Abstract
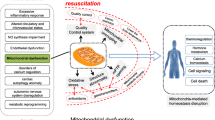
The working heart is a highly metabolic organ that under normal resting conditions extracts nearly 70% of the oxygen supplied by the coronary circulation [1, 2], representing close to 10% of the total body oxygen consumption. However, the heart has minimal capability for extracting additional oxygen, and increases in metabolic demands can only be met by autoregulatory increases in coronary blood flow through vasodilation of the coronary circuit [3]. Consequently, a severe energy imbalance develops when cardiac arrest occurs and coronary blood flow ceases. The severe energy imbalance continues during the ensuing resuscitation effort when current closed-chest resuscitation techniques are used because of the very limited capability for generating systemic and coronary blood flow [4]. The magnitude of energy imbalance is contingent upon the metabolic requirements and is particularly severe in the presence of ventricular fibrillation (VF), when oxygen requirements are comparable with or exceed those of the normally beating heart [5, 6]. A lesser energy deficit is expected during cardiac arrest with a quiescent or minimally active heart (i.e. asystole or pulseless electrical activity precipitated by asphyxia or exsanguination). Moreover, with reperfusion during resuscitation, multiple pathogenic mechanisms — collectively known as reperfusion injury — are activated and further contribute to myocardial injury. Primary contributors to reperfusion injury are mitochondrial calcium (Ca2+) overload [7, 8] and generation of reactive oxygen species (ROS) [9]. Limited oxygen supply and concomitant reperfusion injury compromise the mitochondrial capability for regenerating adenosine triphosphatase (ATP) through oxidative phosphorylation. Limited amounts of ATP, however, are generated at the substrate level from anaerobic glycolysis and breakdown of creatine phosphate. Taking these processes together, the myocardium develops a marked lactic acid increase, rapid creatine phosphate depletion and relatively slow ATP depletion during cardiac arrest and resuscitation [10]. Accordingly, the resuscitation effort typically proceeds — and occasionally succeeds — in the presence of ischaemia and in the midst of reperfusion injury.
Similar content being viewed by others
Keywords
- Ventricular Fibrillation
- Coronary Blood Flow
- Chest Compression
- Spontaneous Circulation
- Coronary Perfusion Pressure
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The working heart is a highly metabolic organ that under normal resting conditions extracts nearly 70% of the oxygen supplied by the coronary circulation [1, 2], representing close to 10% of the total body oxygen consumption. However, the heart has minimal capability for extracting additional oxygen, and increases in metabolic demands can only be met by autoregulatory increases in coronary blood flow through vasodilation of the coronary circuit [3]. Consequently, a severe energy imbalance develops when cardiac arrest occurs and coronary blood flow ceases. The severe energy imbalance continues during the ensuing resuscitation effort when current closed-chest resuscitation techniques are used because of the very limited capability for generating systemic and coronary blood flow [4]. The magnitude of energy imbalance is contingent upon the metabolic requirements and is particularly severe in the presence of ventricular fibrillation (VF), when oxygen requirements are comparable with or exceed those of the normally beating heart [5, 6]. A lesser energy deficit is expected during cardiac arrest with a quiescent or minimally active heart (i.e. asystole or pulseless electrical activity precipitated by asphyxia or exsanguination). Moreover, with reperfusion during resuscitation, multiple pathogenic mechanisms — collectively known as reperfusion injury — are activated and further contribute to myocardial injury. Primary contributors to reperfusion injury are mitochondrial calcium (Ca2+) overload [7, 8] and generation of reactive oxygen species (ROS) [9]. Limited oxygen supply and concomitant reperfusion injury compromise the mitochondrial capability for regenerating adenosine triphosphatase (ATP) through oxidative phosphorylation. Limited amounts of ATP, however, are generated at the substrate level from anaerobic glycolysis and breakdown of creatine phosphate. Taking these processes together, the myocardium develops a marked lactic acid increase, rapid creatine phosphate depletion and relatively slow ATP depletion during cardiac arrest and resuscitation [10]. Accordingly, the resuscitation effort typically proceeds — and occasionally succeeds — in the presence of ischaemia and in the midst of reperfusion injury.
2 Functional Manifestations
Various functional myocardial abnormalities develop consequent to ischaemia and reperfusion during cardiac arrest and resuscitation efforts that exert effects detrimental to cardiac resuscitation. These abnormalities can be grouped into those that manifest during the resuscitation effort and those that manifest after the return of spontaneous circulation. The former include reductions in left ventricular (LV) myocardial distensibility and increased resistance to electrical defibrillation; the latter includes reperfusion arrhythmias and postresuscitation myocardial dysfunction.
2.1 Reductions in Left Ventricular Myocardial Distensibility
Studies in various animal models of VF and resuscitation have shown progressive thickening of the LV wall accompanied by parallel reductions in LV cavity without changes in intracavitary pressures during the resuscitation effort [11, 12]. A functionally similar phenomenon — known as ischaemic contracture — was reported in the early 1970s during open heart surgery when operations were conducted under normothermic conditions and in fibrillating hearts [13, 14] and more recently after prolonged intervals of untreated VF [15]. However, ischaemic contracture is associated with profound reductions in myocardial ATP and often leads to a “stony heart”, heralding irreversible ischaemic injury [16].
Reductions in LV myocardial distensibility observed during cardiac resuscitation is a different phenomenon:
-
1.
it occurs much earlier than the stony heart;
-
2.
onset and subsequent progression coincide with the interval of reperfusion during resuscitation [11, 17];
-
3.
it is associated with less ATP depletion [10];
-
4.
it has been attributed to myocardial energy deficit compounded by cytosolic and mitochondrial Ca2+ overload, precluding complete relaxation of individual cardiomyocytes;
-
5.
it evolves into diastolic dysfunction upon return of spontaneous circulation [18];
-
6.
it is largely reversible [19].
2.1.1 Haemodynamic Consequences of Reductions in Left Ventricular Myocardial Distensibility
As blood returns to the heart during the relaxation phase of chest compression, distensible ventricles are important to properly accommodate the returning blood and establish an adequate preload for the subsequent compression. The larger the distensibility, the larger the preload, and the larger the amount of blood that can be ejected by chest compression. This mechanism is akin to the Frank-Starling law [20] of the beating heart and presumes that blood is ejected from the LV into the aorta during chest compression. Progressive decreases in LV myocardial distensibility during chest compression contribute to progressive decline in haemodynamic efficacy of closed-chest resuscitation. Studies in a VF porcine model have shown that the severity of this phenomenon is proportional to the duration of untreated VF [11].
Work in our laboratory demonstrated that reductions in LV myocardial distensibility can be prevented by pharmacologic interventions targeting reperfusion injury, resulting in more haemodynamically stable closed-chest resuscitation [17, 21]. In one study, progressive LV wall thickening with reductions in cavity size were mitigated by administration of the sodium/hydrogen exchanger 1 (NHE-1) inhibitor cariporide [17]. This effect prevented haemodynamic deterioration that characteristically occurs during chest compression, maintaining a stable coronary perfusion pressure above the resuscitability threshold of 10 mmHg in pigs and yielding higher resuscitation rates [17].
2.1.2 Clinical Evidence of Reductions in Left Ventricular Myocardial Distensibility
Takino and Okada [22] reported in 1996 on 59 adult patients who suffered nontraumatic out-of-hospital cardiac arrest and underwent open-chest direct manual cardiac compression in the emergency department after failure of closed-chest resuscitation. A “firm” myocardium was noticed during manual cardiac compression in 36 cases that predominantly affected the LV. In the remaining 23 cases, the hearts were “soft”. The authors also noted that some hearts became “firm” during compression. The presence of a firm myocardium was associated with reduced haemodynamic efficacy of cardiac compression, as evidenced by a lower end-tidal carbon dioxide (CO2) tension (PETCO2) — which is a well-documented surrogate measurement of systemic and regional blood flow during cardiac resuscitation [4, 23–25]. Hearts with very firm myocardium never regained spontaneous contractions. Hearts with less firm myocardium showed some, albeit insufficient, spontaneous contractions. Hearts with soft myocardium regained contractions and were able to generate a peripheral pulse in most instances.
2.2 Resistance to Defibrillation
Electrical shocks delivered immediately after VF onset are consistently effective in reestablishing cardiac activity. Even short delays (i.e. up to 3 min) may not be substantially detrimental, resulting in >50% likelihood of successful resuscitation [26]. However, longer intervals of untreated VF — as usually occurs after out-of-hospital cardiac arrest — predictably decreases the effectiveness of defibrillation attempts in which electrical shocks — even of higher energy levels — may fail to reverse VF or may precipitate asystole or pulseless electrical activity. Under these conditions, haemodynamic efficacy of the resuscitation technique becomes of paramount importance for successful defibrillation.
2.3 Reperfusion Arrhythmias
Premature ventricular complexes and episodes of ventricular tachycardia and VF commonly occur during the early minutes after return of cardiac activity. Postresuscitation episodes of VF — which require additional electrical shocks — have been reported in up to 79% of patients, with some studies showing and inverse relationship between the number of episodes and survival [27]. The underlying cell mechanisms are complex but prominently involve cytosolic Ca2+ overload and after-depolarisations. There are repolarisation abnormalities that include shortening of the action potential duration, decreased action potential amplitude and development of action potential alternans, creating conditions for re-entry. Experimentally, these repolarisation abnormalities are short-lived (5–10 min) and coincide with the interval of increased propensity for ventricular arrhythmias and recurrent VF [17]. These repolarisation abnormalities and reperfusion arrhythmias can be markedly attenuated by NHE-1 inhibition [17].
2.4 Postresuscitation Myocardial Dysfunction
Variable degrees of systolic [28–31] and diastolic [17, 32] dysfunction develop after resuscitation from cardiac arrest. Dysfunction occurs despite full restoration of coronary blood flow and is largely reversible, conforming to the definition of myocardial stunning. Systolic dysfunction is characterised by decreases in contractility — documented by load-independent indices derived from varying end-systolic pressure-volume relationship — leading to reductions in LV ejection fraction, cardiac index, LV stroke work [29, 30] and poor tolerance to afterload increases [33]. Diastolic dysfunction is characterised by LV wall thickening with reductions in end-diastolic volume and impaired relaxation [17]. Diastolic dysfunction appears to be maximal immediately after spontaneous circulation restoration, with the magnitude of wall thickness closely correlated with wall thickness during VF [18], suggesting a common pathogenic thread. From a functional perspective, diastolic dysfunction may limit the compensatory ventricular dilatation required to overcome decreases in contractility according to the Frank-Starling mechanism [20].
3 Novel Intervention Targeting the Pathophysiology of Myocardial Injury
Two lines of research developed at the Resuscitation Institute at Rosalind Frank University show promising new interventions that can ameliorate myocardial injury and have beneficial effects for initial resuscitation and postresuscitation myocardial dysfunction. One line of research involves using NHE-1 inhibitors in various animal models of cardiac arrest [10, 12, 17, 21, 34–38]. The other relates to more recent work using erythropoietin in a rat model of cardiac arrest [39] and in a small clinical study in patients suffering out-of-hospital cardiac arrest [40]. Both lines of research support the rationale and feasibility of using either an NHE-1 inhibitor or erythropoietin for preserving LV myocardial distensibility during cardiac resuscitation and function after return of spontaneous circulation.
3.1 NHE-1 Inhibitors
3.1.1 Cell Mechanism
Cessation of coronary blood flow during cardiac arrest causes a metabolic shift to anaerobic metabolism, prompting rapid development of intense and sustained intracellular acidosis [41–43]. Intracellular acidosis activates the sarcolemmal NHE-1, initiating an electroneutral sodium/hydrogen (Na+-H+) exchange that brings Na+ into the cell [44]. During the ensuing resuscitation effort, the myocardium is reperfused with blood that typically has a normal pH, resulting in the washout of protons that accumulated in the extracellular space during the preceding interval of no-flow cardiac arrest. This intensifies sarcolemmal Na+-H+ exchange and the resulting Na+ entry [34, 44, 45]. Na+ accumulates in the cytosol because the Na+-K+ ATPase activity is concomitantly reduced [46], such that progressive and prominent increases in cytosolic Na+ occurs. Na+ may also enter the cell through Na+ channels and the Na+-bicarbonate (HCO3 -) cotransporter. The cytosolic Na+ excess, in turn, drives sarcolemmal Ca2+ influx through reverse-mode operation of the sarcolemmal Na+-Ca2+ exchanger, leading to cytosolic and mitochondrial Ca2+ overload [47], causing a myriad of detrimental effects.
Cytosolic Ca2+ overload during ischaemia and reperfusion has been identified as a primary effector of mitochondrial injury. Mitochondria can sequester large amounts of cytosolic Ca2+, a process regulated by the Ca2+ uniporter for influx and by the Na+-Ca2+ exchanger for efflux [48]. However, as matrix Ca2+ levels progressively rise, the mitochondrial Na+-Ca2+ exchanger becomes saturated and mitochondrial Ca2+ overload ensues [48]. Mitochondrial Ca2+ overload can worsen cell injury in part by compromising its capability to sustain oxidative phosphorylation [49] and by promoting the release of proapoptotic factors [50].
The relevance of this mechanism of injury is highlighted by a large preclinical database demonstrating consistent attenuation of myocardial injury caused by ischaemia and reperfusion when Na+ entry to the cell is limited, as when NHE-1 activity is inhibited [44] or when Na+ channels are blocked [51, 52].
3.1.2 Effects of NHE-1 Inhibition on Resuscitation
Research over the last decade in our laboratory using various translational rat and pig models of cardiac arrest has shown consistent myocardial benefit associated with inhibition of NHE-1 activity during resuscitation from VF [10, 12, 17, 21, 34–38, 53–57]. Mechanistically, these benefits are associated with less cytosolic Na+ overload, less mitochondrial Ca2+ overload and preservation of oxidative phosphorylation. Some of these studies, highlighting key aspects of NHE-1 inhibition during resuscitation pertinent to this application, are succinctly discussed below.
3.1.2.1 Effects on Left Ventricular Myocardial Distensibility
The initial findings suggesting that NHE-1 inhibition could attenuate reductions in LV myocardial distensibility during resuscitation and also prevent postresuscitation diastolic dysfunction were made in an isolated (Langendorff) rat model of VF and simulated resuscitation [34, 35], establishing the rational for subsequent studies in intact rat and intact pig models of VF and resuscitation. Findings in one of our pig studies paralleled the aforementioned findings in the isolated rat heart, corroborating in a clinically relevant model preservation of LV myocardial distensibility during closed-chest resuscitation evidenced by preservation of wall thickness and cavity size. Preservation of LV myocardial distensibility enabled the generation of higher coronary perfusion pressures, leading to higher resuscitability rates (2/8 vs 8/8; p < 0.05) [18].
Subsequent studies were conducted in our intact rat model of VF and closed-chest resuscitation designed to measure — using fluorescent microspheres — the effects of NHE-1 inhibition (using cariporide) on systemic and organ blood flow as a function of compression depth [21]. We reasoned that if LV myocardial distensibility — and therefore preload — could be preserved by NHE-1 inhibition, then higher forward blood flows could be generated for a given compression depth, thus shifting the relationship between flow and compression depth to the left. Two series of 14 experiments each were conducted in which rats were subjected to 10 min of untreated VF followed by 8 min of chest compression before attempting defibrillation. Compression depth was adjusted to maintain an aortic diastolic pressure between 26 and 28 mmHg in the first series and between 36 and 38 mmHg in the second series. Within each series, rats were randomised to receive cariporide (3 mg/kg) or NaCl 0.9% (control) before starting chest compression. In rats that received cariporide, the compression depth required to generate a given level of systemic and organ blood flow was markedly reduced compared with in rats that received the vehicle control.
These studies also suggested that higher coronary perfusion pressures could be generated when administering a vasopressor agent, given the larger blood flow generated in the presence of an NHE-1 inhibitor for a given compression depth. This was the case when cariporide was combined with epinephrine in our pig model [12] and when combined with epinephrine and with vasopressin in our rat model [37].
3.1.2.2 Effects on Cytosolic Na+ and Mitochondrial Ca2+
A rat model of VF and closed-chest resuscitation was used to examine the effects of NHE-1 inhibition and of Na+ channel blockade (interventions collectively referred to as Na+-limiting interventions) on intracellular Na+ levels, mitochondrial Ca2+ levels, cardiac function and plasma levels of cardiospecific troponin I (cTnI) after resuscitation [38]. For these studies, hearts were removed at specific time events; namely: (1) at baseline; (2) at 15min of untreated VF; (3) at 15min of VF with chest compression provided during the last 5min of VF; and (4) at 60min postresuscitation. Rats from the last two time events were randomised to receive Na+-limiting intervention immediately before starting chest compression or vehicle control. Na+-limiting interventions included a newly developed NHE-1 inhibitor, AVE4454 (1 mg/kg), lidocaine (5 mg/kg), and the combination of AVE4454 and lidocaine. Limiting sarcolemmal Na+ entry attenuated increases in cytosolic Na+ and mitochondrial Ca2+ overload during chest compression and the postresuscitation phase. Attenuation of cytosolic Na+ and mitochondrial Ca2+ increases was accompanied by preservation of LV myocardial distensibility during chest compression, less postresuscitation myocardial dysfunction and lower levels of cTnI.
3.1.2.3 Effects on Energy Metabolism
An open-chest pig model of electrically induced VF and extracorporeal circulation was developed to study the myocardial energy effects of inhibiting NHE-1 under conditions of controlled coronary perfusion pressure [10]. For this study, VF was induced by epicardial delivery of an alternating current and left untreated for 8min. After this interval, extracorporeal circulation was started and systemic (extracorporeal) blood flow adjusted to maintain a coronary perfusion pressure at 10 mmHg for 10min before attempting defibrillation and restoration of spontaneous circulation. The target coronary perfusion pressure was chosen to mimic the low coronary perfusion pressure generated by closed-chest resuscitation. Two groups of eight pigs each were randomised to receive the NHE-1 inhibitor zoniporide (3 mg/kg) or vehicle control as a right atrial bolus immediately before starting extracorporeal circulation. As in a previous study using the NHE-1 inhibitor cariporide [17], zoniporide also prevented reductions in LV myocardial distensibility during the VF interval and extracorporeal circulation, which in control pigs was characterised by progressive reductions in cavity size and progressive thickening of the LV wall.
Importantly, these effects occurred without changes in coronary blood flow or coronary vascular resistance, indicating that the favourable myocardial effects of NHE-1 inhibition during resuscitation are not likely the result of increased blood flow and oxygen availability (e.g. by less extrinsic compression of the coronary circuit). Instead, myocardial tissue measurements indicated that zoniporide administration prevented progressive loss of oxidative phosphorylation during the interval of simulated resuscitation. Animals that received zoniporide: (1) maintained a higher creatine phosphate to creatine (pCr/Cr) ratio; (2) maintained a higher ATP/adenosine diphosphate (ADP) ratio; and (3) had lesser increases in adenosine. These measurements are consistent with regeneration of ADP into ATP by mitochondria instead of downstream degradation into adenosine, with the newly formed ATP being used to regenerate creatine phosphate. All these findings are indicative of preserved mitochondrial bioenergetic function. These changes were accompanied by prominent amelioration of myocardial lactate increases, attaining levels inversely proportional to the pCr/Cr ratio at 8min of VF and extracorporeal circulation, suggesting a shift away from anaerobic metabolism consequent to preservation of mitochondrial bioenergetic function in pigs treated with zoniporide. After return of spontaneous circulation, pigs treated with zoniporide had higher LV ejection fraction (0.57 ± 0.07 vs 0.29 ± 0.05; p < 0.05) and higher cardiac index (4.8 ± 0.4 vs 3.4 ± 0.2 l/min/m2; p < 0.05) [10], replicating previously reported favourable effects of NHE-1 inhibition on postresuscitation myocardial function [12, 17, 34, 35]. These energy effects are consistent with NHE-1 inhibition protecting mitochondrial bioenergetic function — probably as a result of limiting mitochondrial Ca2+ overload — and supportive of the concept that LV myocardial distensibility during resuscitation is likely to be preserved by activating mitochondrial mechanisms capable.
3.2 Erythropoietin
3.2.1 Cell Mechanism
Erythropoietin is a 30.4-kDa glycoprotein best known for its action on erythroid progenitor cells and regulation of circulating red cell mass. However, several studies have recently shown that erythropoietin also activates potent cell survival mechanisms during ischaemia and reperfusion through genomic and nongenomic signalling pathways in a broad array of organs and tissues, including the heart [58–63], brain [64, 65], spinal cord [66], retina [67], kidney [68], liver [69] and skin [70].
Activation of these protective mechanisms involves binding of erythropoietin to a specifi c cell membrane receptor (epoR) member of the type 1 superfamily of single-transmembrane cytokine receptors, prompting cross-phosphorylation and activation of Janus tyrosine kinases (JAK) 1 and 2. JAK activation causes phosphorylation of tyrosine residues, creating docking sites for recruitment and activation of multiple signalling proteins that have Src-homology-2 (SH2) domains resulting in well-established antiapoptotic [59], anti-inflammatory [71, 72] and proliferative effects (i.e. neovascularisation) [73, 74], with time courses that vary contingent upon the specific signalling mechanism and duration of erythropoietin binding to the EpoR. Although important in other settings, these effects are not likely to play a role for initial cardiac resuscitation. We hypothesise that erythropoietin signalling is important for resuscitation through pathways that result in preservation of mitochondrial bioenergetic function, leading to functional effects similar to those elicited by NHE-1 inhibition (albeit through quite distinct cell mechanisms).
3.2.2 Effects of Erythropoietin on Resuscitation
3.2.2.1 Studies in Rats
The effects of erythropoietin were studied in our rat model of VF and closed-chest resuscitation using human recombinant erythropoietin (epoetin alpha, Amgen, Thousand Oaks, CA, USA) [39]. Rats were subjected to 10 min of untreated VF followed by 8 min of closed-chest resuscitation before attempting defibrillation. The depth of compression was adjusted to maintain an aortic diastolic pressure between 26 and 28 mmHg. This level of diastolic aortic pressure secured a coronary perfusion pressure above the resuscitability threshold of 20 mmHg in rats. The relationship between the coronary perfusion pressure and compression depth (CPP/depth) was used to assess changes in LV myocardial distensibility. Successfully resuscitated rats were observed for 120min before euthanasia.
Three groups of ten rats each were randomised to receive a right atrial bolus of epoetin alpha (5,000 IU/kg) at baseline 15min before induction of VF (EPOBL -15-min), at 10min of VF before starting chest compression (EPOVF 10-min) or to receive 0.9% NaCl solution (control), with the investigators blinded to the treatment assignment. Erythropoietin given coincident with the beginning of chest compression after 10min of untreated VF — but not before inducing VF — promoted haemodynamically more effective chest compression such that the coronary perfusion pressure to compression depth (CPP/depth) ratio averaged during the interval of chest compression was 2.0 ± 0.3 mmHg/mm in EPOVF 10-min, 1.6 ± 0.2 mmHg/mm in EPOBL -15-min and 1.6 ± 0.3 mmHg/mm in the control group (p < 0.05 EPOVF 10-min vs EPOBL -15-min and vs control). This difference represented a 25% improvement in the haemodynamic efficacy of chest compression with erythropoietin given at the beginning of chest compression. Postresuscitation, EPOVF 10-min rats had significantly higher mean aortic pressure associated with numerically higher cardiac index and higher peripheral vascular resistance. The diminished effectiveness of erythropoietin when given before VF is intriguing and worth of additional investigation.
Similar observations were made in a recent series of experiments in the same rat model of VF and closed-chest resuscitation described above. However, the protocol was modified such that the chest was compressed to the maximum depth of 17 mm in rats. Under this clinically more relevant protocol, 5,000 IU/kg of erythropoietin given at the beginning of chest compression prompted haemodynamically more effective chest compression, yielding a coronary perfusion pressure approximately 5 mmHg higher than in control rats and substantial improvement in postresuscitation myocardial dysfunction.
3.2.2.2 Studies in Humans
A clinical study was performed in collaboration with Dr. Štefek Grmec and the Maribor Emergency Medical Services system in the city of Maribor, Slovenia and adjacent rural areas, encompassing a population of approximately 200,000 inhabitants [40]. Resuscitation was attempted using regionally developed protocols that incorporate International Liaison Committee on Resuscitation (ILCOR) 2005 [75] recommendations by a two-tier system composed of basic life support and advanced life support teams, with the latter led by a physician. Patients assigned to erythropoietin received 90,000 IU of beta-epoetin (NeoRecormon, Hoffman La Roche) as a bolus within 1 or 2min after starting chest compression, followed by a 10-ml bolus of 0.9% NaCl. Beta-epoetin was kept refrigerated (2–8°C) in the ambulance until immediately before use. In every instance, erythropoietin was given before any other drug. The primary end-point was intensive care unit (ICU) admission. The secondary end-points were return of spontaneous circulation (ROSC) in the field, survival at 24 h and survival at hospital discharge.
The study was originally designed to be prospective and randomised. However, disruption in the supply of erythropoietin prompted investigators to administer erythropoietin or 0.9% NaCl control based on availability, allocating 24 patients to erythropoietin and 30 to 0.9% NaCl between April 2007 and May 2008. The control group for the analysis was designated as concurrent controls. Post hoc, a second control group was included in which 48 of 126 patients were selected who had out-of-hospital cardiac arrest treated with the same resuscitation protocol the year before. These 48 patients were selected using propensity scores assigning two controls for each erythropoietin-treated patient. Propensity scores were calculated using multiple logistic regression: entering age, male sex, witnessed arrest, time from call to start of cardiopulmonary resuscitation (CPR), pulseless electrical activity, asystole and bystander CPR as pretreatment predictors of outcome. The control group was designated as matched controls. The same variables used to calculate propensity scores were used to adjust odds ratios (OR) for comparison between erythropoietin and the concurrent controls and between erythropoietin and the matched controls.
By univariate analysis, administration of erythropoietin — when compared with concurrent controls — was associated with higher rates of ICU admission, ROSC, 24-h survival and survival to hospital discharge and — when compared with matched controls — was associated with higher rates of ICU admission, ROSC, and 24-h survival. After adjustment by pretreatment covariates (listed above), comparison with concurrent controls reduced the OR but retained statistical significance for ICU admission and ROSC, whereas comparison with matched controls increased the OR, demonstrating statistical significance for all four outcomes.
To assess whether the beneficial effects on resuscitation outcomes could have been linked to beneficial effects on LV myocardial distensibility — as suggested by our preceding study in rats [39] — we examined the effects on PETCO2. As discussed earlier, PETCO2 is a good surrogate measurement of forward blood flow during chest compression [4, 23–25]. In the study, rescuers were trained and retrained to provide consistent compression depth and rate, and the PETCO2 values in both control groups were already indicative of highquality chest compression. If, as hypothesised, erythropoietin preserved myocardial distensibility, for a given compression depth, one would expect higher forward blood flow in the presence of erythropoietin and therefore higher PETCO2. This was indeed the case. Patients who received erythropoietin had significantly higher PETCO2 during chest compression.
4 Conclusions
These preclinical and clinical observations suggest that myocardial injury can be attenuated during resuscitation from cardiac arrest leading to functional benefits that enable haemodynamically more effective chest compression and improved postresuscitation myocardial function. Future effort should focus on the translation of these concepts through additional clinical trials that could not only support these findings but also quantitate their treatment effects paving the way for ultimately clinical implementation.
References
Binak K, Harmanci N, Sirmaci N (1967) Oxygen extraction rate of the myocardium at rest and on exercise in various conditions. Br Heart J 29: 422–427
Yusa T, Obara S (1981) Myocardial oxygen extraction rate under general anesthesia. Tohoku J Exp Med 133: 321–324
Hoffman JIE (1984) Maximal coronary flow and the concept of coronary vascular reserve. Circulation 70: 153–159
Duggal C, Weil MH, Gazmuri RJ et al (1993) Regional blood flow during closed chest cardiac resuscitation in rats. J Appl Physiol 74: 147–152
Ditchey RV, Goto Y, Lindenfeld J (1992) Myocardial oxygen requirements during experimental cardiopulmonary resuscitation. Cardiovasc Res 26: 791–797
Gazmuri RJ, Berkowitz M, Cajigas H (1999) Myocardial effects of ventricular fibrillation in the isolated rat heart. Crit Care Med 27: 1542–1550
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (2006) Calcium in cell injury and death. Annu Rev Pathol 1: 405–434
Halestrap AP (2006) Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 34: 232–237
Weisfeldt ML, Zweier J, Ambrosio G et al (1988) Evidence that free radicals result in reperfusion injury in heart muscle. Basic Life Sci 49: 911–919
Ayoub IM, Kolarova J, Kantola R et al (2007) Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med 35: 2329–2336
Klouche K, Weil MH, Sun S et al (2002) Evolution of the stone heart after prolonged cardiac arrest. Chest 122: 1006–1011
Ayoub IM, Kolarova JD, Sehgal MA et al (2003) Sodium-hydrogen exchange inhibition minimizes adverse effects of epinephrine during cardiac resuscitation. Circulation 108: IV–420 (abstract)
Cooley DA, Reul GJ, Wukasch DC (1972) Ischemic contracture of the heart: “stone heart”. Am J Cardiol 29: 575–577
Katz AM, Tada M (1972) The “stone heart”: A challenge to the biochemist. Am J Cardiol 29: 578–580
Sorrell VL, Altbach MI, Kern KB et al (2005) Images in cardiovascular medicine. Continuous cardiac magnetic resonance imaging during untreated ventricular fibrillation. Circulation 111: e294
Koretsune Y, Marban E (1990) Mechanism of ischemic contracture in ferret hearts: relative roles of [Ca2+]i elevation and ATP depletion. Am J Physiol 258: H9–H16
Ayoub IM, Kolarova JD, Yi Z et al (2003) Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation 107: 1804–1809
Gazmuri RJ (2000) Effects of repetitive electrical shocks on postresuscitation myocardial function. Crit Care Med 28: N228–N232
Gazmuri RJ, Deshmukh S, Shah PR (2000) Myocardial effects of repeated electrical defibrillations in the isolated fibrillating rat heart. Crit Care Med 28: 2690–2696
Starling EH, Visscher MB. The regulation of the energy output of the heart. J Physiol 1927; 62: 243–261.
Kolarova JD, Ayoub IM, Gazmuri RJ (2005) Cariporide enables hemodynamically more effective chest compression by leftward shift of its flow-depth relationship. Am J Physiol Heart Circ Physiol 288:H2904–2911
Takino M, Okada Y (1996) Firm myocardium in cardiopulmonary resuscitation. Resuscitation 33: 101–106
Sanders AB, Atlas M, Ewy GA et al (1985) Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med 3: 147–149
Gudipati CV, Weil MH, Bisera J et al (1988) Expired carbon dioxide: A noninvasive monitor of cardiopulmonary resuscitation. Circulation 77: 234–239
Rubertsson S, Karlsten R (2005) Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation 65: 357–363
Valenzuela TD, Roe DJ, Nichol G et al (2000) Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med 343: 1206–1209
van Alem AP, Post J, Koster RW (2003) VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation 59: 181–188
Gazmuri RJ, Weil MH, Bisera J et al (1996) Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med 24: 992–1000
Kern KB, Hilwig RW, Rhee KH, Berg RA (1996) Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol 28: 232–240
Laurent I, Monchi M, Chiche JD et al (2002) Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 40: 2110–2116
Ruiz-Bailen M, Aguayo dH, Ruiz-Navarro S et al (2005) Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 66: 175–181
Xu T, Tang W, Ristagno G et al (2008) Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med 36: 188–192
Hilwig RW, Berg RA, Kern KB, Ewy GA (2000) Endothelin-1 vasoconstriction during swine cardiopulmonary resuscitation improves coronary perfusion pressures but worsens postresuscitation outcome. Circulation 101: 2097–2102
Gazmuri RJ, Hoffner E, Kalcheim J et al (2001) Myocardial protection during ventricular fibrillation by reduction of proton-driven sarcolemmal sodium influx. J Lab Clin Med 137: 43–55
Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD (2001) Successful ventricular de-fibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation 104: 234–239
Gazmuri RJ, Ayoub IM, Kolarova JD, Karmazyn M (2002) Myocardial protection during ventricular fibrillation by inhibition of the sodium-hydrogen exchanger isoform-1. Crit Care Med 30: S166–S171
Kolarova J, Yi Z, Ayoub IM, Gazmuri RJ (2005) Cariporide potentiates the effects of epinephrine and vasopressin by nonvascular mechanisms during closed-chest resuscitation. Chest 127: 1327–1334
Wang S, Radhakrishnan J, Ayoub IM et al (2007) Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol 103: 55–65
Singh D, Kolarova JD, Wang S et al (2007) Myocardial protection by erythropoietin during resuscitation from ventricular fibrillation. Am J Ther 14: 361–368
Grmec S, Strnad M, Kupnik D et al (2009) Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation 80: 631–637
von Planta M, Weil MH, Gazmuri RJ et al (1989) Myocardial acidosis associated with CO2 production during cardiac arrest and resuscitation. Circulation 80: 684–692
Kette F, Weil MH, Gazmuri RJ et al (1993) Intramyocardial hypercarbic acidosis during cardiac arrest and resuscitation. Crit Care Med 21: 901–906
Noc M, Weil MH, Gazmuri RJ et al (1994) Ventricular fibrillation voltage as a monitor of the effectiveness of cardiopulmonary resuscitation. J Lab Clin Med 124: 421–426
Karmazyn M, Sawyer M, Fliegel L (2005) The na(+)/h(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord 5: 323–335
Imahashi K, Kusuoka H, Hashimoto K et al (1999) Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res 84: 1401–1406
Avkiran M, Ibuki C, Shimada Y, Haddock PS (1996) Effects of acidic reperfusion on arrhythmias and Na(+)-K(+)-ATPase activity in regionally ischemic rat hearts. Am J Physiol 270: H957–H964
An J, Varadarajan SG, Camara A et al (2001) Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol 281: H2398–H2409
Gunter TE, Buntinas L, Sparagna G et al (2000) Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28: 285–296
Yamamoto S, Matsui K, Ohashi N (2002) Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol 39: 569–575
Borutaite V, Brown GC (2003) Mitochondria in apoptosis of ischemic heart. FEBS Lett 541: 1–5
Nasser FN, Walls JT, Edwards WD, Harrison CE, Jr (1980) Lidocaine-induced reduction in size of experimental myocardial infarction. Am J Cardiol 46: 967–975
Hinokiyama K, Hatori N, Ochi M et al (2003) Myocardial protective effect of lidocaine during experimental off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 9: 36–42
Gazmuri RJ, Ayoub IM (2003) Myocardial effects of sodium-hydrogen exchange inhibition during resuscitation from ventricular fibrillation. In: Dhallas NS, Takeda N, Singh M, Lukas A (eds) Myocardial ischemia and preconditioning. Kluwer Academic, Boston, pp. 375–388
Gazmuri RJ, Ayoub IM, Kolarova J (2003) Myocardial protection during resuscitation from cardiac arrest. Curr Opin Crit Care 9: 199–204
Gazmuri RJ, Ayoub IM (2006) The case for sodium-hydrogen exchanger isoform-1 inhibition during cardiac resuscitation remains strong. Crit Care Med 34: 1580–1582
Ayoub IM, Radhakrishnan J, Gazmuri RJ (2008) Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med 36: S440–S446
Radhakrishnan J, Ayoub IM, Gazmuri RJ (2009) Activation of caspase-3 may not contribute to postresuscitation myocardial dysfunction. Am J Physiol Heart Circ Physiol 296: H1164–H1174
Cai Z, Manalo DJ, Wei G et al (2003) Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85
Parsa CJ, Matsumoto A, Kim J et al (2003) A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 112: 999–1007
Tramontano AF, Muniyappa R, Black AD et al (2003) Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun 308: 990–994
Parsa CJ, Kim J, Riel RU et al (2004) Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem 279: 20655–20662
Wright GL, Hanlon P, Amin K et al (2004) Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J 18: 1031–1033
Namiuchi S, Kagaya Y, Ohta J et al (2005) High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol 45: 1406–1412
Brines ML, Ghezzi P, Keenan S et al (2000) Erythropoietin crosses the bloodbrain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97: 10526–10531
Ghezzi P, Brines M (2004) Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ 11: S37–S44
Celik M, Gokmen N, Erbayraktar S et al (2002) Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99: 2258–2263
Junk AK, Mammis A, Savitz SI et al (2002) Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA 99: 10659–10664
Vesey DA, Cheung C, Pat B et al (2004) Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant 19: 348–355
Abdelrahman M, Sharples EJ, McDonald MC et al (2004) Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock 22: 63–69
Buemi M, Vaccaro M, Sturiale A et al (2002) Recombinant human erythropoietin influences revascularization and healing in a rat model of random ischaemic flaps. Acta Derm Venereol 82: 411–417
Rui T, Feng Q, Lei M et al (2005) Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res 65: 719–727
Li Y, Takemura G, Okada H et al (2006) Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardio- vasc Res 71: 684–694
van der Meer P, Lipsic E, Henning RH et al (2005) Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol 46: 125–133
Hirata A, Minamino T, Asanuma H et al (2006) Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol 48: 176–184
2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 112(Suppl I):IV-1–IV-5
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Italia
About this paper
Cite this paper
Gazmuri, R.J., Ayoub, I.M., Radhakrishnan, J. (2011). Physiopathology and Severity of Postresuscitation Myocardial Dysfunction: Effects of Sodium-Hydrogen Exchanger Isoform-1 (NHE-1) Inhibitors and Erythropoietin. In: Gullo, A. (eds) Anaesthesia, Pharmacology, Intensive Care and Emergency Medicine A.P.I.C.E.. Springer, Milano. https://doi.org/10.1007/978-88-470-2014-6_14
Download citation
DOI: https://doi.org/10.1007/978-88-470-2014-6_14
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-2013-9
Online ISBN: 978-88-470-2014-6
eBook Packages: MedicineMedicine (R0)