Abstract
Background: Elevated pulmonary arterial pressure may increase right ventricular (RV) oxidative metabolism in patients with pulmonary hypertension (PH) and heart failure (HF). 11C- acetate positron emission tomography (PET) can be used to measure RV oxidative metabolism noninvasively. The combination of RV oxidative metabolism and cardiac work, as estimated by cardiac magnetic resonance imaging (CMR), indicates RV efficiency and can provide new insights. However, the clinical importance of these markers has not been fully studied. The purpose of this study was to investigate the possible impacts of these markers in patients with PH through 11C-acetate PET.
Methods: Ventricular function was assessed using magnetic resonance imaging (MRI). Dynamic 11C-acetate PET was used to simultaneously measure RV and left ventricular (LV) oxidative metabolism (kmono). The RV myocardial work per oxygen consumption index was calculated as follows: [RV stroke volume index (SVI)/RV kmono]. PH patients who had additional or increased doses of PH-specific vasodilator(s), based on the joint guidelines of the European Society of Cardiology (ESC) and European Respiratory Society (ERS) for the diagnosis and management of PH, were compared with five PH patients who had no treatment modification (control group).
Results: PH patients showed higher RV kmono than did controls (P < 0.05). RV oxidative metabolism was correlated with the mean pulmonary arterial pressure (mPAP) (r = 0.44, P = 0.021), pulmonary vascular resistance (PVR) (R = 0.56, P = 0.002), and brain natriuretic peptide (BNP) (R = 0.42, P = 0.029). Members of the PH therapy group had reduced RV oxidative metabolism (P = 0.002) and improved RV work/oxygen consumption index (P < 0.001).
Conclusions: RV oxidative metabolism increased as the correlation between mPAP and PVR increased. PH-specific treatments reduced RV oxidative metabolism. Therefore, RV metabolism and its efficiency can provide new pathophysiological insights and will be a new therapeutic marker in patients with PH.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The right ventricle (RV) is the anterior cardiac chamber located just behind the sternum. The RV receives systemic venous return and pumps it into the pulmonary arteries [1]. Under normal conditions, the RV requires little energy to pump venous blood to the pulmonary arteries since pulmonary circulation has a much lower vascular resistance than does systemic circulation [2]. The RV therefore usually has low oxygen consumption.
Until recently, most cardiology research was concerned with left ventricle (LV) physiologies and pathologies. In fact, in the early twentieth century, some groups hypothesized that human circulation could be maintained without RV function [3]. However, cardiovascular surgeons recognized the importance of RV function, and RV dysfunction appeared to be associated with major cardiovascular events in heart failure (HF) [4]. In addition, development of state-of-the-art cardiovascular imaging modalities such as echocardiography, cardiac magnetic resonance (CMR) imaging, and positron emission tomography (PET) have made it possible to noninvasively evaluate the functional and physiological characteristics of RV myocardium [5, 6]. Based on this background, research on the RV has progressed intensively in recent years.
One of the major causes of RV dysfunction is pulmonary hypertension (PH). PH is categorized into five groups as follows: Group 1, pulmonary artery hypertension (PAH); Group 2, PH with LV disease; Group 3, PH associated with lung disease and/or hypoxia; Group 4, PH due to chronic thrombosis and/or embolism; and Group 5, miscellaneous [7]. Elevated pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) may induce RV dysfunction in patients with PH [7]. However, the RV has a high capacity to adapt to pressure or volume overload before failing in its function [8]. Before myocardial fibrosis and RV heart failure develop, moderate to severe PH often leads to initial RV adaptation [9]. Given the increased arterial pressure in pulmonary circulation, the RV may require a significant power output in response to increased afterload, and this may increase the demand for oxygen in the RV [10]. In this circumstance, the RV may require improved mechanical efficiency in order to increase the power output. A study by Wong et al. showed that idiopathic PH with RV dysfunction was associated with increased RV myocardial oxygen consumption (MVO2) and reduced RV efficiency [11], a situation possibly mirroring the case of LV energetics in heart failure [12–14]. However, the study by Wong et al. lacked control subjects [11]. Yoshinaga et al. evaluated the RV oxidative metabolism in patients with PH and showed that patients with PH had increased oxidative metabolism in comparison with that of normal individuals [15]. Increasing RV oxidative metabolism was associated with several prognostic markers such as mean PAP (mPAP), PVR, and brain natriuretic peptide (BNP). These findings suggest that elevated RV oxidative metabolism may be a prognostic marker.
11C-acetate PET is a noninvasive technique for measuring regional and global myocardial oxidative metabolism [13, 16–19], which is correlated with tricarboxylic acid cycle flux and MVO2 [16]. RV myocardial oxidative metabolism can also be measured noninvasively using 11C-acetate PET [15, 17, 20–22]. HF therapies such as beta-blockers that reduce MVO2 are considered to improve the survival of patients with HF [23]. LV cardiac efficiency can be estimated noninvasively [12] by combining the mechanical work with the oxidative metabolism as measured by 11C-acetate PET. This form of LV efficiency measurement has been applied to evaluate several new HF treatments including beta-blockers, cardiac resynchronized therapy, surgical reconstruction therapy, and obstructive sleep apnea (OSA) syndrome treatments [13, 14, 24–26].
PH-specific vasodilators such as endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostacyclin decrease pulmonary arterial pressure [7, 27]. These new vasodilators indeed improve survival in PH patients. Oikawa et al. reported that reducing mPAP through the use of epoprostenol was associated with reduced RV glucose metabolism observed by 18F-fluorodeoxyglucose (FDG) PET [28]. However, it is not clear whether such a treatment favorably affects RV myocardial oxidative metabolism.
The purpose of this study was to investigate the possible impacts of the intensified PH-specific vasodilator therapy on myocardial energetics using 11C-acetate PET.
2 Materials and Methods
2.1 Study Subjects
Study subjects were recruited from the first department of medicine at the Hokkaido University Hospital. The patient inclusion criteria were as follows: (1) a resting mPAP ≥ 25 mmHg and a pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg [7], (2) symptoms of PH in accordance with World Health Organization (WHO) functional class II or III, (3) PH subtypes chronic thromboembolic pulmonary hypertension (CTEPH) or PAH [29], and (4) stable condition (unchanged for > 4 weeks).
Exclusion criteria were secondary PH due to LV disease, unstable condition of patients, and permanent pacemaker implantation.
The study was approved by the Hokkaido University Graduate School of Medicine Human Research Ethics Board. Written informed consent was obtained from all patients.
2.2 Experimental Protocol
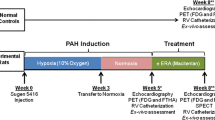
14 PH patients underwent right heart catheterization. Precapillary PH was diagnosed based on a mPAP ≥ 25 mmHg at rest and a PCWP ≤ 15 mmHg [7]. Among the 14 PH patients, nine patients started or added the PH-specific vasodilators based on the combined guidelines of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), and these nine patients were categorized as the intensified treatment group [30]. The remaining five patients did not change their treatments and served as a control group.
Within 7 days of right heart catheterization, all patients underwent blood sampling, CMR, and 11C-acetate PET at rest on the same day [15]. CMR and 11C-acetate PET were repeated after 10.7 ± 6.5 months for the intensified treatment group (Fig. 15.1). Patients with PH but without OSA served as the control group and underwent CMR and 11C-acetate PET at rest at baseline and after 10.6 ± 6.3 months (P = NS).
WHO functional class evaluation and blood tests including BNP were measured at baseline and follow-up.
2.3 Right Heart Catheterization
A complete hemodynamic evaluation was performed in all PH patients using the standard technique for right heart catheterization. The hemodynamic measurements included mean right atrial pressure, mPAP, and mean PCWP. Cardiac output was determined through thermodilution. PVR was calculated as (mPAP – mean PCWP) divided by cardiac output.
2.4 Measurements of BNP
Blood samples were obtained for fasting conditions the morning before the PET studies. BNP data were obtained for all patients. Plasma BNP was measured using immunoradiometric assay [31].
2.5 Cardiac Magnetic Resonance Imaging
CMR imaging studies were performed using a 1.5-T Achieva magnetic resonance imaging system (Philips Medical Systems, Best, the Netherlands) equipped with master gradients (maximum gradient amplitude 33 mT/m and maximum slew rate 100 mT/m/msec). CMR data acquisition was performed on patients in the supine position with breath-holding in expiration. We used a five-element cardiac phased-array coil and a vector cardiographic method for electrocardiogram (ECG)-gating images. We performed localizing scans with breath-hold cine imaging and axial orientation. Coronal images were used for cardiac anatomy evaluation. CMR images spanned from bronchial bifurcation to diaphragm level thus allowing for whole-heart analysis. We obtained 12 short-axis cine slices during a steady-state free precession pulse sequence. The precession pulse sequence was as follows: repetition time = 2.8 msec, echo time = 1.4 msec, flip angle = 60, acquisition matrix = 192 × 256, field of view = 380 mm, slice thickness = 10 mm, inter-slice gap = 0 mm, and phases/cardiac cycle = 20 [32].
2.6 CMR Data Analysis
CMR images were analyzed using commercially available software (Extended MR Work Space: version 2.6.3, Philips Medical Systems, Amsterdam, the Netherlands). RV and LV volumes were measured using cine long-axis images. RV and LV endocardial borders were semiautomatically traced. We then calculated the RV ejection fraction (EF), LVEF, and RV volumes using this information [15]. RVEF was measured in transverse (axial) orientation. LVEF was measured in short-axial orientation [33]. All CMR images were analyzed by clinicians blinded to the clinical and imaging data.
2.7 Positron Emission Tomography
All patients were instructed to fast overnight. PH patients who had vasodilator treatments took their morning medications before the PET and CMR studies. Patients were positioned with the heart centered in the field of view in a whole-body PET scanner (ECAT HR+, Siemens/CTI Knoxville, TN) [34]. Dynamic PET acquisition was initiated (10 × 10 s (s); 2 × 30s; 5 × 100 s; 3 × 180 s; 2 × 300 s) [14] followed by intravenous administration of 20 mCi (mCi) [740 megabecquerel (MBq)] 11C-acetate.
2.8 11C-Acetate PET Data Analysis
The reconstructed dynamic PET images were analyzed by applying a region of interest over the whole LV and free wall of the RV myocardium in 3–5 midventricular transaxial planes [15, 20, 26]. A mono-exponential function was fit to the myocardial time-activity data. The clearance rate constant (kmono) was determined as described previously [13, 14, 34]. The mono-exponential fit began at the point when the blood pool was stable (usually 2–4 min after injection) (Fig. 15.2).
Example of left and right myocardial time-activity data from a 11C-acetate PET acquisition. A mono-exponential function fit to the myocardial clearance yields a clearance rate constant; kmono represents the rate of oxidative metabolism and reflects MVO2. Blue line represents left ventricular (LV) myocardium time-activity curve. Orange line represents right ventricular (RV) myocardium time-activity curve. Red line indicates blood time-activity curve
All data were analyzed by clinicians blinded to the clinical and imaging data.
2.9 RV Work Per MVO2 Index Calculation
The 11C-acetate clearance data were combined with the stroke work data to derive myocardial efficiency using the LV work metabolic index (WMI) using the following equation [13, 14, 26].
WMI = SVI × SBP × HR/kmono; where SVI is the stroke volume index determined by CMR, SBP is systolic blood pressure, HR is heart rate, and kmono is the mono-exponential rate constant for 11C-acetate clearance from the myocardium.
As a modified LV work metabolic index equation, the RV work per MVO2 index was calculated as the ratio of RV SVI measured by CMR divided by RV MVO2. The equation was as follows [35]:
2.10 Statistical Analysis
Continuous variables (using a logarithmic transformation on skewed distributions as appropriate) are presented as mean and standard deviations. Categorical variables are presented as frequencies with percentages. Differences in results for the PH group and the control group were examined for statistical significance with a two-sample t-test for continuous variables. A P value of less than 0.05 was considered indicative of a statistically significant difference. Statistical calculations were carried out using SAS software version 9.2 (SAS Institute, Inc., Cary, NC).
3 Results
3.1 Patient Characteristics
A total of 14 WHO functional class II or III PH patients were prospectively enrolled in the study between December 2009 and July 2011, and all PH patients completed the study protocol.
The baseline characteristics of the participants are shown in Table 15.1. There was no significant difference in mean age between members of the intensified PH treatment patient group and those of the control group.
3.2 Right Heart Catheterization
The baseline right heart catheterization data is shown in Table 15.1. There was no significant difference in the systolic PAP, diastolic PAP, mPAP, and PVR of the intensified PH treatment group and those of the control group.
3.3 LV and RV Systolic Function
The RVEF and LVEF were similar in both the intensified PH treatment group and the control group (Table 15.2).
3.4 LV and RV Oxidative Metabolism
There was no statistically significant difference between the LV oxidative metabolism, kmono, of the intensified PH treatment group and that of the controls (P = 0.72). There was also no statistically significant difference between the RV oxidative metabolism, kmono, of the intensified PH treatment group and that of the controls (P = 0.18) (Table 15.2). In addition, the RV SVI per RV kmono was similar in both the intensified PH treatment group and the control group (P = 0.31).
3.5 Longer-Term Effects of Intensified PH-Specific Therapy on RV Metabolism and Work per Oxygen Consumption
The intensified PH therapy group tended to have reduced PVR compared with that of the control group (−20.6 ± 41.7 vs. −3.4 ± 33.2 %, P = 0.29). Intensified PH therapy tended to reduce BNP (377.2 ± 694.5 to 70.8 ± 132.6 mol/mL, P = 0.18).
Intensified PH therapy significantly reduced RV oxidative metabolism (0.044 ± 0.008 to 0.039 ± 0.007/min, P = 0.002) while increasing SVI (31.4 ± 10.1 to 39.3 ± 9.6 mL/m2, P = 0.017) (Fig. 15.3). As a result, the intensified PH-specific therapy significantly improved the RV work/oxygen consumption index (730.8 ± 253.6 to 1038.5 ± 262.7 mL/min + m2, P < 0.001) (Fig. 15.4). There was no significant change in LV kmono in this group (0.052 ± 0.008 to 0.050 ± 0.006/min, P = 0.35). The control group showed no changes in these parameters (RV kmono: 0.041 ± 0.006 to 0.049 ± 0.01/min, P = 0.15, RV work/oxygen consumption index: 829.1 ± 362.9 to 721.5 ± 241.2 mL/min + m2, P = 0.69).
Effects of intensified PH-specific vasodilator therapy on RV work per oxygen consumption index. Patients who had intensified PH-specific vasodilator therapy had a significantly improved RV work per oxygen consumption index. In contrast, there was no significant difference in RV work per oxygen consumption index in control group. PH pulmonary hypertension, RV right ventricle, TX therapy
4 Discussion
Intensified PH-specific vasodilator therapy tended to reduce PVR and BNP. The intensive PH-specific therapy led to reduction in right ventricular oxidative metabolism and a significant improvement in right ventricular work per oxygen consumption index in patients with pulmonary hypertension.
4.1 Increased RV Oxidative Metabolism in Patients with PH
The major determinants of myocardial metabolism are heart rate, contractility, and wall stress [36, 37]. Oxygen demand in the RV may also increase with elevated PAP [10].
In LV, heart failure is considered to be an energy-depleted state. Improved survival through the use of beta-blockers and vasodilator therapy is in part associated with the energy-sparing effects of these medications [38].
RV MVO2 was originally measured through right coronary venous blood sampling [10]. As noninvasive approaches, 11C-acetate and 15O-labeled tracers PET have also been applied for RV oxidative measurements in HF and PH [15, 17, 22, 26, 39, 40]. The current data revealed increased RV oxidative metabolism in patients with PH and reduced RV contraction. The current data agreed with findings of our previous study and the study by Wong et al. [15, 39].
4.2 Study Population and Effects of PH-Specific Vasodilator Therapy
In the current study, eight PH patients did not have PH-specific vasodilator therapy. One patient had a PH-specific vasodilator, but the PH control did not reach the standard control level. Therefore, these nine patients either started or added the PH-specific vasodilator. The remaining five patients already received PH-specific vasodilator treatments and they were stable. These five patients did not meet the criteria to add further vasodilators to control their PH based on ESC and ERS guidelines [30].
The intensified PH therapy group tended to have reduced mPAP. Although the mPAP did not reach statistically significant levels, this finding may be due to the small sample size, but it may still indicate that intensified PH-specific therapy had beneficial effects. In fact, BNP also tended to be reduced after the therapy, a finding that may indicate a secondary reduction in wall stress due to reduced mPAP. It may therefore be appropriate to evaluate the therapeutic effects on RV energetics in this study population.
4.3 Effects of PH-Specific Vasodilator Therapy on RV Oxidative Metabolism and Work per MVO2 Index
In the previous study, we evaluated RV power and RV efficiency using the following equation: RV efficiency = HR × mPAP × SV × 1.33 × 10−4/RV kmono × RV mass × 20 [35]. Using this equation, WHO functional classification II and III PH patients actually had increased RV power and RV efficiency compared to patients in the control group [15]. The increasing power and RV efficiency may be adaptive processes associated with increasing mPAP. In the LV, the LV efficiency is estimated using the WMI. The WMI was calculated as follows: WMI = SVI × SBP × HR/kmono [13]. LVHF treatments usually do not significantly change the HR and SBP [14]. In contrast, PH-specific vasodilators significantly reduce the mPAP [27]. Therefore, if the equation proposed by Sun et al. is simply applied to RV efficiency measurements [35], RV efficiency may be reduced after PH-specific vasodilator therapy since the reduction of mPAP would play a significant role over the increasing SVI. The afterload of pulmonary circulation is usually lower than that of systemic circulation [1, 41]. Therefore, the RV may have volume efficiency rather than pressure efficiency as would apply in the case of the LV. Therefore, we applied SVI per MVO2 index instead of Sun’s equation in the current study.
The SVI per MVO2 index shows that the intensified PH-specific vasodilator therapy significantly improved this parameter in comparison with that of controls. The trend of mPAP reduction may contribute to these improvements. Therefore, improving volume efficiency in the RV may be one of the therapeutic effects of PH-specific vasodilators, and doing so may contribute to the improvement of outcomes in patients with PH. This hypothesis should be tested in a larger study population in the future.
4.4 Limitations
The thickness of the RV free wall is usually 4–5 mm, which is thinner than the LV free wall. However, the current study population had thicker RV walls. When radiotracer uptake is measured, the partial volume effect may significantly attenuate the data. However, we analyzed the oxidative metabolism using mono-exponential fitting. This approach measures clearance of the radiotracer from the myocardium. Thus, the partial volume effect should be minimal.
5 Conclusion
Intensified PH-specific vasodilator therapy led to a reduction in right ventricular oxidative metabolism and a significant improvement in right ventricular work per oxygen consumption index in patients with pulmonary hypertension. These effects may contribute to the benefits of intensified PH-specific vasodilator therapy.
References
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. doi:10.1161/CIRCULATIONAHA.107.653576.
Dell’Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol. 1991;16:653–720.
Lee FA. Hemodynamics of the right ventricle in normal and disease states. Cardiol Clin. 1992;10:59–67.
Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–8. doi:10.1161/CIRCULATIONAHA.109.887570.
Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi:10.1161/CIRCULATIONAHA.106.674028.
Valsangiacomo Buechel ER, Mertens LL. Imaging the right heart: the use of integrated multimodality imaging. Eur Heart J. 2012;33:949–60. doi:10.1093/eurheartj/ehr490.
Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–38.
Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–31. doi:10.1161/CIRCULATIONAHA.107.653584.
Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–8.
Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/supply balance in the right ventricle. Exp Biol Med (Maywood). 2005;230:507–19.
Wong YY, Ruiter G, Lubberink M, Raijmakers PG, Knaapen P, Marcus JT, et al. Right ventricular failure in idiopathic pulmonary arterial hypertension is associated with inefficient myocardial oxygen utilization. Circ Heart Fail. 2011;4:700–6. doi:10.1161/CIRCHEARTFAILURE.111.962381.
Ukkonen H, Beanlands R. Oxidative metabolism and cardiac efficiency. In: Wahl R, editor. Principles and practice of PET and PET/CT. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 589–606.
Beanlands RS. Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, et al. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: A double-blind, placebo-controlled, positron-emission tomography study. Circulation. 2000;102:2070–5.
Yoshinaga K, Burwash IG, Leech JA, Haddad H, Johnson CB. deKemp RA, et al. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol. 2007;49:450–8.
Yoshinaga K, Ohira H, Tsujino I, Oyama-Manabe N, Mielniczuk L, Beanlands RS, et al. Attenuated right ventricular energetics evaluated using (1)(1)C-acetate PET in patients with pulmonary hypertension. Eur J Nucl Med Mol Imaging. 2014;41:1240–50. doi:10.1007/s00259-014-2736-4.
Armbrecht JJ, Buxton DB, Brunken RC, Phelps ME, Schelbert HR. Regional myocardial oxygen consumption determined noninvasively in humans with [1-11C]acetate and dynamic positron tomography. Circulation. 1989;80:863–72.
Stolen KQ, Kemppainen J, Ukkonen H, Kalliokoski KK, Luotolahti M, Lehikoinen P, et al. Exercise training improves biventricular oxidative metabolism and left ventricular efficiency in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:460–7.
Yoshinaga K, Chow BJ, Dekemp RA, Thorn S, Ruddy TD, Davies RA, et al. Application of cardiac molecular imaging using positron emission tomography in evaluation of drug and therapeutics for cardiovascular disorders. Curr Pharm Des. 2005;11:903–32.
Yoshinaga K, Tamaki N. Imaging myocardial metabolism. Curr Opin Biotechnol. 2007;18:52–9. doi:10.1016/j.copbio.2006.11.003.
Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000;68:522–31.
Wong YY, Raijmakers P, van Campen J, van der Laarse WJ, Knaapen P, Lubberink M, et al. 11C-Acetate clearance as an index of oxygen consumption of the right myocardium in idiopathic pulmonary arterial hypertension: a validation study using 15O-labeled tracers and PET. J Nucl Med. 2013;54:1258–62. doi:10.2967/jnumed.112.115915.
Ohira H, Beanlands RS, Davies RA, Mielniczuk L. The role of nuclear imaging in pulmonary hypertension. J Nucl Cardiol. 2015;22:141–57. doi:10.1007/s12350-014-9960-y.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi:10.1056/NEJM199605233342101.
Chiba S, Naya M, Iwano H, Yoshinaga K, Katoh C, Manabe O, et al. Interrelation between myocardial oxidative metabolism and diastolic function in patients undergoing surgical ventricular reconstruction. Eur J Nucl Med Mol Imaging. 2013;40:349–55. doi:10.1007/s00259-012-2297-3.
Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130:892–901. doi:10.1161/CIRCULATIONAHA.113.005893.
Ukkonen H, Beanlands RS, Burwash IG, de Kemp RA, Nahmias C, Fallen E, et al. Effect of cardiac resynchronization on myocardial efficiency and regional oxidative metabolism. Circulation. 2003;107:28–31.
Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. doi:10.1016/j.jacc.2013.10.031.
Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–55.
Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi:10.1016/j.jacc.2009.04.012.
Task Force for D, Treatment of Pulmonary Hypertension of European Society of C, European Respiratory S, International Society of H, Lung T, Galie N, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi:10.1183/09031936.00139009.
Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–70.
Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. doi:10.1002/jmri.10329.
Oyama-Manabe N, Sato T, Tsujino I, Kudo K, Manabe O, Kato F, et al. The strain-encoded (SENC) MR imaging for detection of global right ventricular dysfunction in pulmonary hypertension. Int J Cardiovasc Imaging. 2013;29:371–8. doi:10.1007/s10554-012-0105-6.
Yoshinaga K, Katoh C, Beanlands RS, Noriyasu K, Komuro K, Yamada S, et al. Reduced oxidative metabolic response in dysfunctional myocardium with preserved glucose metabolism but with impaired contractile reserve. J Nucl Med. 2004;45:1885–91.
Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, et al. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39:272–80.
Braunwald E. Control of myocardial oxygen consumption: physiologic and clinical considerations. Am J Cardiol. 1971;27:416–32.
Laine H, Katoh C, Luotolahti M, Yki-Jarvinen H, Kantola I, Jula A, et al. Myocardial oxygen consumption is unchanged but efficiency is reduced in patients with essential hypertension and left ventricular hypertrophy. Circulation. 1999;100:2425–30.
Katz AM. Potential deleterious effects of inotropic agents in the therapy of chronic heart failure. Circulation. 1986;73:III184–90.
Wong YY, Westerhof N, Ruiter G, Lubberink M, Raijmakers P, Knaapen P, et al. Systolic pulmonary artery pressure and heart rate are main determinants of oxygen consumption in the right ventricular myocardium of patients with idiopathic pulmonary arterial hypertension. Eur J Heart Fail. 2011;13:1290–5. doi:10.1093/eurjhf/hfr140.
Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, et al. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–27. doi:10.1161/CIRCULATIONAHA.106.660639.
Sheehan F, Redington A. The right ventricle: anatomy, physiology and clinical imaging. Heart. 2008;94:1510–5. doi:10.1136/hrt.2007.132779.
Acknowledgments
The authors thank Keiichi Magota, PhD; Ken-ichi Nishijima, PhD; Daisuke Abo, MSc; and Eriko Suzuki for their support for this study. This manuscript has been reviewed by a North American English-language professional editor, Ms. Holly Beanlands. The authors also thank Ms. Holly Beanlands for critical reading of the manuscript.
This study was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Category B, No. 23390294), Hokkaido Heart Association (H-20) (Sapporo, Japan), Adult Vascular Disease Research Foundation (#H22-23) (Kyoto, Japan), North-Tech Research Foundation (#H23-S2-17, Sapporo, Japan), and grants from the Innovation Program of the Japan Science and Technology Agency. Dr. Yoshinaga is supported by the Imura Clinical Research Award (Adult Vascular Disease Research Foundation).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Conflicts of Interest
None
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/) which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this paper
Cite this paper
Yoshinaga, K. et al. (2016). Right Ventricular Metabolism and Its Efficiency. In: Kuge, Y., Shiga, T., Tamaki, N. (eds) Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55894-1_15
Download citation
DOI: https://doi.org/10.1007/978-4-431-55894-1_15
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55892-7
Online ISBN: 978-4-431-55894-1
eBook Packages: MedicineMedicine (R0)