Abstract
Dietary fibre (DF) has been shown to be a vital component of diet for human health, decreasing the risk of cardiovascular disease, type II diabetes and possibly bowel cancer. DF in wheat flour is derived from the cell walls of the starchy endosperm, which is principally composed (~70 %) of the polysaccharide arabinoxylan (AX). Diversity screens of elite wheat germplasm have established that variation in total and water-extractable AX within flour exists and has high heritability. Identification of genes which determine AX content will assist in introduction of high DF alleles into appropriate backgrounds. We identified candidate genes for the synthesis and feruloylation of AX from bioinformatics approaches. Using RNAi suppression of genes in wheat endosperm, we have shown that a glycosyl transferase (GT) family 61 gene is responsible for nearly all mono-substitution of xylose by arabinose on AX, and that genes in GT43 and GT47 families are responsible for the synthesis of the xylan backbone in AX. Using mapping populations derived from crosses of high AX x normal AX varieties, we are also seeking to identify QTLs for high AX. This combination of forward and reverse genetics will accelerate the introduction of the high fibre alleles into modern commercial wheat varieties.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.

The opinions expressed and arguments employed in this publication are the sole responsibility of the authors and do not necessarily reflect those of the OECD or of the governments of its Member countries.The Special Session was sponsored by the OECD Co-operative Research Programme on Biological Resource Management for Sustainable Agricultural Systems, whose financial support made it possible for most of the invited speakers to participate in the Special Session.
Wheat as a Source of Dietary Fibre
In addition to providing calories and protein to much of the world’s population, wheat is also an important source of dietary fibre (DF), with bread products alone contributing 20 % of the DF in the adult UK diet. There is also a significant shortfall in the intake of DF in many countries. For example, in the UK the daily intake of DF is only about 13 g per day (Buttress and Stokes 2008) compared with a recommended daily intake of 25–40 g a day in most other countries. Most of the DF consumed is non-starch polysaccharides, with resistant starch contributing about 20–25 % of the total in European diets (Cummings 1983). There is strong evidence that DF in wholegrain provides protection against the risk of a number of chronic diseases, including type 2 diabetes, obesity and cardiovascular disease, with both soluble and insoluble forms contributing to the beneficial effects (Slavin 2004; Topping 2007; Wood 2007; Lunn and Buttriss 2007; Buttress and Stokes 2008; Anderson et al. 2009; Fardet 2010). DF is not digested in the upper gastrointestinal (GI) tract and many of the beneficial effects result from fermentation in the colon. However, it also has effects throughout the GI tract, with insoluble fibre providing faecal bulk to speed up transit, while soluble fibre may increase the viscosity of the digesta to reduce the rates digestion and the uptake of nutrients in the small intestine, and hence lower the glycaemic load. The wide consumption and low cost of staple foods made from wheat mean that it is an excellent vehicle to deliver the health benefits conferred by DF to large populations at low cost. However, in order to achieve this it is necessary to increase the fibre content of wheat and, in particular, in the part of the grain that is most widely consumed: the starchy endosperm which forms white flour on milling.
Cell Wall Composition of Wheat
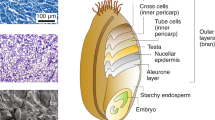
The wheat grain comprises three main parts which differ in their contents and composition of cell wall polysaccharides. The major storage tissue is the endosperm, which accounts for about 90 % of the dry weight of the mature grain (Barron et al. 2007). The outer layer of endosperm cells, called the aleurone layer, have thick cell walls which account for about 40 % of the dry weight. These surround the starchy endosperm cells, which have thin walls (about 2–3 % dry wt.) and contain mainly starch and storage proteins. The second major tissue is the embryo, which accounts for about 3 % of the mature grain (Barron et al. 2007). These tissues are sounded by several outer layers, the nucellar epidermis, testa and inner and outer pericarps. On milling the outer layers, embryo and aleurone form the bran fraction and the starchy endosperm cells the white flour.
The major cell wall polysaccharides in the cell walls of wheat grain are arabinoxylan (AX) and (1,3; 1,4)-β-D-glucan (β-glucan). AX comprises a backbone of β-D-xylopyranosyl residues linked through (1,4) glycosidic linkages, with some residues being substituted with α-L-arabinofuranosyl residues, while β-glucan comprises only glucose residues which are linked by (1,4) and (1,3) bonds. AX accounts for about 70 % of the total cell wall polysaccharides in white flour and 62–65 % in aleurone, while β-glucan accounts for about 20 % and 29–35 % in these tissues, respectively (Table 46.1). Cellulose is a minor component in both tissues (2–4 %) while the starchy endosperm cell walls also contain about 7 % glucomannan. By contrast, the composition of the outer bran layers has high contents of cellulose, lignin and complex glucuronarabinoxylans but no β-glucan (Table 46.1).
Genetic Variation and Heritability of AX Content
Analyses of wheat genotypes have shown extensive variation in the content and composition of AX in whole grain and white flour. A study carried out under the EU FP6 HEALTHGRAIN programme (Poutanen et al. 2008), showed that total DF varied from 11.5 % to 15.5 % dry wt (mean 13.4 %) in wholegrain of 129 wheat lines grown on the same site, while total AX (TOT-AX) ranged from 5.53 % to 7.42 % (mean 6.49 %) (Andersson et al. 2013). A more detailed study of 151 lines grown on the same site (including those studied by Andersson et al. 2013) compared the contents of TOT-AX and water-extractable AX (WE-AX) in flour and bran fractions (Gebruers et al. 2008; Ward et al. 2008). Although the bran fractions contained 13–22 % TOT-AX, WE-AX only ranged from 0.30 % to 0.85 %, corresponding to between 2 % and 5 % of the whole fraction. By contrast, white flour contained only 1.35–2.75 % TOT-AX but between 20 % and 50 % of this was water soluble (0.30–1.4 % dry wt. WE-AX). A small number of lines from this study were subsequently grown in an additional five environments (sites and/or years), allowing the broad sense heritability of the AX fractions to be determined. The ratios of genetic variance to total variance were 0.39 and 0.71 for TOT-AX in bran and flour, respectively, and 0.47 and 0.59 for WE-AX in bran and flour (Gebruers et al. 2010; Shewry et al. 2010). These high heritabilities, particularly for the flour fractions, indicate that the contents of both TOT-AX and WE-AX should be amenable to manipulation by plant breeding.
Variation in AX Structure
AX comprises a backbone of β-D-xylopyranosyl residues linked through (1,4) glycosidic linkages, with some residues being substituted with α-L-arabinofuranosyl residues at either position 3 or positions 2 and 3. Another key attribute of AX structure is the degree of feruloylation of some of the three-linked arabinose. The solubility of AX is dictated by degree of arabinose substitution (increasing solubility), cross-linking of AX chains by intermolecular dimerization of ferulate (decreasing solubility) and the chain length of AX backbone (longer chains are less soluble) (Saulnier et al. 2007). Any of these attributes may influence the benefits that AX confers in the diet, either directly or by altering the solubility of AX. There is genetic variation in the proportions of monosubstituted and disubstituted xylose residues (Saulnier et al. 2007) but less is known about genetic variation of the other AX structural attributes. AX feruloylation, measured as total bound ester-linked ferulic acid, is highly variable with low heritability, but the extent of genetic variation within white flour (where levels are much lower) is unknown. More research is needed both of the structural attributes of AX which confer greatest benefit in human diet and on the wheat genes responsible for them to determine the importance and feasibility of genetic improvement of AX structure.
Genes Responsible for AX Synthesis in Wheat Endosperm
Identification of Candidate Genes
Xylan is more abundant in primary cell walls of grasses than those of dicots and arabinose substitution is far more common (the main substitution in dicot xylan being glucuronic acid). Feruloylation of AX occurs exclusively in grasses and other commelinid monocots. Since xylan is a highly abundant polymer within plant biomass, we expect transcripts encoding the synthetic enzymes to be highly abundant; exploiting this fact and the differences between grasses and dicots led us to identify candidate genes for the synthesis and feruloylation of AX (Mitchell et al. 2007). The results are summarized in Fig. 46.1; we identified clades within the glycosyl transferase (GT) gene families 43, 47 and 61 as likely involved in the synthesis of AX, and a clade from the BAHD acyl coA transferase superfamily as likely responsible for AX feruloylation.
Histogram of results of analyses in Mitchell et al. (2007). The number of orthologous groups are shown for each bin of log ratio of expression in cereals to that in dicots. Clades from families with greater expression in cereals and correct characteristics for AX synthesis (inverting GT families) and feruloylation (acyl transferases) are indicated
Using RNA-Seq transcriptome analyses of developing wheat starchy endosperm, we found transcripts within all of these clades during the period of greatest AX synthesis (Pellny et al. 2012). In order to demonstrate their function, we transformed wheat to suppress their expression using an endosperm-specific promoter driving RNAi constructs.
Role of GT61 Genes in AX Synthesis
We compared five homozygous transgenic lines suppressing the most highly expressed GT61 gene in wheat endosperm with null segregant controls. Digestion of AX with xylanase and HPAEC quantification of resultant oligosaccharides showed that those oligosaccharides with single three-linked arabinose substitution were substantially decreased and 1H-NMR showed almost complete abolition of this linkage in undigested soluble AX (Anders et al. 2012; Fig. 46.2). Furthermore our collaborators (Dupree group, University of Cambridge, UK) showed that expression of similar wheat and rice genes in Arabidopsis introduced arabinose linkages onto Arabidopsis xylan (Anders et al. 2012). Therefore these GT61 genes encode xylan arabinosyl transferases, consistent with their far greater expression in grasses than dicots (Fig. 46.1).
Analysis of xylan structure in endosperm samples from homozygous TaGT61_1 RNAi transgenic wheat. (a) Oligosaccharide abundance from transgenic samples relative to corresponding azygous controls after xylanase digest; mean of five independent lines ±95 % confidence intervals. Columns are colored according to oligosaccharide substitution: unsubstituted (green), mono-substituted only (red), di-substituted only (blue), and mono- and di-substituted (purple). (b) 1H-NMR spectra for transgenic (red) and azygous control (blue) samples showing H1 signals for arabinose in AX: A3-Xmono denotes α-(1,3)–linked to mono-substituted xylose (Redrawn from Anders et al. 2012)
Role of GT43 and GT47 Genes in AX Synthesis
We compared homozygous transgenic lines suppressing either the most highly expressed GT43 (TaGT43_2) or GT47 (TaGT47_2) genes in wheat endosperm with their null segregant controls. Suppression of either gene had similar effects with AX content decreased to about 50 % of controls as determined by monosaccharide composition of non-starch polysaccharide (Fig. 46.3a; Lovegrove et al. 2013). In contrast to suppression of TaGT61_1, the amount of arabinose substitution was increased (Fig. 46.3b). The TaGT43_2 and TaGT47_2 genes are orthologous to IRX9 and IRX10 in Arabidopsis, respectively. Mutations in IRX9 and IRX10 lead to decreased xylan and xylan chain length in Arabidopsis (Pena et al. 2007; Brown et al. 2009) and we obtained similar results by suppressing their orthologues in wheat endosperm. However, size-exclusion profiles of AX showed that suppressing TaGT43_2 had a greater effect on the longest chains of AX than suppressing TaGT47_2 (Fig. 46.3c).
Summary of results in Lovegrove et al. (2013) from RNAi suppression of TaGT43_2 and TaGT47_2 genes in wheat endosperm. (a, b) Total AX abundance (a) and arabinose to xylose ratio (b) from transgenic samples and null-segregant controls determined by monosaccharide analyses of non-starch polysaccharide; mean of three independent lines ±SE. (c) Log intrinsic viscosity of soluble AX fractions (which is proportional to log AX chain length; Dervilly-Pinel et al. 2001) separated by SE-HPLC versus concentration for samples from TaGT43_2 and a TaGT47_2 RNAi lines and corresponding controls
Combining Forward and Reverse Genetics to Develop Wheat with Enhanced Health Benefits
Rapidly improving bioinformatics resources for wheat, e.g. the International Wheat Genome Sequencing Consortium (http://www.wheatgenome.org/) chromosome-sorted survey sequences, make it possible to start relating genes to traits in silico. In Table 46.2, the genes shown to be responsible for AX synthesis in wheat endosperm above are shown with their putative chromosome location, together with some published QTLs for DF or related traits in wheat. The causative alleles for these QTLs may be cis-elements controlling these genes or the genes themselves. As our understanding of which genes dictate DF content in wheat products improves, there are excellent prospects for efficient breeding of wheat varieties which deliver enhanced health benefits in the near future.
References
Anders N, Wilkinson MD, Lovegrove A et al (2012) Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci U S A 109:989–993
Anderson JW, Baird P, Davis RH Jr et al (2009) Health benefits of dietary fiber. Nutr Rev 67:188–205
Andersson AAM, Andersson R, Piironen V et al (2013) Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem 136:1243–1248
Antoine C, Peyron S, Mabille F et al (2003) Individual contribution of grain outer layers and their cell wall structure to the mechanical properties of wheat bran. J Agric Food Chem 51:2026–2033
Bacic A, Stone BA (1981) Chemistry and organization of aleurone cell wall components from wheat and barley. Aust J Plant Physiol 8:475–495
Barron C, Surget A, Rouau X (2007) Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J Cereal Sci 45:88–96
Brown DM, Zhang ZN, Stephens E et al (2009) Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J 57:732–746
Buttress JL, Stokes CS (2008) Dietary fibre and health: an overview. Nutr Bull 33:186–200
Cummings JH (1983) Fermentation in the human large intestine: evidence and implications for health. Lancet 321:1206–1209
Dervilly-Pinel G, Thibault JF, Saulnier L (2001) Experimental evidence for a semi-flexible conformation for arabinoxylans. Carbohydr Res 330:365–372
Du Pont MS, Selvendran RR (1987) Hemicellulosic polymers from the cell walls of beeswing wheat bran: part I, polymers solubilised by alkali at 2°. Carbohydr Res 163:99–113
Fardet A (2010) New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 23:65–134
Gebruers K, Dornez E, Boros D et al (2008) Variation in the content of dietary fiber and components thereof in wheats in the HEALTHGRAIN diversity screen. J Agric Food Chem 56:9740–9749
Gebruers K, Dornez E, Bedo Z et al (2010) Environment and genotype effects on the content of dietary fiber and its components in wheat in the HEALTHGRAIN diversity screen. J Agric Food Chem 58:9353–9361
Lovegrove A, Wilkinson MD, Freeman J et al (2013) RNA interference suppression of genes in glycosyl transferase families 43 and 47 in wheat starchy endosperm causes large decreases in arabinoxylan content. Plant Physiol 163:95–107
Lunn J, Buttriss JL (2007) Carbohydrates and dietary fibre. Nutr Bull 32:21–64
Mares DJ, Stone BA (1973) Studies on wheat endosperm. I. Chemical composition and ultrastructure of the cell walls. Aust J Biol Sci 26:793–812
Mitchell RAC, Dupree P, Shewry PR (2007) A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol 144:43–53
Nguyen VL, Huynh BL, Wallwork H, Stangoulis J (2011) Identification of quantitative trait Loci for grain arabinoxylan concentration in bread wheat. Crop Sci 51:1143–1150
Pellny TK, Lovegrove A, Freeman J et al (2012) Cell walls of developing wheat starchy endosperm: comparison of composition and RNA-seq transcriptome. Plant Physiol 158:612–627
Pena MJ, Zhong RQ, Zhou GK et al (2007) Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19:549–563
Poutanen K, Shepherd R, Shewry PR et al (2008) Beyond whole grain: the European HEALTHGRAIN project aims at healthier cereal foods. Cereal Foof World 53:32–35
Quraishi UM, Murat F, Abrouk M et al (2011) Combined meta-genomics analyses unravel candidate genes for the grain dietary fiber content in bread wheat (Triticum aestivum L.). Funct Integr Genomics 11:71–83
Rhodes DI, Stone BA (2002) Proteins in walls of wheat aleurone cells. J Cereal Sci 2002(36):83–101
Saulnier L, Sado PE, Branlard G et al (2007) Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J Cereal Sci 46:261–281
Selvendran RR, Ring SG, O’Neill MA, Du Pont MS (1980) Composition of cell wall material from wheat bran used in clinical feeding trials. Chem Ind 22:885–888
Shewry PR, Piironen V, Lampi A-M et al (2010) The HEALTHGRAIN wheat diversity screen: effects of genotype and environment on phytochemicals and dietary fiber components. J Agric Food Chem 58:9291–9298
Slavin J (2004) Dietary fiber and the prevention of cancer. In: Remacle C, Reusens B (eds) Functional foods, ageing and degenerative disease. Woodhead Publishing Ltd, Cambridge, pp 628–644
Topping D (2007) Cereal complex carbohydrates and their contribution to human health. J Cereal Sci 46:220–229
Ward JL, Poutanen K, Gebruers K et al (2008) The HEALTHGRAIN cereal diversity screen: concept, results, and prospects. J Agric Food Chem 56:9699–9709
Wood J (2007) Cereal β-glucans in diet and health. J Cereal Sci 46:230–23
Acknowledgments
Our research described here was funded by the Biotechnology and Biological Science Research Council of the UK.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Mitchell, R.A.C., Shewry, P.R. (2015). Dietary Fibre: Wheat Genes for Enhanced Human Health. In: Ogihara, Y., Takumi, S., Handa, H. (eds) Advances in Wheat Genetics: From Genome to Field. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55675-6_46
Download citation
DOI: https://doi.org/10.1007/978-4-431-55675-6_46
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55674-9
Online ISBN: 978-4-431-55675-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)