Abstract
This chapter summarizes a newly developed method (quadripulse stimulation (QPS)) to induce neural plasticity in the human brain.
What Is QPS? One stimulation burst consisting of four monophasic pulses is given every 5 s for 30 min. In total, 360 bursts (1440 pulses) are given in one session. Short-interval QPS potentiates excitability and longer-interval QPS depresses the target area. QPS at intervals of 5 ms (QPS5) induces long-term potentiation (LTP) most efficiently and QPS50 induces long-term depression (LTD) most effectively in the human primary motor cortex (M1).
After QPS, no changes were found in the threshold, GABAergic function of M1, or acetylcholine function. In contrast, EPSP summation and sharpness of the IO curve are bidirectionally modulated by QPS. These findings indicate that excitatory synaptic efficacy is bidirectionally modulated by QPS. The effect is specific to the activated neurons. These all are consistent with synaptic LTP/LTD.
Dopamine enhanced both LTP of QPS5 and LTD of QPS50. It is again compatible with plasticity induction in animals. These are consistent with the concept that LTD was enhanced by the D1 agonist and LTD by the D1 and D2 agonists together, but the D1 agonist alone or the D2 agonist alone induced no changes in LTD.
In PD patients, even at an early stage, QPS induced neither LTP- nor LTD-like effects in the motor cortex. This lack of plasticity was normalized by L-Dopa intake in parallel with motor symptom improvements.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Synaptic plasticity
- Axonal plasticity
- Human motor cortex
- Transcranial magnetic stimulation (TMS)
- Quadripulse stimulation (QPS)
- Dopamine
- Dopamine agonist
- Parkinson’s disease
- Long-term potentiation (LTP)
- Long-term depression (LTD)
Introduction
Neural plasticity is one main mechanism to support the flexibility of the human brain. It underlies several important human brain functions such as memory (hippocampus), motor learning (cerebellum), motor programming (basal ganglia), and so on. It has also some relation to reward learning, decision making, and addiction. It is well known that plasticity plays some roles also in the recovery of damaged function in some neurological disorders, such as cerebrovascular disease, Parkinson disease, dystonia, and so on. This chapter summarizes a newly developed stimulation method (quadripulse stimulation (QPS)) to induce neural plasticity in the human brain.
What Is QPS? [1]
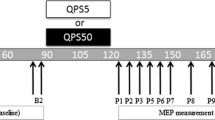
There are several stimulation methods to induce plastic changes in the human brain. QPS must be the most powerful and reliable one for human plasticity induction. Figure 1 shows the protocol of QPS, and Fig. 2 demonstrates a set of four stimulators for QPS. After baseline (motor evoked potential) MEP recordings, one session of QPS stimulation was performed and MEPs were followed by single-pulse transcranial magnetic stimulation (TMS) with the same intensity used in the baseline recordings (Fig. 1). In QPS, one stimulation burst consisting of four monophasic TMS pulses is given every 5 s for 30 min. In total, 360 bursts (1,440 pulses) are given in one session. The interval of TMS pulses was changed in different QPS conditioning sessions from 1.5 to 1250 ms. We evaluated the motor cortical excitability using the size of MEPs to TMS pulses at the same intensity.
Figure 3 shows typical MEPs before and after QPS5 (inter-pulse interval (IPI) 5 ms), QPS30, and QPS50. MEPs were enlarged after QPS5, whereas they were decreased in size after QPS30 and PQS50. After QPS, in total, the stimulated cortical area excitability is bidirectionally modulated depending on the interval of magnetic pulses in one burst. Short-interval QPS potentiates excitability, and longer-interval QPS depresses the target area. QPS at an interval of 5 ms (QPS5) induces long-term potentiation (LTP) most efficiently, and QPS50 induces long-term depression (LTD) most effectively in the human primary motor cortex (M1). Figure 4 shows the relation between the QPS effect and the IPI of TMS pulses, which fits well to the well-known Bienenstock–Cooper–Munro (BCM) curve. Their physiological characteristics, bidirectional modulation, dependence on the interval of pulses, and spatial specificity are all compatible with the plasticity reported previously. We conclude that QPS can induce neuronal plasticity in the human brain.
Mechanisms Underlying QPS [1]
To elucidate the mechanisms underlying QPS, we compared several physiological features between the baseline (before QPS) and after QPS. No changes were found in the threshold (Fig. 5a, d), GABAergic function of M1, or acetylcholine function. In contrast, both intracortical facilitation (ICF) and short-latency cortical facilitation (SICF) were bidirectionally modulated by QPS5 and QPS50 (Fig. 6a, b). Both of them may reflect glutamatergic EPSP summation. The sharpness of the IO curve was also bidirectionally modulated by QPS (Fig. 5c, f). The sharpness of the IO curve should also reflect the excitatory synaptic function of M1. These indicate that excitatory synaptic efficacy is bidirectionally modulated by QPS. Figure 5c, f show the special specificity of the QPS effect to the area conditioned by QPS. These are all consistent with the synaptic LTP/LTD (Fig. 7)
Physiological characteristics before and after QPS. The threshold is shown in (a) and (d), the input-output curves in (b) and (e), and somatotopic specificity in (c) and (f). They revealed no changes in the threshold, bidirectional modulation of the IO curves, and somatotopic specificity to the target muscle. These are all consistent with bidirectional changes in the excitatory glutamatergic synapses
Nakatani-Enomoto et al. [2] supported the safety of QPS by recoding EEGs and measuring serum prolactin levels before and after QPS. Nakamura et al. [3] showed the independency of QPS effects on brain-derived nerve growth factor (BDNF) polymorphism on which some plasticity induction methods depend. It is one superior point of QPS compared with other methods, especially when applying these methods to patients, because we do not need to consider BDNF polymorphism in judging the results in the patients.
Dopamine/Dopamine Agonists and Plasticity [5]
Dopamine/Dopamine Agonists in Normal Subjects [5]
It is well known that dopamine enhances both LTP and LTD, and the former is one of the D1 effects and the latter is a kind of D2 effect. To study the relation of dopamine or a dopamine agonist with QPS, we compared LTP/LTD effects between the conditions after placebo, L-Dopa, or pramipexole intake in normal volunteers. Figure 8 shows typical MEPs 30 min after QPS5 and QPS50 under the conditions of placebo, L-Dopa, or pramipexole intake. Under the placebo condition, QPS5 enlarged MEPs and QPS50 made them smaller, as shown above. Under the L-Dopa intake condition, both LTP-like and LTD-like effects were enhanced as compared with the placebo condition. In contrast, neither an LTP-like effect nor an LTD-like effect was modulated by pramipexole. This is again compatible with plasticity induction in animals. Pramipexole, almost purely a D2 agonist, may not enhance LTP because D1 activation strengthens LTP but D2 activation does not. It did not enhance the LTD-like effects, probably because both D1 and D2 coactivation is required for LTD [4].
Dopamine/Dopamine Agonists in PD
In PD patients, even at an early stage, QPS induced neither LTP nor LTD-like effects in the motor cortex. This lack of plasticity was normalized by L-Dopa intake in parallel with motor symptom improvements.
Meta-plasticity [6]
Flexibility plays critical roles in brain function, as shown above. However, flexibility or plasticity is sometimes harmful to the brain. Hyperpotentiation of the cerebral cortices may induce epilepsy, and excessive reward-seeking may produce an addiction to drugs or gambling. The brain, therefore, has several mechanisms to maintain the stable state to some extent. In other words, the brain is as flexible as possible, but within a certain safety range. To maintain this safety, the brain has meta-plasticity, which is called “plasticity of plasticity”. The meta-plasticity function was studied with some priming stimulation over the primary motor cortex (M1), premotor cortex (PM), or supplementary motor cortex (SMA). Figure 9 shows the experimental protocol of the meta-plasticity experiment. Before the usual QPS experiments, we gave a priming stimulation for a short period (10 min), which had no influence on MEPs when given alone. Figure 10 shows the MEP time courses with and without priming stimulation (QPS5 for 10 min). In all QPS protocols, the QPS5 priming stimulation modulated the main QPS effects in the depressive direction. This is compatible with the meta-plasticity of animal experiments. It indicates that the human motor cortex has a safety-maintaining mechanism, just like animal M1.
Time courses of QPS 1.5, 5, 10, 30, 50, and 100 with and without QPS5 priming over M1. All time courses without priming (purple) were depressed by the QPS5 priming stimulation. The facilitatory priming stimulation depressed the main conditioning effects, which is compatible with the meta-plastic influence of the priming stimulation
Sensory Cortical Plasticity [ 7, 8]
The above studies were all done on M1. We studied the neural plasticity of the sensory cortex in humans, using sensory evoked potentials (SEPs). SEPs were bidirectionally modulated by QPS over M1. QPS5 over M1 potentiated SEPs and QPS50 depressed SEPs, but neither QPS5 nor QPS50 over the sensory cortex (S1) had any influence on SEPs. This indicates that S1 is more influenced by motor cortical excitability changes than S1 excitability itself, through heterotopic synaptic plasticity. This finding may have some relation to sensory gating of motor performance. This directionality of sensory cortical plasticity was broken in myoclonus epilepsy patients. Any QPS protocols induced LTP-like effects in patients with cortical myoclonus but did not induce LTD. The safety-maintaining mechanisms may be broken in these epileptic disorders.
Axonal Plasticity in the Human Motor Cortex
All of the above works studied synaptic plasticity in the human brain, which is produced by synaptic efficacy changes without any axonal excitability changes (threshold for axonal membrane or the resting membrane potential). In some situations, axonal excitability changes are also induced in association with synaptic plasticity in animal experiments. We studied whether axonal plasticity is also induced in the human motor cortex after QPS over M1 using high-voltage electric stimulation (transcranial electrical stimulation (TES)) of M1. TES activates corticospinal tract axons directly, whereas TMS activates them through at least one synapse in M1 (Fig. 11). We compared the QPS effects on MEPs to TMS and those on MEPs to TES. QPS5 over M1 induced axonal depression in association with synaptic potentiation, and QPS50 induced axonal potentiation and synaptic depression (Fig. 12). This is the first report of axonal plasticity in the human brain.
Future Projects for QPS
We would like to finally use QPS for treatment of neurological and psychiatric disorders. Several projects are now ongoing, such as in Parkinson disease, depression, and cerebrovascular diseases. To show clinical efficacy of these methods as treatment, we need to take care of several points: what sham stimulation to use, axonal plasticity effects in addition to synaptic plasticity effects. We are also considering the effects on some distant areas after QPS in treatment applications [9]. I believe QPS will be used as some sort of treatment in the near future.
References
Hamada M, Terao Y, Hanajima R et al (2008) Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol 586:3927–3947
Nakatani-Enomoto S, Hanajima R, Hamada M et al (2011) Some evidence supporting the safety of quadri-pulse stimulation (QPS). Brain Stimul 4:303–305
Nakamura K, Enomoto H, Hanajima R et al (2011) Quadri-pulse stimulation (QPS) induced LTP/LTD was not affected by Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene. Neurosci Lett 487:264–267
Calabresi P, Maj R, Mercuri NB, Bernardi G (1992) Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett 142:95–99
Enomoto H, Terao Y, Kadowaki S, Nakamura K, Moriya A, Nakatani-Enomoto S, Kobayashi S, Hanajima R, Ugawa Y (2015) Effects of L-Dopa and pramipexole on plasticity induced by QPS in human motor cortex. J Neural Transm. doi: 10.1007/s00702-015-1374-8
Hamada M, Hanajima R, Terao Y et al (2009) Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol 587:4845–4862
Hirose M, Mochizuki H, Tanji Y (2011) On-line effects of quadripulse transcranial magnetic stimulation (QPS) on the contralateral hemisphere studied with somatosensory evoked potentials and near infrared spectroscopy. Exp Brain Res 214(4):577–586
Nakatani-Enomoto S, Hanajima R, Hamada M et al (2012) Bidirectional modulation of sensory cortical excitability by quadripulse magnetic stimulation (QPS) in humans. Clin Neurophysiol 123:1415–1421
Tsutusmi R, Hanajima R, Terao Y et al (2014) Effects of the motor cortical quadripulse tarancranial magnetic stimulation (QPS) on the contralateral motor cortex and interhemispheric interactions. J Neurophysiol 111:26–35
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Ugawa, Y. (2015). Synaptic and Axonal Plasticity Induction in the Human Cerebral Cortex. In: Nakao, K., Minato, N., Uemoto, S. (eds) Innovative Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55651-0_24
Download citation
DOI: https://doi.org/10.1007/978-4-431-55651-0_24
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55650-3
Online ISBN: 978-4-431-55651-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)