, fatty change, hepatomas and myelodysplastic infiltration but tend to be species- and strain-specific (Van Bezooijen 1984; Popper 1986; Lipman et al. 1999a). The loss of mitochondrial number was exacerbated by a high fat diet (Baur et al. 2006), while acute severe caloric restriction (one third normal food intake) in rats was associated with a decrease in hepatocyte size and glycogen content, and an increase in mitochondria number (Herdson et al. 1964).
The detailed effects of long term caloric restriction on organ morphology in rodents were reported recently (Lipman et al. 1999b, a). In B6C3F1 mice, there was an age-related increase in vacuolated hepatocytes, which was reduced by caloric restriction. Vacuolated hepatocytes were a key characteristic of aging in these mice. The presence of lymphocytes in the liver was also a feature of aging that was reversed by caloric restriction and presumably reflects the pro-inflammatory nature of old age (Lipman et al. 1999b). In a similar study of three strains of rat, aging was associated with bile duct hyperplasia and the presence of lymphoid nodules. As well as liver tumors in all strains, large granular cell leukemia was very common in old F344 rats. All these pathological changes were delayed by caloric restriction (Lipman et al. 1999a).
5 Sinusoidal Cells
Old age is associated with morphological changes in the hepatic sinusoidal endothelium and space of Disse from intact livers of many species including humans (Le Couteur et al. 2001; Cogger et al. 2003; McLean et al. 2003; Hilmer et al. 2005; Warren et al. 2005; Ito et al. 2007; Jamieson et al. 2007; Le Couteur et al. 2008; Stacchiotti et al. 2008). These changes have been called “pseudocapillarization” (Le Couteur et al. 2001). On electron microscopy there is an increase in endothelial thickness and a reduction in the porosity and number of fenestrations, which are pores in the endothelial cell that facilitate transport of particulate substrates (Le Couteur et al. 2008). These changes are associated with perisinusoidal basal lamina deposition in many old livers and some scattered collagen in the space of Disse (Lipman et al. 1999b, a; Le Couteur et al. 2008). The age-related changes in the ultrastructure of the sinusoidal endothelium are associated with altered expression of several antigens and stains used to identify pathology in the hepatic sinusoid (Le Couteur et al. 2008). In most studies the expression of von Willebrands factor is increased in old age, one study showed a reduction in caveolin-1 expression (Jamieson et al. 2007) and while another report showed increased ICAM-1 expression (Ito et al. 2007). There is upregulation of markers of extracellular matrix, such as collagen IV and Sirius red. These findings, together with the presence of collagen in the space of Disse on electron microscopy, are consistent with the development of patchy perisinusoidal fibrosis in old age. The age-related reduction in fenestration porosity is substantial (around 50%) and has several implications. In particular, the effect of the loss of fenestrations on the hepatic disposition of lipoproteins such as chylomicron remnants and consequent risk of systemic vascular disease has been reported (Le Couteur et al. 2002; Hilmer et al. 2005). The loss of fenestrations and altered diffusional properties of the aged sinusoidal endothelium and space of Disse also influence the hepatic clearance of medications, hence contributing to adverse drug reactions (Le Couteur et al. 2005). The effects of caloric restriction on pseudocapillarization have been investigated. In old caloric restricted rats, endothelial thickness was significantly less (190±7 nm vs 211±6 nm) and fenestration porosity was significantly greater (3.9±0.3% vs 2.4±0.1%) than in old ad libitum fed rats. In young rats, caloric restriction increased fenestration porosity above normal levels. Caloric restriction prevented the age-related decrease in liver caveolin-1 expression and content (Jamieson et al. 2007; Martini et al. 2007) and increase in perisinusoidal collagen IV staining (Jamieson et al. 2007).
The liver sinusoid also contains Kupffer cells (macrophages) and stellate cells (fat storing cells). There have been some studies of aging in these cell types, although none on the effect of caloric restriction. Two early studies of Kupffer cells in humans noted an increase in their numbers and activity in old age (Schaffner and Popper 1959; Findor et al. 1973). In rats, an increase in Kupffer cell numbers has been reported (Hilmer et al. 2007) whereas another study reported a reduction in their volume density (Martin et al. 1992). Kupffer cells from old rat livers have fewer pseudopodia and a reduction in the actin and myosin cytoskeleton (Sun et al. 1998) and an increased number of lysosomes (Knook and Sleyster 1976). The reported effects of age on Kupffer cell phagocytic activity are inconsistent. The uptake of colloidal carbon is either reduced (Videla et al. 2001) or unchanged (Vomel et al. 1981; De Leeuw et al. 1983; Yamano et al. 2000) whereas the uptake of denatured albumin has been reported to be reduced (Caperna and Garvey 1982; Heil et al. 1984; Brouwer et al. 1985). The uptake of large microspheres by Kupffer cells is either largely unchanged (De Leeuw et al. 1983; Vollmar et al. 2002), decreased (Sun et al. 1998) or increased (Hilmer et al. 2007; Ito et al. 2007). Kupffer cell uptake of other substrates such as mitochondria (Martin et al. 1994) and copper-ceruloplasmin (Vomel et al. 1984) is reduced in old age. The responsiveness of Kupffer cells to stimuli is diminished in old age as determined by carbon-induced oxygen consumption (Videla et al. 2001) and cadmium-induced phagocytosis (Yamano et al. 2000). Despite these inconsistencies, it is likely that the ability of the Kupffer cell to mount an effective immune response will be impaired given the established negative effects of aging on systemic immunity (Weng 2006). Furthermore, any increase in Kupffer cell number and basal activity is not unexpected (Hilmer et al. 2007) because aging is a pro-inflammatory state associated with increased systemic markers of inflammation (Johnson 2006).
Recently it has been found that stellate cells become swollen and engorged with fat in old age (Durham et al. 1990; Martin et al. 1992; Vollmar et al. 2002; Cogger et al. 2003; Grizzi et al. 2003; Warren et al. 2005; Ito et al. 2007). This is obvious on electron microscopy but also can be identified by a signet ring appearance on light microscopy (Cogger et al. 2003; Warren et al. 2005). The stellate cells in old age are not activated because they are engorged with fat, demonstrate increased vitamin A autofluorescence (Vollmar et al. 2002) and the expression of α-SMA and desmin, protein markers of stellate cell activation, is not increased (Cogger et al. 2003). Some of the stellate cells in rodents are so swollen that they protrude into the sinusoidal lumen it has been suggested that this will reduce sinusoidal blood flow (Ito et al. 2007).
6 Gene and Protein Expression
The effects of aging on gene and protein expression have become an increasing focus of research as a result of microarray and bioinformatic technologies (Raghothama et al. 2005). Patterns of altered gene expression with aging vary substantially between tissues and species, and results in the aging liver also vary between studies (Fu et al. 2006; Zahn et al. 2007; Lee et al. 2008a). Overall, it seems that there is much less age-related change in gene expression in the liver compared with other tissues (Zahn et al. 2007). Even so, expression of specific genes and proteins have been found to change in the liver. These have tended to show that there is decreased expression of genes involved with xenobiotic metabolism, mitochondria, apoptosis, and cell cycle/nucleic acid metabolism whereas there is increased expression of genes involved with inflammation. Caloric restriction reversed or prevents some but not certainly all of these aging changes in the liver (Cao et al. 2001; Fu et al. 2006; Pearson et al. 2008; Swindell 2008).
In livers from two rat strains, about 5% of 28,000 genes studied changed with old age. Apart from xenobiotic metabolism, the main gene groups different between young and old rats included those involved with metabolism of steroids and lipids, and the electron transfer chain (Lee et al. 2008a). In old Sprague Dawley rat livers, there was 1.7-fold or more increase in the expression of 17 and a 0.6-fold or less decrease expression of 30 genes out of the 1,000 expressed in the liver. These were mostly involved in energy metabolism including a reduction in genes involved in mitochondrial respiration and an increase of those involved with glucose and fatty acid oxidation (Tollet-Egnell et al. 2001). In a study of aging mouse livers, it was found that mRNA expression of only 20 out of 11,000 genes increased 1.7-fold or more with age. These were mainly pro-inflammatory genes (including lysozyme, biglycan, serum amyloid P component, cystatin B) and various other genes coding for stress response proteins and chaperones. Expression of another 26 genes decreased with age including those involved with cell cycle/DNA replication, IGF binding protein-1, xenobiotic metabolizing genes (N-sulfotransferase, 3 cytochrome P450s, glutathione-S-transferase omega-1) and apolipoprotein E (Cao et al. 2001). In another report of old mice livers, there was a statistically significant increase in the mRNA expression of 828 and decreased expression of 991 genes out of 12,000 tested (Fu et al. 2006). There has been at least one study in old humans, and in this study aging changes in gene expression were compared with those seen in rats. There was a threefold or greater change in expression was found in 582 genes in the old rat livers and 192 genes in old human livers. Antioxidant and the cytochrome P450 genes were influenced by aging in both rats and humans (Thomas et al. 2002). In addition there are age-related changes in liver microRNA (miRNA), which are short, non-coding RNA segments that suppress gene expression. With old age in mice there is an increase in the expression of 4 miRNAs. Approximately one quarter of the proteins down-regulated with age are targets of these up-regulated miRNAs, including proteins involved with cytoskeleton, proliferation, extracellular matrix, mRNA processing and ubiquination (Maes et al. 2008). However the total miRNA content did not change with age and the effect of age on miRNA is variable in other tissues and species.
Short term and long term caloric restriction reversed only some of the changes in gene expression that occurred in the aging mouse (Cao et al. 2001; Fu et al. 2006; Swindell 2008). For example, long term caloric restriction normalized the expression of 14 out of 20 genes that had increased expression in old age, and 13 of the 26 genes with decreased expression (Cao et al. 2001). In another study, there was little overlap between the effects of aging and caloric restriction on liver mRNA expression, in fact only 6 of 35 pairwise comparisons showed any overlap (Swindell 2008). Therefore, caloric restriction has a discrete effect on hepatic gene expression, which is not simply a reversal of age-related changes. On the other hand it is of particular interest that the SIRT1 agonist, resveratrol, closely mimics the effects of caloric restriction on hepatic gene expression in mice (Pearson et al. 2008; Swindell 2008). In the Pearson study, the gene expression patterns of 28 month old mice on caloric restriction or treated with resveratrol showed an 87 and 90% correlation with the expression patterns of mice 9 months younger (Pearson et al. 2008).
7 Hepatic Metabolism
The liver is responsible for coordinating much of the response to dietary manipulation. The hepatic metabolism of carbohydrates, protein and fat undergoes marked changes in response to starvation and feeding (Bauer et al. 2004). Insulin is the key hormone involved in regulating this response. In summary, old age is associated with hepatic insulin resistance, reduced protein and amino acid metabolism, reduced gluconeogenesis with increased glycolysis, and increased hepatic fat synthesis and storage. Caloric restriction has opposite effects as the liver utilizes protein for gluconeogenesis and liberates fat for systemic energy requirements.
7.1 Protein and Amino Acid Metabolism
Animal studies have shown an overall, but not consistent, reduction in total liver protein synthesis and degradation in old age (Van Bezooijen 1984; Ward and Richardson 1991; Salive et al. 1992; Fu and Nair 1998). Protein synthesis measured by valine uptake was found to decrease by 70% between 6 and 24 months in rats (Ward and Richardson 1991). Caloric restriction significantly increases the rate of protein synthesis by rodent livers (Ward and Richardson 1991). Albumin is the major protein produced by the liver and its blood level has usually been reported to decrease slightly in old age but remain within the normal range (Fu and Nair 1998; Thalacker-Mercer and Campbell 2008). In one human cohort study, albumin fell by 0.8 g/dL every decade of life and low levels correlated with mortality and frailty (Salive et al. 1992). Yet the fractional synthesis of albumin and its response to dietary feeding are not influenced by age in humans (Thalacker-Mercer and Campbell 2008). There is a reduction of the fractional synthesis rate of fibrinogen despite increased blood levels in older humans, indicating that there must also be an age-related reduction in fibrinogen clearance (Fu and Nair 1998).
The utilization of amino acids for gluconeogenesis is reduced in old age. This is secondary in part to the age-related reduction of protein production by skeletal muscle (Salive et al. 1992). Even so, hepatic alanine and aspartate transaminase decrease with old age (Hagopian et al. 2003a) and low circulating blood levels of alanine transaminase are associated with higher mortality in humans (Elinav et al. 2006). The hepatic uptake of amino acids (proline, serine, arginine) and urea production are also reduced in old rat livers. Alanine was taken up by young livers but in contrast was released by old livers where the hepatic content of alanine was increased fourfold (Jourdan et al. 2008). Caloric restriction is associated with increased utilization of protein for energy and there is increased expression and activity of enzymes involved in nitrogen metabolism. Caloric restriction increases the activity of hepatic transaminases in mice, presumably in order to increase gluconeogenesis (Hagopian et al. 2003a). Likewise, acute starvation also increases expression of hepatic transaminases and other urea cycle genes in mice reflecting the utilization of protein for energy production (Bauer et al. 2004). Long term caloric restriction in mice increased the expression of glutaminase and carbomyl phosphate synthetase 1, while reducing the expression of glutamine synthetase (Dhahbi et al. 2001). Given such changes in protein and amino acid metabolism with caloric restriction, it is surprising that many aspects of caloric restriction can be replicated by protein restriction, at least in rodents (Pamplona and Barja 2006). Protein restriction also generated longevity gains in drosophila independent of caloric intake (Lee et al. 2008b). Intriguingly, the changes in longevity caused by manipulating the protein content of the diet were inversely related to fecundity, which provides experimental verification of the disposable soma theory of aging proposed by Kirkwood (Kirkwood and Holliday 1979).
7.2 Carbohydrate and Glucose Metabolism
In general, the ability of the liver to supply glucose for systemic metabolism is diminished in old age. This is secondary to a combination of increased glycolysis and reduced gluconeogenesis. Old age is also associated with insulin resistance (Elahi and Muller 2000; Dhahbi et al. 2001) and this has been mechanistically correlated with abdominal fat that accumulates in old age (Gupta et al. 2000). There are increased fasting levels of insulin in old humans and rodents and there is probably a slight increase in blood glucose levels and glycosylated hemoglobin (HbA1c) in humans (Elahi and Muller 2000). In the liver, insulin resistance is secondary to altered phosphorylation of the insulin receptor or early insulin signal transduction (Carvalho et al. 1996; Zhu et al. 2005). Age-related mitochondrial dysfunction also contributes to insulin resistance (Kim et al. 2008) and increasing circulating levels of insulin are, in part, secondary to altered hepatic clearance of insulin (Pacini et al. 1990). Caloric restriction preserves hepatic insulin sensitivity, particularly the ability of insulin to suppress hepatic glycogenolysis (Gupta et al. 2000; Dhahbi et al. 2001). Caloric restriction is associated with increased mRNA expression of the insulin receptor and increased phosphorylation of the insulin receptor following insulin stimulation (Zhu et al. 2004, 2005). The expression of insulin receptor and GLUT4 is not increased by caloric restriction (Masternak et al. 2005).
A consistent and important observation is the age-related reduction in the expression and activity of glucose-6-phosphatase, which is the final step in gluconeogenesis and glycogenolysis and has a pivotal role in regulating peripheral blood glucose levels (Tollet-Egnell et al. 2001; Hagopian et al. 2003a; Pearson et al. 2008; Swindell 2008). In old mice, there is increased expression of several key enzymes involved in glycolysis (phosphofructokinase-1, triosephosphate isomerase, enolase, pyruvate kinase) with an elevation of hepatic ketone bodies and reduction in hepatic lactate levels (Hagopian et al. 2003b). Microarray studies in the rat have also found reduced mRNA expression of gluconeogenic enzymes and increased expression of glycolytic enzymes (Tollet-Egnell et al. 2001) and there is reduced activity of enzymes involved in gluconeogenesis (glucose-6-phosphatase, fructose-1,6-bisphosphatase and transaminases) with an elevation of acetyl CoA (Hagopian et al. 2003a; Swindell 2008). Lifelong caloric restriction in mice is associated with increased activity and expression of key hepatic enzymes involved with gluconeogenesis (pyruvate carboxylase, phosphoenolpyruvate carboxykinase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase) while hepatic glucose and enzymes involved with glycolysis are reduced (Dhahbi et al. 2001; Hagopian et al. 2003b, a).
7.3 Lipid and Lipoprotein Metabolism
Liver fat and the levels of circulating cholesterol and triglycerides increase with old age. In one study total liver lipid content increased in old rats nearly threefold (Dawson et al. 2000) and in other studies in mice, there were increases in the liver content of cholesterol (Bose et al. 2005; Martin et al. 2008). As a result there is an increased prevalence of hepatosteatosis or non-alcoholic fatty liver disease in old age (Clark 2006). Hepatosteatosis is prevented in mice lacking either the peroxisome proliferator-activated receptor-alpha (PPARα) or L-fatty acid binding protein despite age-related obesity (Martin et al. 2008).
There are changes in expression of many proteins involved in hepatic lipid metabolism. There are increases in hepatic lipoprotein lipase, scavenger receptor B1 and apolipoprotein B but no change in HMG CoA reductase (Tollet-Egnell et al. 2001; Martin et al. 2008). PPARα participates in the control of fatty acid metabolism and beta oxidation through its effects on many genes involved in fatty acid metabolism. Therefore any age-related change in the expression of mRNA is likely to be a key factor in age-related changes in lipid metabolism. However, the expression of PPARα has been reported to be increased (Tollet-Egnell et al. 2001; Martin et al. 2008) or reduced in old age (Sanguino et al. 2005).
There is a reduction in the hepatic fractional catabolic rate of LDL in older humans by about 15% (Ericsson et al. 1991) and a reduction in the hepatic metabolism of chylomicron remnants by about 50% (Krasinski et al. 1990; Cassader et al. 1996). There are a variety of changes in enzymes involved in lipoprotein metabolism and disposition that may contribute to this impaired metabolism. For example, there is an increase in acyl-CoA:cholesterol transferase (Bose et al. 2005). Reports on the effect of old age on the LDL receptor vary although there is a consistent increase of LDL and LDL cholesterol (Bose et al. 2005; Galman et al. 2007) Although there is no change in total apolipoprotein E expression in old livers, the distribution is altered suggestive of impaired hepatocyte trafficking (Hilmer et al. 2004). Loss of fenestrations in the sinusoidal endothelium impairs the transfer of lipoproteins between blood and hepatocytes (Hilmer et al. 2005). Caloric restriction stimulates the use of fat for energy by increasing mitochondrial β-oxidation. Long term caloric restriction reduced total, LDL- and HDL-cholesterol towards levels seen in younger rats (Martini et al. 2007). Short term caloric restriction in rats enhanced the gene expression involved in fatty acid synthesis and those involved in mitochondrial β-oxidation, probably by increasing the expression of PPARα (Higami et al. 2006).
8 Hepatic Detoxification Pathways
Old age has usually been shown to be associated with reduced hepatic antioxidant, proteolytic and xenobiotic detoxification pathways, which potentially increases systemic exposure to toxins implicated in age-related disease. Caloric restriction has generally reversed such changes although the effects are variable.
8.1 Xenobiotic Metabolism
Xenobiotic metabolism occurs via two major pathways: the phase I pathway which is primarily the cytochrome P450 system, and the phase II pathway, which is conjugation. Both processes transform lipophilic substrates to hydrophilic substrates that can be excreted in bile or urine. Generally hepatic xenobiotic metabolism converts toxic substrates to their nontoxic metabolites however in some cases, substrates are activated to their active form. In old male rats there is a marked reduction in cytochrome P450 expression and activity, secondary to “feminization” of the male liver (Kitagawa et al. 1985; Kitani 1986; Birnbaum 1991). Cytochrome P450 content and most phase I pathways are normalized by caloric restriction, as are most of the phase II pathways. However the age-related decline in the hydroxysteroid metabolizing activity of UDP-glucuronyl transferase and sulfotransferase was not ameliorated, and some phase I pathways (microsomal testosterone 6β-hydroxylase, lauric acid 12-hydroxylase, 4-nitrophenol hydroxylase) were actually increased above young levels (Leakey et al. 1989a, b; Lee and Yu 1991).
In humans, the reduction in hepatic drug clearance has been ascribed primarily to age-related reductions in liver mass and blood flow rather than changes in enzyme content and activity (James 1985; Wynne et al. 1989a, b; Durnas et al. 1990; Schmucker et al. 1990; Wynne and James 1990). However, a recent detailed study of liver tissue from 226 humans found a 32% reduction in hepatic cytochrome P450 content after 70 years of age (Sotaneimi et al. 1997). In addition, impaired ability of albumin-bound drugs to cross the pseudocapillarized liver sinusoidal endothelial cell will contribute to altered drug metabolism (Le Couteur et al. 2005). It has been concluded that phase I drug metabolism is preferentially reduced in healthy old age while phase II metabolism is paradoxically preserved (Le Couteur and McLean 1998). However, in contrast to fit older people, phase II acetaminophen glucuronidation is reduced in the frail elderly (Wynne et al. 1990a). More recently a detailed re-analysis of hepatic metabolism has shown that old age is associated with a reduction in all metabolic pathways once free (non-protein bound) clearance is calculated (Butler and Begg 2008). Although conclusions regarding hepatic drug metabolism in aging humans have changed substantially over the last decades, current data suggest that most drug metabolism pathways are reduced by up to 50% in old age depending upon comorbidity and frailty, and that this is secondary to reduced hepatic blood flow and also probably hepatic mass and enzyme content.
8.2 Proteolysis and Autophagy
There are two major proteolytic systems, the ubiquitin-proteasomal and the lysosomal systems and these are present in all tissues. Autophagy refers to lysosomal proteolysis that is undertaken either to remove damaged proteins or to convert protein to energy in times of nutritional undersupply. An age-related decrease in autophagy occurs in most tissues including the liver and this presumably contributes to the increased levels of damaged proteins that accumulate within cells (Cuervo and Dice 1998; Vittorini et al. 1999; Cuervo 2008a, b). In particular, expression of the lysosomal receptor for chaperone mediated autophagy (LAMP2A) is reduced in the aged liver and transgenic upregulation of this receptor has been shown to delay age-related changes in hepatocyte and liver mitochondrial function and structure (Mizushima et al. 2008; Zhang and Cuervo 2008). The effect of caloric restriction on the age-related decline in lysosomal proteolysis in the liver was to partially restore its activity (Vittorini et al. 1999; Cavallini et al. 2001) and to maintain the responsiveness of hepatic autophagy to insulin and glucagon (Donati et al. 2008).
8.3 Endogenous Oxidant Metabolism
Old age is associated with increased oxidative stress and oxidative injury while endogenous antioxidant systems (such as superoxide dismutase, catalase, glutathione peroxidase, ascorbic acid, reduced glutathione) tend to decrease with age (Muller et al. 2007). Old age causes reduced expression of many hepatic antioxidant enzymes and increased evidence of oxidative stress in most studies, but such results are variable and probably influenced by strain and gender (Ji et al. 1990; Rikans et al. 1991; Amicarelli et al. 1997; Sanz et al. 1997; Martin et al. 2002; Muradian et al. 2002). In rat liver, there are reports of lower mRNA expression and activity of Cu/Zn superoxide dismutase, GSH reductase, catalase and glutathione peroxidase (Rikans et al. 1991; Sanz et al. 1997; Martin et al. 2002). The changes are likely to be significant, for example Cu/Zn superoxide dismutase activity was decreased to about 25–45% of that seen in young rats (Sanz et al. 1997; Kiray et al. 2004). Another study reported reduced activity of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase in livers of old rats and also lower levels of ascorbate, α-tocopherol and glutathione associated with consequential increased levels of oxidative stress determined from lipoperoxides and protein carbonyls (Navarro and Boveris 2004; Senthil Kumaran et al. 2008). Reduced glutathione and the GSH:GSSG ratio in the liver decline with age (Sanz et al. 1997). As a consequence of the reduced antioxidant defenses, aging increases the susceptibility of hepatocytes to oxidative stress. There is more rapid loss of viability at lower concentrations of tert-butylhydroperoxide in hepatocytes isolated from old versus young rats (Hagen et al. 2000) with similar results obtained using hydrogen peroxide (Ikeyama et al. 2002; Li and Holbrook 2003). A decline in the ratio of coenzymes Q 10:9 and alpha-tocopherol in liver cell membranes was found in aged rat livers, and this change was attenuated by caloric restriction (de Cabo et al. 2004). In addition coenzyme Q-dependent NADPH dehydrogenases were also increased by caloric restriction and, as a result, liver cell membranes were more resistant to oxidative stress (de Cabo et al. 2004).
8.4 Endocytosis
Liver sinusoidal endothelial cells play a critical role in the removal of macromolecular waste products from the systemic circulation and are the most active endocytic cells in the body (Smedsrod et al. 1990; Smedsrod 2004). They clear many substrates of relevance to old age including connective tissue macromolecules, oxidized and acetylated low density lipoproteins, advanced glycation end products and immune complexes (Skogh et al. 1985; Smedsrod et al. 1997; Martin-Armas et al. 2006). In vivo microscopy was used to detect endocytosis of two fluorescent labeled scavenger receptor ligands, advanced glycation end product-modified albumin and formaldehyde-treated albumin in aged mice (Ito et al. 2007). There was suppressed endocytic function in old mice, especially in the pericentral area. It was concluded that such an age-related reduction in this activity increases extrahepatic deposition and deleterious effects of circulating waste macromolecules. The effect of caloric restriction on endocytosis has not been reported.
9 Hepatocyte Mitochondria
There are well established aging changes in the structure and number of liver mitochondria as well as increased free radical production and decreased ATP production and respiration. These changes parallel those found in other tissues (Hagen et al. 1997, 1999). In hepatocytes, there is a 30% reduction in mitochondrial potential (Sastre et al. 1996) and a 25% reduction in ATP measured using magnetic resonance spectroscopy (Le Couteur et al. 2001). The activity of many mitochondrial enzymes (eg mitochondrial nitric oxide synthase, Mn-superoxide dismutase, complex I, complex IV) is decreased in old age by 25–75% (Navarro and Boveris 2004). These dysfunctional mitochondria are a potent source of endogenous reactive oxygen species and contribute to age-related oxidative stress (Sastre et al. 2003; Lopez-Lluch et al. 2008).
There are aging changes in genes related to fatty acid oxidation and oxidative capacity and point mutations in mitochondrial (mt)DNA at specific regions that control replication of mtDNA (Ames et al. 1995; Sastre et al. 2000; Kujoth et al. 2005). The number of copies of mtDNA is reduced by about 50% (Barazzoni et al. 2000) and the age-associated 4,834 bp mtDNA deletion seen in other tissues also occurs in the aging liver (Cassano et al. 2004). Microarray studies of rat liver show additional age-related decline in expression of several nuclear genes involved in mitochondrial respiration (Tollet-Egnell et al. 2001).
The effects of caloric restriction on hepatic mitochondria have been extensively studied. The lactate:pyruvate ratio, a measure of cytoplasmic redox status and the hydroxybutyrate:acetoacetate, a measure of mitochondrial redox status did not change in the livers of aged mice, but are both increased by lifelong caloric restriction (Hagopian et al. 2003a). Caloric restriction increases the number of mitochondria in hepatocytes towards levels seen in young rats (Lopez-Lluch et al. 2006) and leads to less free radical production (Lopez-Torres et al. 2002; Lopez-Lluch et al. 2006) and less evidence of oxidative injury (Lambert et al. 2004; Lopez-Lluch et al. 2006). Despite lower oxygen consumption and free radical production, the mitochondria after caloric restriction maintain higher levels of ATP production (Lopez-Lluch et al. 2006). The effects of caloric restriction are mostly on complex I of the electron transfer chain (Pamplona and Barja 2006) and are mediated by increased activity of PPAR-1α (Lopez-Lluch et al. 2006). Caloric restriction also reversed the age-related accumulation of the 4,834 bp mtDNA deletion (Cassano et al. 2004) and oxidative damage to mtDNA (Lopez-Torres et al. 2002). It should be noted that as well as contributing to the aging process, impaired hepatic mitochondria are key factors in the pathogenesis of hepatosteatosis and hepatic insulin resistance, which are very common conditions in older people (Wei et al. 2008).
10 Regenerative Capacity and Transplantation
Hepatic regeneration following partial hepatectomy is complete but slower in old rats, taking up to 3–4 weeks rather than 1 week to fully reconstitute (Bucher et al. 1964; Beyer et al. 1991; Smanik et al. 1991). This slower regeneration is associated with decreased DNA synthesis and hepatocyte mitotic activity (Schapiro et al. 1982; Beyer et al. 1991; Bland et al. 1991; Chou et al. 1995; Shaddock et al. 1996). Recently it has been shown that the proliferative response in old age is reduced because of inhibition of c-myc mediated by C/EBPα (Iakova et al. 2003), reduced activation of extracellular signal-regulated kinase (ERK), reduced phosphorylation of the epidermal growth factor receptor (EGFR) and increased activation of p38 (Li and Holbrook 2003).
Livers transplanted from 1 year old rats were associated with less growth and lower albumin levels in recipient rats, compared with livers from 11 week old rats (Koh et al. 2006). In humans, transplantation of donor livers is associated with a 1% rate of primary graft failure when the donor is less than 55 years, compared with 10% with older donors (Serste and Bourgeois 2006). On the other hand, re-transplantation of livers in rats found that transplanted livers survived until maximum 52 months (compared with the maximum life of rat 39.9 months) and recipients had same survival whether given livers from 5 or 28 month donors. However, there was a higher incidence of fibrosis, pigment deposition and biliary proliferation in old transplanted livers (Sakai et al. 1997). Parabiosis experiments showed rejuvenation of old livers following union of young and old rats (Tauchi and Sato 1978) indicating that the re-transplantation studies might be a result of the rejuvenating milieu of the young recipients rather than an inherent quality of the old livers. Overall, it appears that old age impairs hepatic regeneration but transplantation of old livers is still feasible.
Caloric restriction accelerates liver regeneration in old age. Following partial hepatectomy in old rats, the percentage of liver regenerated after 24 h was 21% in control rats and this was improved to 29% with caloric restriction (Chou et al. 1995). Caloric restriction was associated with larger increases in DNA synthesis and hepatocytes in S phase following partial hepatectomy (Chou et al. 1995) and enhanced proliferative response to epidermal growth factor (EGF) (Ikeyama et al. 2003).
11 Mechanisms for the Hepatic Effects of Caloric Restriction
Although caloric restriction delays the aging phenotype and extends longevity, the data presented here show that caloric restriction can not be interpreted as simply reversing or slowing age-related changes in the liver. For example, caloric restriction does not normalize the effects of aging with respect to liver mass, apoptosis, endothelial fenestrations, xenobiotic metabolism and gene expression. Rather than being “anti-aging”, caloric restriction, at least in the liver, can be considered as an intervention that ameliorates some of the deleterious phenotypic effects of old age. From an evolutionary perspective, it has been proposed that the beneficial effects of caloric restriction evolved as a positive switch to increase cellular resilience in times of famine (Holliday 2006). By contrast, in the past there has not been any evolutionary pressure to generate strategies to delay aging changes.
Recent studies have shown that the sirtuin pathway mediates many of the beneficial effects of caloric restriction and as such is the positive switch that responds to dietary deprivation (Cohen et al. 2004; Guarente and Picard 2005; Sinclair 2005; Haigis and Guarente 2006; Sinclair and Guarente 2006; Guarente 2008) (See Chapter 9). Sirtuins are one of the three classes of histone deacetylases, which are NAD+-dependent enzymes that deacetylase lysine groups found on histones. The sirtuins are named for their effect on histones and hence gene transcription (Silent Information Regulator) but also deacetylate lysine moieties on many other cellular proteins including p53, tubulin and PPARγ coactivator 1 (PGC-1α). Amongst the sirtuins, the SIR2 enzymes (consisting of SIR2 in yeast and SIRT1 in mammals) are most closely linked with longevity, caloric restriction and aging (Guarente and Picard 2005; Sinclair 2005; Haigis and Guarente 2006; Sinclair and Guarente 2006). Following long term caloric restriction in rats, there was increased expression of SIRT1 in all tissues including liver (Cohen et al. 2004). However, short term caloric restriction in mice showed reduced hepatic expression of SIRT1, while other tissues had the expected increase in SIRT1 expression (Chen et al. 2008). It was noted in this study that caloric restriction was associated with an increase in the NAD+/NADH ratio in muscle but a decreased ratio in liver, thereby potentially explaining the differential effects on SIRT1 expression. Resveratrol is an agonist of SIRT1 that recapitulates many of the effects of caloric restriction, and more potent agonists such as SRT1720 have recently been published (Howitz et al. 2003; Wood et al. 2004; Baur et al. 2006; Milne et al. 2007; Feige et al. 2008; Pearson et al. 2008). In the liver, resveratrol mimicked the transcriptional profile generated by caloric restriction (Pearson et al. 2008). In aged mice fed a high caloric diet, resveratrol prevented the development of fatty liver and its sequelae such as insulin resistance and hyperlipidemia (Baur et al. 2006).
12 Conclusions
Contrary to past assumptions, the liver does indeed undergo changes in structure and function in old age. Albeit, the changes in liver structure are subtle but the changes in function are often substantial, often in the order of 50% reductions in metabolic activity. Because of the central role of the liver in metabolism and detoxification, changes in liver function have potential implications for systemic aging and age-related diseases in particular cardiovascular disease. The effects of caloric restriction in the liver are beneficial but do not simply reverse or delay age-related changes, instead acting primarily through discrete cellular mechanisms such as the sirtuin pathway. Given the key role of the liver in responding to dietary perturbations, it is likely that many of the effects of caloric restriction are mediated by the liver.
References
Ames, B. N., Shigenaga, M. K. and Hagen, T. M., 1995. Mitochondrial decay in aging. Biochim Biophys Acta 1271, 165–170.
Amicarelli, F., Di Ilio, C., Masciocco, L., Bonfigli, A., Zarivi, O., D’Andrea, M. R., Di Giulio, C. and Miranda, M., 1997. Aging and detoxifying enzymes responses to hypoxic or hyperoxic treatment. Mech Ageing Dev 97, 215–226.
Ando, K., Higami, Y., Tsuchiya, T., Kanematsu, T. and Shimokawa, I., 2002. Impact of aging and life-long calorie restriction on expression of apoptosis-related genes in male F344 rat liver. Microsc Res Tech 59, 293–300.
Bach, B., Hansen, J. M., Kampmann, J. P., Rasmussen, S. N. and Skovsted, L., 1981. Disposition of antipyrine and phenytoin correlated with age and liver volume in man. Clin Pharmacokinet 6, 389–396.
Barazzoni, R., Short, K. R. and Nair, K. S., 2000. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275, 3343–3347.
Bauer, M., Hamm, A. C., Bonaus, M., Jacob, A., Jaekel, J., Schorle, H., Pankratz, M. J. and Katzenberger, J. D., 2004. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol Genomics 17, 230–244.
Baur, J. A., Pearson, K. J., Price, N. L. et al., 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342.
Bevilacqua, L., Ramsey, J. J., Hagopian, K., Weindruch, R. and Harper, M. E., 2005. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab 289, E429–E438.
Beyer, H. S., Sherman, R. and Zieve, L., 1991. Aging is associated with reduced liver regeneration and diminished thymidine kinase mRNA content and enzyme activity in the rat. J Lab Clin Med 117, 101–108.
Birnbaum, L. S., 1991. Pharmacokinetic basis of age-related changes in sensitivity to toxicants. Annu Rev Pharmacol Toxicol 31, 101–128.
Bland, G., Banks, A., Nicholson, M. and Jones, A. L., 1991. Decreased liver DNA synthesis in old rats following partial hepatectomy is restored by epidermal growth factor. In Kitani, K. (ed), Liver and Aging – 1990. Elsevier Science Publishers, Amsterdam, pp. 279–290.
Bose, C., Bhuvaneswaran, C. and Udupa, K. B., 2005. Age-related alteration in hepatic acyl-CoA: cholesterol acyltransferase and its relation to LDL receptor and MAPK. Mech Ageing Dev 126, 740–751.
Boyer, J. L., 2001. The liver does age! Pseudocapillarization of the sinusoids in older rats. Hepatology 33, 487.
Brouwer, A., Barelds, R. J. and Knook, D. L., 1985. Age-related changes in the endocytic capacity of rat liver Kupffer and endothelial cells. Hepatology 5, 362–366.
Bucher, N. L., Swaffield, M. N. and Ditroia, J. F., 1964. The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res 24, 509–512.
Butler, J. M. and Begg, E. J., 2008. Free drug metabolic clearance in elderly people. Clin Pharmacokinet 47, 297–321.
Calloway, N. O., Foley, C. F. and Lagerbloom, P., 1965. Uncertainties in geriatric data. II Organ size. J Am Geriatr Soc 13, 20–28.
Cao, S. X., Dhahbi, J. M., Mote, P. L. and Spindler, S. R., 2001. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A 98, 10630–10635.
Caperna, T. J. and Garvey, J. S., 1982. Antigen handling in aging. II. The role of Kupffer and endothelial cells in antigen processing in Fischer 344 rats. Mech Ageing Dev 20, 205–221.
Carvalho, C. R., Brenelli, S. L., Silva, A. C., Nunes, A. L., Velloso, L. A. and Saad, M. J., 1996. Effect of aging on insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of rats. Endocrinology 137, 151–159.
Cassader, M., Gambino, R., Rulu, G., Marena, S., Bodoni, P. and Pagano, G., 1996. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging Clin Exp Res 8, 421–428.
Cassano, P., Lezza, A. M., Leeuwenburgh, C., Cantatore, P. and Gadaleta, M. N., 2004. Measurement of the 4,834-bp mitochondrial DNA deletion level in aging rat liver and brain subjected or not to caloric restriction diet. Ann N Y Acad Sci 1019, 269–273.
Cavallini, G., Donati, A., Gori, Z., Pollera, M. and Bergamini, E., 2001. The protection of rat liver autophagic proteolysis from the age-related decline co-varies with the duration of anti-ageing food restriction. Exp Gerontol 36, 497–506.
Chen, D., Bruno, J., Easlon, E., Lin, S. J., Cheng, H. L., Alt, F. W. and Guarente, L., 2008. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22, 1753–1757.
Chou, M. W., Shaddock, J. G., Kong, J., Hart, R. W. and Casciano, D. A., 1995. Effect of dietary restriction on partial hepatectomy-induced liver regeneration of aged F344 rats. Cancer Lett 91, 191–197.
Clark, J. M., 2006. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40(Suppl 1), S5–S10.
Cogger, V. C., Warren, A., Fraser, R., Ngu, M., McLean, A. J. and Le Couteur, D. G., 2003. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol 38, 1101–1107.
Cohen, H. Y., Miller, C., Bitterman, K. J., Wall, N. R., Hekking, B., Kessler, B., Howitz, K. T., Gorospe, M., de Cabo, R. and Sinclair, D. A., 2004. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392.
Crandall, D. L., Goldstein, B. M., Huggins, F. and Cervoni, P., 1984. Adipocyte blood flow: influence of age, anatomic location, and dietary manipulation. Am J Physiol 247, R46–R51.
Cuervo, A. M., 2008a. Autophagy and aging: keeping that old broom working. Trends Genet 24, 604–612.
Cuervo, A. M., 2008b. Calorie restriction and aging: the ultimate “cleansing diet”. J Gerontol A Biol Sci Med Sci 63, 547–549.
Cuervo, A. M. and Dice, J. F., 1998. How do intracellular proteolytic systems change with age?. Front Biosci 3, d25–d43.
Dawson, H. D., Yamamoto, Y., Zolfaghari, R., Rosales, F. J., Dietz, J., Shimada, T., Li, N. and Ross, A. C., 2000. Regulation of hepatic vitamin A storage in a rat model of controlled vitamin A status during aging. J Nutr 130, 1280–1286.
de Cabo, R., Cabello, R., Rios, M., Lopez-Lluch, G., Ingram, D. K., Lane, M. A. and Navas, P., 2004. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol 39, 297–304.
De Leeuw, A. M., Brouwer, A., Barelds, R. J. and Knook, D. L., 1983. Maintenance cultures of Kupffer cells isolated from rats of various ages: ultrastructure, enzyme cytochemistry, and endocytosis. Hepatology 3, 497–506.
Dhahbi, J. M., Mote, P. L., Wingo, J., Rowley, B. C., Cao, S. X., Walford, R. L. and Spindler, S. R., 2001. Caloric restriction alters the feeding response of key metabolic enzyme genes. Mech Ageing Dev 122, 1033–1048.
Donati, A., Recchia, G., Cavallini, G. and Bergamini, E., 2008. Effect of aging and anti-aging caloric restriction on the endocrine regulation of rat liver autophagy. J Gerontol A Biol Sci Med Sci 63, 550–555.
Durham, S. K., Brouwer, A., Barelds, R. J., Horan, M. A. and Knook, D. L., 1990. Comparative endotoxin-induced hepatic injury in young and aged rats. J Pathol 162, 341–349.
Durnas, C., Loi, C. M. and Cusack, B. J., 1990. Hepatic drug metabolism and aging. Clin Pharmacokinet 19, 359–389.
Elahi, D. and Muller, D. C., 2000. Carbohydrate metabolism in the elderly. Eur J Clin Nutr 54(Suppl 3), S112–S120.
Elinav, E., Ackerman, Z., Maaravi, Y., Ben-Dov, I. Z., Ein-Mor, E. and Stessman, J., 2006. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 54, 1719–1724.
Engelmann, G. L., Richardson, A., Katz, A. and Fierer, J. A., 1981. Age-related changes in isolated rat hepatocytes. Comparison of size, morphology, binucleation, and protein content. Mech Ageing Dev 16, 385–395.
Ericsson, S., Eriksson, M., Vitols, S., Einarsson, K., Berglund, L. and Angelin, B., 1991. Influence of age on the metabolism of plasma low density lipoproteins in healthy males. J Clin Invest 87, 591–596.
Feige, J. N., Lagouge, M., Canto, C., Strehle, A., Houten, S. M., Milne, J. C., Lambert, P. D., Mataki, C., Elliott, P. J. and Auwerx, J., 2008. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8, 347–358.
Findor, J., Perez, V., Bruch Igartua, E., Giovanetti, M. and Fioravanti, N., 1973. Structure and ultrastructure of the liver in aged persons. Acta Hepato Gastroent 20, 200–204.
Fu, C., Hickey, M., Morrison, M., McCarter, R. and Han, E. S., 2006. Tissue specific and non-specific changes in gene expression by aging and by early stage CR. Mech Ageing Dev 127, 905–916.
Fu, A. and Nair, K. S., 1998. Age effect on fibrinogen and albumin synthesis in humans. Am J Physiol 275, E1023–E1030.
Galman, C., Matasconi, M., Persson, L., Parini, P., Angelin, B. and Rudling, M., 2007. Age-induced hypercholesterolemia in the rat relates to reduced elimination but not increased intestinal absorption of cholesterol. Am J Physiol Endocrinol Metab 293, E737–E742.
Geller, A. M. and Zenick, H., 2005. Aging and the environment: a research framework. Environ Health Perspect 113, 1257–1262.
Grasl-Kraupp, B., Bursch, W., Ruttkay-Nedecky, B., Wagner, A., Lauer, B. and Schulte-Hermann, R., 1994. Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci U S A 91, 9995–9999.
Greenberg, J. A. and Boozer, C. N., 2000. Metabolic mass, metabolic rate, caloric restriction, and aging in male Fischer 344 rats. Mech Ageing Dev 113, 37–48.
Grizzi, F., Franceschini, B., Gagliano, N., Moscheni, C., Annoni, G., Vergani, C., Hermonat, P. L., Chiriva-Internati, M. and Dioguardi, N., 2003. Mast cell density, hepatic stellate cell activation and TGF-beta1 transcripts in the aging Sprague-Dawley rat during early acute liver injury. Toxicol Pathol 31, 173–178.
Guarente, L., 2008. Mitochondria – a nexus for aging, calorie restriction, and sirtuins?. Cell 132, 171–176.
Guarente, L. and Picard, F., 2005. Calorie restriction – the SIR2 connection. Cell 120, 473–482.
Gupta, G., Cases, J. A., She, L., Ma, X. H., Yang, X. M., Hu, M., Wu, J., Rossetti, L. and Barzilai, N., 2000. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol Endocrinol Metab 278, E985–E991.
Hagen, T. M., Ingersoll, R. T., Lykkesfeldt, J., Liu, J., Wehr, C. M., Vinarsky, V., Bartholomew, J. C. and Ames, A. B., 1999. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J 13, 411–418.
Hagen, T. M., Vinarsky, V., Wehr, C. M. and Ames, B. N., 2000. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid Redox Signal 2, 473–483.
Hagen, T. M., Yowe, D. L., Bartholomew, J. C., Wehr, C. M., Do, K. L., Park, J. Y. and Ames, B. N., 1997. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci U S A 94, 3064–3069.
Hagopian, K., Ramsey, J. J. and Weindruch, R., 2003a. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp Gerontol 38, 267–278.
Hagopian, K., Ramsey, J. J. and Weindruch, R., 2003b. Influence of age and caloric restriction on liver glycolytic enzyme activities and metabolite concentrations in mice. Exp Gerontol 38, 253–266.
Haigis, M. C. and Guarente, L. P., 2006. Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes Dev 20, 2913–2921.
Heil, M. F., Dingman, A. D. and Garvey, J. S., 1984. Antigen handling in ageing. III. Age-related changes in antigen handling by liver parenchymal and nonparenchymal cells. Mech Ageing Dev 26, 327–340.
Herdson, P. B., Garvin, P. J. and Jennings, R. B., 1964. Fine structural changes produced in rat liver by partial starvation. Am J Pathol 45, 157–181.
Higami, Y., Tsuchiya, T., Chiba, T., Yamaza, H., Muraoka, I., Hirose, M., Komatsu, T. and Shimokawa, I., 2006. Hepatic gene expression profile of lipid metabolism in rats: impact of caloric restriction and growth hormone/insulin-like growth factor-1 suppression. J Gerontol A Biol Sci Med Sci 61, 1099–1110.
Hilmer, S. N., Cogger, V. C., Fraser, R., McLean, A. J., Sullivan, D. and Le Couteur, D. G., 2005. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology 42, 1349–1354.
Hilmer, S. N., Cogger, V. C. and Le Couteur, D. G., 2007. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci 62, 973–978.
Hilmer, S. N., Warren, A., Cogger, V. C., Fraser, R., McLean, A. J., Sullivan, D. and Le Couteur, D. G., 2004. The effect of aging on the immunohistochemistry of apolipoprotein E in the liver. Exp Gerontol 39, 53–57.
Holliday, R., 2006. Food, fertility and longevity. Biogerontology 7, 139–141.
Howitz, K. T., Bitterman, K. J., Cohen, H. Y., Lamming, D. W., Lavu, S., Wood, J. G., Zipkin, R. E., Chung, P., Kisielewski, A., Zhang, L. L., Scherer, B. and Sinclair, D. A., 2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196.
Iakova, P., Awad, S. S. and Timchenko, N. A., 2003. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell 113, 495–506.
Ikeyama, S., Kokkonen, G., Martindale, J. L., Wang, X. T., Gorospe, M. and Holbrook, N. J., 2003. Effects of aging and calorie restriction of Fischer 344 rats on hepatocellular response to proliferative signals. Exp Gerontol 38, 431–439.
Ikeyama, S., Kokkonen, G., Shack, S., Wang, X. T. and Holbrook, N. J., 2002. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J 16, 114–116.
Ito, Y., Sorensen, K. K., Bethea, N. W., Svistounov, D., McCuskey, M. K., Smedsrod, B. H. and McCuskey, R. S., 2007. Age-related changes in the hepatic microcirculation of mice. Exp Gerontol 48, 789–797.
James, O. F. W., 1985. Drugs and the ageing liver. J Hepatol 1, 431–435.
James, S. J., Muskhelishvili, L., Gaylor, D. W., Turturro, A. and Hart, R., 1998. Upregulation of apoptosis with dietary restriction: implications for carcinogenesis and aging. Environ Health Perspect 106(Suppl 1), 307–312.
Jamieson, H., Hilmer, S. N., Cogger, V. C., Warren, A., Cheluvappa, R., Abernethy, D. R., Everitt, A., Fraser, R., de Cabo, R. and Le Couteur, D. G., 2007. Caloric restriction reduces age-related pseudocapillarization of the hepatic sinusoid. Exp Gerontol 42, 374–378.
Ji, L. L., Dillon, D. and Wu, E., 1990. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol 258, R918–R923.
Johnson, T. E., 2006. Recent results: biomarkers of aging. Exp Gerontol 41, 1243–1246.
Jourdan, M., Cynober, L., Moinard, C., Blanc, M. C., Neveux, N., De Bandt, J. P. and Aussel, C., 2008. Splanchnic sequestration of amino acids in aged rats: in vivo and ex vivo experiments using a model of isolated perfused liver. Am J Physiol Regul Integr Comp Physiol 294, R748–R755.
Kennedy, M. A., Rakoczy, S. G. and Brown-Borg, H. M., 2003. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol 38, 997–1008.
Kim, J. A., Wei, Y. and Sowers, J. R., 2008. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102, 401–414.
Kiray, M., Uysal, N., Sonmez, A., Acikgoz, O. and Gonenc, S., 2004. Positive effects of deprenyl and estradiol on spatial memory and oxidant stress in aged female rat brains. Neurosci Lett 354, 225–228.
Kirkwood, T. B. and Holliday, R., 1979. The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci 205, 531–546.
Kitagawa, H., Fujita, S., Suzuki, T. and Kitani, K., 1985. Disappearance of sex difference in rat liver drug metabolism in old age. Biochem Pharmacol 34, 579–581.
Kitani, K., 1986. Hepatic drug metabolism in the elderly. Hepatology 6, 316–319.
Knook, D. L. and Sleyster, E. C., 1976. Lysosomal enzyme activities in parenchymal and nonparenchymal liver cells isolated from young, adult and old rats. Mech Ageing Dev 5, 389–398.
Koh, M., Okamoto, E., Yamanaka, J. and Fujimoto, J., 2006. Impact of donor age on the growth of young recipient rats after liver transplantation. Surg Today 36, 457–464.
Krasinski, S. D., Cohn, J. S., Schaefer, E. J. and Russell, R. M., 1990. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest 85, 883–892.
Kujoth, G. C., Hiona, A., Pugh, T. D., Someya, S., Panzer, K., Wohlgemuth, S. E., Hofer, T., Seo, A. Y., Sullivan, R., Jobling, W. A., Morrow, J. D., Van Remmen, H., Sedivy, J. M., Yamasoba, T., Tanokura, M., Weindruch, R., Leeuwenburgh, C. and Prolla, T. A., 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484.
Lambert, A. J., Portero-Otin, M., Pamplona, R. and Merry, B. J., 2004. Effect of ageing and caloric restriction on specific markers of protein oxidative damage and membrane peroxidizability in rat liver mitochondria. Mech Ageing Dev 125, 529–538.
Le Couteur, D. G., Cogger, V. C., Markus, A. M. A., Harvey, P. J., Yin, Z. L., Ansselin, A. D. and McLean, A. J., 2001. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 33, 537–543.
Le Couteur, D. G., Fraser, R., Cogger, V. C. and McLean, A. J., 2002. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet 359, 1612–1615.
Le Couteur, D. G., Fraser, R., Hilmer, S., Rivory, L. P. and McLean, A. J., 2005. The hepatic sinusoid in aging and cirrhosis – Effects on hepatic substrate disposition and drug clearance. Clin Pharmacokinet 44, 187–200.
Le Couteur, D. G. and McLean, A. J., 1998. The aging liver – Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet 34, 359–373.
Le Couteur, D. G., Rivory, L. P., Roberts, M. S. and Pond, S. M., 1992. Aging and the response of the isolated perfused rat liver to vasoactive drugs. Biochem Pharmacol 43, 913–915.
Le Couteur, D., Rivory, L. P., Yi, C. and Pond, S. M., 1995. Aging, acute oxidative injury and hepatocellular glucose transport in the rat. Int Hepatol Commun 3, 244–253.
Le Couteur, D. G., Warren, A., Cogger, V. C., Smedsrod, B., Sorensen, K., de Cabo, R., Fraser, R. and McCuskey, R. S., 2008. Old age and the hepatic sinusoid. Anat Rec 291, 672–683.
Leakey, J. E., Cunny, H. C., Bazare, J., Jr., Webb, P. J., Feuers, R. J., Duffy, P. H. and Hart, R. W., 1989b. Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome P-450 dependent monooxygenase system. Mech Ageing Dev 48, 145–155.
Leakey, J. A., Cunny, H. C., Bazare, J., Jr., Webb, P. J., Lipscomb, J. C., Slikker, W., Jr., Feuers, R. J., Duffy, P. H. and Hart, R. W., 1989a. Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. II: effects on conjugating enzymes. Mech Ageing Dev 48, 157–166.
Lee, K. P., Simpson, S. J., Clissold, F. J., Brooks, R., Ballard, J. W., Taylor, P. W., Soran, N. and Raubenheimer, D., 2008b. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A 105, 2498–2503.
Lee, J. S., Ward, W. O., Wolf, D. C., Allen, J. W., Mills, C., DeVito, M. J. and Corton, J. C., 2008a. Coordinated changes in xenobiotic metabolizing enzyme gene expression in aging male rats. Toxicol Sci 106, 263–283.
Lee, D. W. and Yu, P. Y., 1991. The age-related alterations in liver microsomal membranes: the effects of lipid peroxidation and dietary restriction. In Kitani, K. (ed), Liver and Aging – 1990. Elsevier Science Publishers, Amsterdam, pp. 17–26.
Li, J. and Holbrook, N. J., 2003. Common mechanisms for declines in oxidative stress tolerance and proliferation with aging. Free Radic Biol Med 35, 292–299.
Lipman, R. D., Dallal, G. E. and Bronson, R. T., 1999a. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. J Gerontol A Biol Sci Med Sci 54, B478–B491.
Lipman, R. D., Dallal, G. E. and Bronson, R. T., 1999b. Lesion biomarkers of aging in the B6C3F1 hybrid mice. J Gerontol 54A, B466–B477.
Lopez-Lluch, G., Hunt, N., Jones, B., Zhu, M., Jamieson, H., Hilmer, S., Cascajo, M. V., Allard, J., Ingram, D. K., Navas, P. and de Cabo, R., 2006. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 103, 1768–1773.
Lopez-Lluch, G., Irusta, P. M., Navas, P. and de Cabo, R., 2008. Mitochondrial biogenesis and healthy aging. Exp Gerontol 43, 813-819.
Lopez-Torres, M., Gredilla, R., Sanz, A. and Barja, G., 2002. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med 32, 882–889.
Lynch, C. D., Cooney, P. T., Bennett, S. A., Thornton, P. L., Khan, A. S., Ingram, R. L. and Sonntag, W. E., 1999. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging 20, 191–200.
Maes, O. C., An, J., Sarojini, H. and Wang, E., 2008. Murine microRNAs implicated in liver functions and aging process. Mech Ageing Dev 129, 534–541.
Martin, G. G., Atshaves, B. P., McIntosh, A. L., Mackie, J. T., Kier, A. B. and Schroeder, F., 2008. Liver fatty acid-binding protein gene-ablated female mice exhibit increased age-dependent obesity. J Nutr 138, 1859–1865.
Martin, R., Fitzl, G., Mozet, C., Martin, H., Welt, K. and Wieland, E., 2002. Effect of age and hypoxia/reoxygenation on mRNA expression of antioxidative enzymes in rat liver and kidneys. Exp Gerontol 37, 1481–1487.
Martin, G., Sewell, R. B., Yeomans, N. D., Morgan, D. J. and Smallwood, R. A., 1994. Hepatic Kupffer cell function: the efficiency of uptake and intracellular degradation of 14C-labelled mitochondria is reduced in aged rats. Mech Ageing Dev 73, 157–168.
Martin, G., Sewell, B., Yeomans, N. D. and Smallwood, R. A., 1992. Ageing has no effect on the volume density of hepatocytes, reticulo-endothelial cells or the extracellular space in livers of female Sprague-Dawley rats. Clin Exp Pharmacol Physiol 19, 537–539.
Martin-Armas, M., Simon-Santamaria, J., Pettersen, I., Moens, U., Smedsrod, B. and Sveinbjornsson, B., 2006. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol 44, 939–946.
Martini, C., Pallottini, V., Cavallini, G., Donati, A., Bergamini, E. and Trentalance, A., 2007. Caloric restrictions affect some factors involved in age-related hypercholesterolemia. J Cell Biochem 101, 235–243.
Masternak, M. M., Al-Regaiey, K. A., Del Rosario Lim, M. M., Jimenez-Ortega, V., Panici, J. A., Bonkowski, M. S. and Bartke, A., 2005. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol 40, 679–684.
McLean, A. J., Cogger, V. C., Chong, G. C., Warren, A., Markus, A. M., Dahlstrom, J. E. and Le Couteur, D. G., 2003. Age-related pseudocapillarization of the human liver. J Pathol 200, 112–117.
Milne, J. C., Lambert, P. D., Schenk, S. et al., 2007. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716.
Mizushima, N., Levine, B., Cuervo, A. M. and Klionsky, D. J., 2008. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075.
Muller, F. L., Lustgarten, M. S., Jang, Y., Richardson, A. and Van Remmen, H., 2007. Trends in oxidative aging theories. Free Radic Biol Med 43, 477–503.
Muradian, K. K., Utko, N. A., Fraifeld, V., Mozzhukhina, T. G., Pishel, I. N. and Litoshenko, A. Y., 2002. Superoxide dismutase, catalase and glutathione peroxidase activities in the liver of young and old mice: linear regression and correlation. Arch Gerontol Geriatr 35, 205–214.
Muskhelishvili, L., Turturro, A., Hart, R. W. and James, S. J., 1996. Pi-class glutathione-S-transferase-positive hepatocytes in aging B6C3F1 mice undergo apoptosis induced by dietary restriction. Am J Pathol 149, 1585–1591.
Navarro, A. and Boveris, A., 2004. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 287, R1244–R1249.
Pacini, G., Beccaro, F., Valerio, A., Nosadini, R. and Crepaldi, G., 1990. Reduced beta-cell secretion and insulin hepatic extraction in healthy elderly subjects. J Am Geriatr Soc 38, 1283–1289.
Pamplona, R. and Barja, G., 2006. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta 1757, 496–508.
Pearson, K. J., Baur, J. A., Lewis, K. N. et al., 2008. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8, 157–168.
Popper, H., 1986. Aging and the liver. Prog Liver Dis 8, 659–683.
Porta, E. A., 1991. Ceroid and lipofuscin in the liver. History and state of the art. In Kitani, K. (ed), Liver and Aging – 1990. Elsevier Science Publishers, Amsterdam, pp. 89–105.
Raghothama, C., Harsha, H. C., Prasad, C. K. and Pandey, A., 2005. Bioinformatics and proteomics approaches for aging research. Biogerontology 6, 227–232.
Rikans, L. E., Moore, D. R. and Snowden, C. D., 1991. Sex-dependent differences in the effects of aging on antioxidant defense mechanisms of rat liver. Biochim Biophys Acta 1074, 195–200.
Sakai, Y., Zhong, R., Garcia, B., Zhu, L. and Wall, W. J., 1997. Assessment of the longevity of the liver using a rat transplant model. Hepatology 25, 421–425.
Salive, M. E., Cornoni-Huntley, J., Phillips, C. L., Guralnik, J. M., Cohen, H. J., Ostfeld, A. M. and Wallace, R. B., 1992. Serum albumin in older persons: relationship with age and health status. J Clin Epidemiol 45, 213–221.
Sanguino, E., Roglans, N., Alegret, M., Sanchez, R. M., Vazquez-Carrera, M. and Laguna, J. C., 2005. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol 145, 853–861.
Sanz, N., Diez-Fernandez, C., Alvarez, A. and Cascales, M., 1997. Age-dependent modifications in rat hepatocyte antioxidant defense systems. J Hepatol 27, 525–534.
Sastre, J., Pallardo, F. V., Pla, R., Pellin, A., Juan, G., O’Connor, J. E., Estrela, J. M., Miquel, J. and Vina, J., 1996. Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology 24, 1199–1205.
Sastre, J., Pallardo, F. V. and Vina, J., 2000. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49, 427–435.
Sastre, J., Pallardo, F. V. and Vina, J., 2003. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med 35, 1–8.
Schaffner, F. and Popper, H., 1959. Nonspecific reactive hepatitis in aged and infirm people. Am J Dig Dis 4, 389–399.
Schapiro, H., Hotta, S. S., Outten, W. E. and Klein, A. W., 1982. The effect of aging on rat liver regeneration. Experientia 38, 1075–6.
Schmucker, D. L., 1976. Age-related changes in hepatic fine structure: a quantitative analysis. J Gerontol 31, 135–143.
Schmucker, D. L., 1978. A quantitative morphological evaluation of hepatocytes in young mature and senescent fischer 344 male rats. In Kitani, K. (ed), Liver and Aging. Elsevier North Holland, Amsterdam, pp. 21–38.
Schmucker, D. L., 1998. Aging and the liver: an update. J Gerontol 53A, B315–B320.
Schmucker, D. L., 2001. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging 18, 837–851.
Schmucker, D. L., 2005. Age-related changes in liver structure and function: implications for disease?. Exp Gerontol 40, 650–659.
Schmucker, D. L., Woodhouse, K. W., Wang, R. K., Wynne, H., James, O. F., McManus, M. and Kremers, P., 1990. Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clin Pharmacol Ther 48, 365–374.
Schnegg, M. and Lauterburg, B. H., 1986. Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation and caffeine clearance. J Hepatol 3, 164–171.
Senthil Kumaran, V., Arulmathi, K., Srividhya, R. and Kalaiselvi, P., 2008. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol 43, 176–183.
Serste, T. and Bourgeois, N., 2006. Ageing and the liver. Acta Gastroenterol Belg 69, 296–298.
Shaddock, J. G., Chou, M. W. and Casciano, D. A., 1996. Effects of age and caloric restriction on cell proliferation in hepatocyte cultures from control and hepatectomized Fischer 344 rats. Mutagenesis 11, 281–284.
Sinclair, D. A., 2005. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126, 987–1002.
Sinclair, D. and Guarente, L., 2006. Unlocking the secrets of longevity genes. Sc Am 294, 48–57.
Skogh, T., Blomhoff, R., Eskild, W. and Berg, T., 1985. Hepatic uptake of circulating IgG immune complexes. Immunology 55, 585–594.
Smanik, E. J., Yue, C. C. and Mullen, K. D., 1991. “Old” livers don’t regenerate in a week. J Lab Clin Med 117, 89–90.
Smedsrod, B., 2004. Clearance function of scavenger endothelial cells. Comp Hepatol 3(Suppl 1), S22.
Smedsrod, B., Melkko, J., Araki, N., Sano, H. and Horiuchi, S., 1997. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J 322(Pt 2), 567–573.
Smedsrod, B., Pertoft, H., Gustafson, S. and Laurent, T. C., 1990. Scavenger functions of the liver endothelial cell. Biochem J 266, 313–327.
Sotaneimi, E. A., Arranto, A. J., Pelkonen, O. and Pasanen, M., 1997. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathological conditions. Clin Pharmacol Ther 61, 331–339.
Stacchiotti, A., Lavazza, A., Ferroni, M., Sberveglieri, G., Bianchi, R., Rezzani, R. and Rodella, L. F., 2008. Effects of aluminium sulphate in the mouse liver: similarities to the aging process. Exp Gerontol 43, 330–338.
Sun, W. B., Han, B. L., Peng, Z. M., Li, K., Ji, Q., Chen, J., Wang, H. Z. and Ma, R. L., 1998. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol 4, 77–79.
Swindell, W. R., 2008. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev 129, 138–153.
Tauchi, H. and Sato, T., 1975. Effect of environmental conditions upon age changes in the human liver. Mech Ageing Dev 4, 71–80.
Tauchi, H. and Sato, T., 1978. Hepatic cells of the aged. In Kitani, K. (ed), Liver and Aging – 1978. Elsevier North Holland, Amsterdam, pp. 3–20.
Thalacker-Mercer, A. E. and Campbell, W. W., 2008. Dietary protein intake affects albumin fractional synthesis rate in younger and older adults equally. Nutr Rev 66, 91–95.
Thomas, R. P., Guigneaux, M., Wood, T. and Evers, B. M., 2002. Age-associated changes in gene expression patterns in the liver. J Gastrointest Surg 6, 445-453; discussion 454.
Tollet-Egnell, P., Flores-Morales, A., Stahlberg, N., Malek, R. L., Lee, N. and Norstedt, G., 2001. Gene expression profile of the aging process in rat liver: normalizing effects of growth hormone replacement. Mol Endocrinol 15, 308–318.
Tuchweber, B., Messier, M., Yousef, I. M., Perea, A. and Audet, M., 1991. Effect of dietary restriction on bile formation and lvier plasma membrane function and fluidity in aging female rats. In Kitani, K. (ed), Liver and Aging – 1990. Elsevier Science Publishers, Amsterdam, pp. 167–176.
Uchida, K., Igimi, H., Takase, H., Numura, Y., Nakao, Y., Chikai, T., Ichihashi, T., Kayahara, T. and Takeuchi, N., 1991. Bile acids and aging in relation to hyeprcholesterolemia in rats. In Kitani, K. (ed), Liver and Aging – 1990. Elsevier Science Publishers, Amsterdam, pp. 153–164.
Valle, A., Silvestri, E., Moreno, M., Chambery, A., Oliver, J., Roca, P. and Goglia, F., 2008. Combined effect of gender and caloric restriction on liver proteomic expression profile. J Proteome Res 7, 2872–2881.
Van Bezooijen, C. F. A., 1984. Influence of age-related changes in rodent liver morphology and physiology on drug metabolism – a review. Mech Ageing Dev 25, 1–22.
Van Bezooijen, C. F., Van Noord, M. J. and Knook, D. L., 1974. The viability of parenchymal liver cells isolated from young and old rats. Mech Ageing Dev 3, 107–119.
Varga, F. and Fischer, E., 1978. Age dependent changes in blood supply of the liver and in the biliary excretion of eosine in rats. In Kitani, K. (ed), Liver and Aging. Elsevier North Holland, Amsterdam, pp. 327–342.
Videla, L. A., Tapia, G. and Fernandez, V., 2001. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep 6, 155–159.
Vittorini, S., Paradiso, C., Donati, A., Cavallini, G., Masini, M., Gori, Z., Pollera, M. and Bergamini, E., 1999. The age-related accumulation of protein carbonyl in rat liver correlates with the age-related decline in liver proteolytic activities. J Gerontol A Biol Sci Med Sci 54, B318–B323.
Vollmar, B., Pradarutti, S., Richter, S. and Menger, M. D., 2002. In vivo quantification of ageing changes in the rat liver from early juvenile to senescent life. Liver 22, 330–341.
Vomel, T., Platt, D. and Schnorr, B., 1981. Phagocytosis activity of the isolated perfused liver in rats of different ages. Biochemical and ultrastructural examination after perfusion with colloidal ink. Fortschr Med 99, 1511–1515.
Vomel, T., Platt, D. and Schnorr, B., 1984. Phagocytic activity of isolated perfused liver of rats of different ages. Biochemical and structural studies following perfusion with Cu-ceruloplasmin. Z Gerontol 17, 69–72.
Ward, W. and Richardson, A., 1991. Effect of age on liver protein synthesis and degradation. Hepatology 5, 935–949.
Warren, A., Bertolino, P., Cogger, V. C., McLean, A. J., Fraser, R. and Le Couteur, D. G., 2005. Hepatic pseudocapillarization in aged mice. Exp Gerontol 40, 807–812.
Wei, Y., Rector, R. S., Thyfault, J. P. and Ibdah, J. A., 2008. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol 14, 193–199.
Weng, N. P., 2006. Aging of the immune system: how much can the adaptive immune system adapt?. Immunity 24, 495–499.
Wiener, E. and Rabinovici, N., 1961. Liver haemodynamics and age. Proc Soc Exp Biol Med 108, 752–754.
Wilkinson, G. R., 1997. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev 27, 123–159.
Wood, J. G., Rogina, B., Lavu, S., Howitz, K., Helfand, S. L., Tatar, M. and Sinclair, D., 2004. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689.
Wynne, H. A., Cope, L. H., Herd, B., Rawlins, M. D., James, O. F. and Woodhouse, K. W., 1990a. The association of age and frailty with paracetamol conjugation in man. Age Ageing 19, 419–424.
Wynne, H. A., Cope, L. H., James, O. F. W., Rawlins, M. D. and Woodhouse, K. W., 1989a. The effect of age and frailty upon acetanilide clearance in man. Age Ageing 18, 415–418.
Wynne, H. A., Cope, L. H., Mutch, E., Rawlins, M. D., Woodhouse, K. W. and James, O. F. W., 1989b. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 9, 297–301.
Wynne, H. A., Goudevenos, J., Rawlins, M. D., James, O. F. W., Adams, P. C. and Woodhouse, K. W., 1990b. Hepatic drug clearance: the effect of age using indocyanine green as a model compound. Brit J Clin Pharmacol 30, 634–637.
Wynne, H. A. and James, O. F. W., 1990. The aging liver. Age Ageing 19, 1–3.
Yamano, T., DeCicco, L. A. and Rikans, L. E., 2000. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol Appl Pharmacol 162, 68–75.
Yang, M. C., McLean, A. J. and Le Couteur, D. G., 2002. Age-related alteration in hepatic disposition of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and pesticides. Pharmacol Toxicol 90, 203–207.
Zahn, J. M., Poosala, S., Owen, A. B. et al., 2007. AGEMAP: a gene expression database for aging in mice. PLoS Genet 3, e201.
Zeeh, J. and Platt, D., 1990. Age related changes in the liver. Consequences for drug therapy. Fortschr Med 108, 651–653.
Zhang, C. and Cuervo, A. M., 2008. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14, 959–965.
Zhu, M., de Cabo, R., Anson, R. M., Ingram, D. K. and Lane, M. A., 2005. Caloric restriction modulates insulin receptor signaling in liver and skeletal muscle of rat. Nutrition 21, 378–388.
Zhu, M., de Cabo, R., Lane, M. A. and Ingram, D. K., 2004. Caloric restriction modulates early events in insulin signaling in liver and skeletal muscle of rat. Ann N Y Acad Sci 1019, 448–452.
Acknowledgments
We acknowledge the support of the Australian National Health and Medical Research Council, Canadian Institutes of Health Research and the Ageing and Alzheimers Research Foundation (a division of the Medical Foundation of the University of Sydney). DAS is a consultant to GSK and Genocea, and research is supported by grants from the National Institutes of Health, The Ellison Medical Foundation, and the Glenn Foundation for Medical Research.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Le Couteur, D.G. et al. (2010). The Aging Liver and the Effects of Long Term Caloric Restriction. In: Everitt, A., Rattan, S., le Couteur, D., de Cabo, R. (eds) Calorie Restriction, Aging and Longevity. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8556-6_11
Download citation
DOI: https://doi.org/10.1007/978-90-481-8556-6_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-8555-9
Online ISBN: 978-90-481-8556-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)