Abstract
On April 1, 2012, new Standard Limits for radionuclide concentrations in food were promulgated, superseding the Provisional Regulation Values in Japan set in 2011. The new Standard Limits are calculated based on 1 mSv y−1 of annual internal radiation dose through food ingestion of 134Cs, 137Cs, 90Sr, Pu and 106Ru, which were detected or possibly released into the environment from the accident at the TEPCO Fukushima Daiichi Nuclear Power Stations (FDNPS). The concentrations of the radionuclides were based on the values of radiocesium (134, 137Cs) and of the other radionuclides (90Sr, Pu and 106Ru); the ratio observed in the determination or predicted concentrations in the soils from the FDNPS accident was used for estimating the concentration of the other radionuclides by means of the ratio against 137Cs. The new Standard Limit of radiocesium in general foods was defined to be 100 Bq kg−1 fresh weight by the Ministry of Health, Labour and Welfare. In the present study the concentration of radiocesium was measured in agricultural products collected mostly in Fukushima-shi and Date-shi, Fukushima Prefecture, in 2012 and 2013. The average concentration of radiocesium in agricultural plants in 2012 was 7.6 (<0.2–40) Bq kg−1 fresh weight, decreasing to 2.0 (<0.1–14) Bq kg−1 fresh weight in 2013, which was approximately one-fourth of the concentration in 2012. The concentration of 90Sr in agricultural products collected in Fukushima Prefecture in 2013 was 0.0047–0.31 Bq kg−1 fresh weight, which was a similar range to those collected throughout Japan. The concentration ratio of 90Sr/137Cs in the agricultural plants collected from the area 5 km west from the Nuclear Power Stations (difficult-to-return zone) was lower than the predicted 90Sr/137Cs ratio, which was calculated using the ratio in the soils and soil-to-plant transfer factors.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Significant quantities of radionuclides were released into the environment from the TEPCO’s Fukushima Daiichi Nuclear Power Stations (FDNPS) accident in March 2011. Radiocesium (134, 137Cs) is the major radionuclide released by the accident and an important radionuclide for the assessment of radiation exposure to the public. Other relatively long half-life radionuclides such as 90Sr, Pu, etc. are also important radionuclides for radiation dose estimation through long-term food ingestion. The new Standard Limits for radionuclides in foods was established by the Ministry of Health, Labour and Welfare on April 1, 2012. The limits were determined on the basis of 1 mSv y−1. The limit in general foods is 100 Bq kg−1 of radiocesium, including the contribution of 90Sr, Pu and 106Ru, determined by using the actual concentration in the soils from the accident (estimated data for 106Ru) and soil-to-transfer factor. However, the public has been concerned about food contamination, especially 90Sr. In the present study the concentrations of radiocesium and 90Sr in agricultural and animal products produced in Fukushima Prefecture were determined, and compared with the values of the new allowable Standard Limits.
2 Materials and Methods
Agricultural and animal products, limited to those produced within the Fukushima Prefecture, were collected from markets located mostly in Fukushima-shi and Date-shi. Table 16.1 contains a list of 120–5000 g samples of 11 spices in 2012 (40 samples) and 2013 (42 samples). The agricultural plants were washed, peeled, and then the edible parts were cut into small pieces. Each sample was dried at 70 °C for 1 week and pulverized in a stainless steel cutter blender before being analyzed for radiocesium. The animal samples were also cut into small pieces. The samples were compressed into a plastic container (47 mm in diameter and 50 mm in height) and the concentration of radiocesium and 40K determined with a Ge detector connected to a multichannel analyzer system by counting for 9400–33,000 s. The detection efficiency of the Ge detector was dependent on the sample thickness and was obtained using the mixed standard radionuclides material made by the Japan Radioisotope Association. Counting statistics standard deviations for 137Cs concentration in the sample were less than 10 % of the value.
Soil and agricultural samples were collected from 10 agricultural fields located both outside and within the more than 50 mSv y−1 of the external radiation dose zone (difficult-to-return zone, Okuma) in Fukushima Prefecture in 2013 and 2014 (Fig. 16.1)~. Shiitake mushroom and its mushroom bed for cultivation was also collected. A stainless steel core sampler was used to collect soil cores 5 cm in diameter and 20 cm in depth at 5 points evenly distributed in each field. Twenty kilogram of each agricultural sample was collected from each field. The soil core samples collected from each field were dried at 50 °C for 1 week and then passed through a 2 mm sieve. The soil samples in each field were thoroughly mixed. The agricultural samples were washed, peeled, and then the edible parts were cut into small pieces. Approximately 100 g of dried sample was pulverized in a stainless steel cutter blender before being analyzed for radiocesium. The rest of the dried agricultural samples were washed at a temperature below 450 °C for analysis of 90Sr. The dried soil and agricultural samples were compressed into plastic containers and the concentrations of radiocesium and 40K determined. A radioanalytical method for 90Sr was performed according to the previously reported method [1]. The ash plant samples (20–50 g) were decomposed with HNO3, H2O2 and HCl after the addition of the Sr carrier. The soil samples (100 g) were heated at 450 °C and then extracted with 12 M HCl after the addition of the Sr carrier. The solution was filtered and the residue discarded. The solution was adjusted to >pH 10 with NaOH and then SrCO3 precipitated by adding Na2CO3. The SrCO3 precipitate was dissolved in HCl and then the oxalates re-precipitated at pH 4.2 by adding oxalic acid. The supernatant was decanted and the oxalate precipitation dissolved in HNO3. Strontium in the solution was separated from Ca by the cation ion-exchange method, and then the filtrated precipitate was dissolved with water. Any radioactive impurity was eliminated by scavenging on BaCrO4 and Fe(OH)3. An ammonium carbonate solution was added to the solution after scavenging, and the SrCO3 precipitate was filtered using a cellulose filter paper. The recovery of Sr was estimated by measuring the stable Sr with ICP-AES and then a disk sample prepared for beta-counting for 6000–60,000 s.
Concentrations of 137Cs and 90Sr (Bq kg−1 dry weight) in cultivated soil (n = 10) collected from Fukushima Prefecture in 2014. Point numbers show the sampling agricultural plants indicated in Table 16.3 and the values in parentheses are the concentrations of 137Cs (former) and 90Sr (latter) in cultivated soil
3 Results and Discussion
The concentrations of radiocesium in agricultural products collected in Fukushima Prefecture in 2012 and 2013 are listed in Table 16.2. The average concentration of radiocesium collected in 2013 was 2.0 ± 2.7 (<0.046–14) Bq kg−1 fresh weight, which was one-fourth of that in 2012 (7.6 ± 10, <0.11–40 Bq kg−1 fresh weight), and the concentrations of radiocesium in all the samples were less than the new Standard Limits. The concentrations of radiocesium in agricultural products decrease with time elapsed, and the reasons are as follows:
-
1.
Decay of radiocesium activities, especially 134Cs (half-life, 2.1 y)
-
2.
Countermeasure with the application of K fertilizer for reducing uptake of radiocesium in plants
-
3.
Aging effect in soil, which radiocesium in exchangeable fraction decreases and that in strongly bound fraction increases with time elapsed [2, 3]
-
4.
Decreasing radiocesium contents in soil by erosion, etc.
The concentration of radiocesium in agricultural plants drastically decreases immediately after the accident, and the rate of the decrease of radiocesium concentration in plants has gradually slowed as more time has lapsed. The reported mean concentration of radiocesium in a duplicate diet collected from Fukushima Prefecture in December 2011 was 1.5 Bq kg−1 [4], which was lower than that in 2013 as determined in this study. This is because the concentration of radiocesium in foods determined by a duplicate method decreased during processing and cooking and market dilution effect.
The concentrations of 134, 137Cs and 90Sr were determined in surface soils and agricultural plants collected from both outside and within the difficult-to-return zone in Fukushima Prefecture. The concentration of radiocesium in the agricultural soils outside the zone is decreasing because of plowing, migration, erosion, etc. However, the soil within the difficult-to-return zone (experimental field 5 km west from the Nuclear Power Stations in Okuma) is still highly contaminated with radiocesium (Fig. 16.1). The concentration of 90Sr in the soils collected outside the zone is low with no differences among the values. In contrast, 90Sr concentration in the soils collected within the zone was several times higher than that outside the zone. However, the concentration of 90Sr in the soils collected both outside and within the zone is within the range collected throughout Japan except Fukushima (ND-5.9 Bq kg−1, data from Environmental Radioactivity and Radiation in Japan [6]). The concentration of radiocesium in the plants collected outside of the difficult-to-return zone (Table 16.3) is similar to the range shown in Table 16.2. The concentration of radiocesium in the agricultural plants within the zone was still higher than that outside the zone (Table 16.3); however, part of the plants cultivated in the experimental field was lower than the new Standard Limits [7]. The range of 90Sr concentration in the plants collected outside the zone is 0.0047–0.30 Bq kg−1 fresh weight, and those collected within the zone were 0.31 (pumpkin) and 0.21 (cabbage). These data are also within the range collected throughout Japan except Fukushima (ND-0.91 Bq kg−1 fresh weight, data from Environmental Radioactivity and Radiation in Japan [6]).
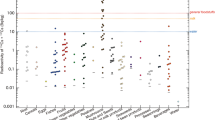
The new Standard Limits of radiocesium include the contribution of 90Sr concentration in general foods. The concentration ratio of 90Sr/137Cs in the foods was predicted by using observed 90Sr/137Cs concentration ratio (0.003) [8] in the soils from the FDNPS accident and the reported soil-to-plant transfer factor [9, 10], and the concentration of 90Sr in foods was determined by multiplying the predicted 90Sr/137Cs ratio in foods by the measured 137Cs value in foods. Therefore, the propriety between the predicted and the measured 90Sr/137Cs ratio in foods (Fig. 16.2) needs to be evaluated. The measured concentration ratio of 90Sr/137Cs in the plants, except for three samples (AP-1, komatsuna; AP-2, cucumber; AP-8, edible chrysanthemum), was lower than the predicted 90Sr/137Cs ratio, and the determined ratio in the two samples collected within the difficult-to-return zone (Okuma), which may have a large contribution from the accident, was also lower than the predicted 90Sr/137Cs ratio. The concentration of 90Sr in the soils collected from the three fields, where the 90Sr/137Cs concentration ratio in the plants overestimated the predicted ratio, was similar in range to the global fallout deposited in the soil. Therefore it is necessary to attribute the 90Sr contents in the plants as being derived from the global fallout from several decades ago.
Comparison of 90Sr/137Cs concentration ratio between the estimated value and the determined value in agricultural plants. Plotted numbers indicate sample no. in Table 16.2. The solid-black circles (AP-10 and AP-11) were collected within the difficult-to-return zone
Internal radiation doses from radiocesium through food ingestion for adult males and females (over the age of 19) were estimated. Measured and predicted data for the radiocesium concentration in food categories were used. The concentration of 137Cs in the animal products including milk collected in 2012 and 2013 was not detected, and the average value of the detection limits (0.6 Bq kg−1) in the animal products was used for the dose estimation. Drinking water pathway was not included for the dose estimation because it was lower than the detection limit. The estimated internal radiation doses through food ingestion for males and females were 0.066 and 0.052 mSv y−1 in 2012, and those in 2013 were 0.016 and 0.012, respectively, reflecting the decreases in the concentration of radiocesium in foods with time elapsed. It was also reported that the internal radiation dose from radiocesium in Fukushima Prefecture in 2012 was 0.0039–0.0066 mSv y−1 by the market basket method [11], which was one order of magnitude lower than that in this study. This is attributed to the fact that the collected foods by the market basket method usually included products both within and outside of Fukushima Prefecture, and the concentration of radiocesium in the foods decreased by market dilution. On the other hand, the samples collected in this study were produced only in Fukushima Prefecture and were not influenced by the market dilution effect. The internal radiation doses from radiocesium by the duplicate diet method in Fukushima Prefecture were reported as 0.026 mSv y−1 in 2011 [4] and 0.0022 mSv y−1 in 2012 [11]. The internal radiation dose from radiocesium through food ingestion determined by the duplicate diet method is lower than that by the market basket method because of processing and cooking, and it is assumed that those values decrease with time elapsed.
References
Tsukada H, Takeda A, Takahashi T, Hasegawa H, Hisamatsu S, Inaba J (2005) Uptake and distribution of 90Sr and stable Sr in rice plants. J Environ Radioact 81:221–231
Takeda A, Tsukada H, Nakao A, Takaku Y, Hisamatsu S (2013) Time-dependent changes of phytoavailability of Cs added to allophanic Andosols in laboratory cultivations and extraction tests. J Environ Radioact 122:29–36
Tsukada H (2014) Behavior of radioactive cesium in soil with aging. Jpn J Soil Sci Plant Nutr 85:77–79 (in Japanese)
Harada KH, Fujii Y, Adachi A, Tsukidate A, Asai F, Koizumi A (2013) Dietary intake of radiocesium in adult residents in Fukushima Prefecture and neighboring regions after the Fukushima nuclear power plant accident: 24-h food-duplicate survey in December 2011. Environ Sci Tech 47:2520–2526
Nuclear Regulation Authority. Monitoring information of environmental radioactivity level. http://ramap.jmc.or.jp/map/eng/
Environmental Radioactivity and Radiation in Japan. http://www.kankyo-hoshano.go.jp/en/index.html
Ohse K, Kitayama K, Suenaga S, Matsumoto K, Kanno A, Suzuki C, Kawatsu K, Tsukada H (2014) Concentration of radiocesium in rice, vegetables, and fruits cultivated in evacuation area in Okuma Town, Fukushima. J Radioanal Nucl Chem 303:1533–1537
Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/stf/shingi/2r98520000023nbs-att/2r98520000023ng2.pdf
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environment, vol 472, Technical report series. International Atomic Energy Agency, Vienna
Tsukada H, Nakamura Y (1998) Transfer factors of 31 elements in several agricultural plants collected from 150 farm fields in Aomori, Japan. J Radioanal Nucl Chem 236:123–131
Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/shinsai_jouhou/shokuhin.html
Acknowledgement
This work was supported by MHLW KAKENHI Grant. We are grateful to Messrs. A. Sato (Ichii Co. Ltd.) and M. Kanno (Citizens for Revitalization of Oguni after Nuclear Disaster) for sample collection, and Dr. P. T. Lattimore for his useful suggestion and comments. We thank Mr. A. Kanno, Mses. C. Suzuki, W. Horiuchi and M. Kato for sample pretreatment.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Tsukada, H., Takahashi, T., Fukutani, S., Ohse, K., Kitayama, K., Akashi, M. (2016). Concentrations of 134, 137Cs and 90Sr in Agricultural Products Collected in Fukushima Prefecture. In: Takahashi, T. (eds) Radiological Issues for Fukushima’s Revitalized Future. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55848-4_16
Download citation
DOI: https://doi.org/10.1007/978-4-431-55848-4_16
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55847-7
Online ISBN: 978-4-431-55848-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)