Abstract
Studies using nonhuman primates have made marked contributions to our understanding of the anatomy and function of the primary somatosensory cortex (SI). Its ventral or inferolateral part (vSI) represents orofacial structures, such as the lips, periodontium, tongue, palate, chewing musculature, etc. This brain region is neurally interconnected with the ventral part of the primary motor cortex that executes voluntary orofacial movements. Also within the vSI, regions representing different orofacial structures are interconnected with each other. Therefore, in self-generated actions, the vSI plays a crucial role in coordinating motor control of a set of structures that are functionally related: the vSI serves as an interface not only between orofacial structures and the external environment but also between the orofacial structures themselves. In this article, we will chiefly review the neuroanatomical and neurophysiological studies on the monkey vSI from the viewpoint of motor control or stereognostic ability. Future physiological studies that analyze the spatiotemporal spiking pattern of vSI neurons during various behaviors in monkeys should reveal the principles of information coding and might significantly benefit future applications of brain-machine-brain interface (BMBI) technology.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
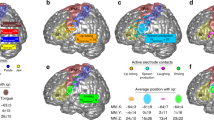
The primary somatosensory area (SI) of primates is located in the anterior-most part of the parietal lobe; i.e., the postcentral gyrus in Old World monkeys and humans. Its ventral or inferolateral part (vSI; Fig. 24.1a, b) represents orofacial structures and the larynx and plays a crucial role in the motor coordination of those structures. Its highly sophisticated form allows for speech motor control in humans. Neuroimaging studies of voice and speech production have consistently reported activation of the vSI, as well as the primary motor cortex, in both hemispheres (for review see [1, 2]). For example, the vSI was activated bilaterally even in the simplest speech task, such as overt speaking of a single syllable [3]. Similar results were reported in singing, another sophisticated orofacial and laryngeal function in humans (for review see [4]): the vSI is activated bilaterally in a simple singing condition where subjects were asked to produce bisyllabic words melodically intoned at a certain pitch, and even during humming [5]. Moreover, several lines of evidence suggest that trained singers may rely more on somatosensory feedback than auditory feedback to make sure that notes are produced properly [4]. In one study, professional opera singers and vocal students showed increased activation in the bilateral vSI compared to laymen (medical students), but not in the auditory cortex [6]. The investigators interpreted this to mean that somatosensory feedback via the vSI towards the motor cortex might play a particularly important role in the development of classical singing skills. Chewing, a more vital orofacial function, was also reported to activate the vSI bilaterally [7, 8]. This suggests that chewing also requires information processing in the vSI to control or monitor a series of movements despite its semi-automatic nature.
Overview of the ventral or inferolateral part of the primary somatosensory cortex (vSI). (a) (b) Lateral view of the cerebral hemisphere of humans (a) and macaques (b). The dark-shaded area roughly indicates the location of the vSI that represents the orofacial structures. The solid line in b corresponds to the section in c. (c) Cytoarchitectonic areas (Brodmann areas 3a, 3b, 1, 2) are shown in a parasagittal section. The broken line indicates layer IV. Dotted lines indicate the boundaries of cytoarchitectonic areas. CS central sulcus, LS lateral sulcus
Non-invasive brain imaging techniques have made significant contributions to our understanding of the brain mechanisms involved in orofacial functions, particularly those specific to humans. However, because of the limitation in spatial and temporal resolution, these techniques are unable to address finer anatomical and physiological details; e.g., the precise location of regions representing each orofacial structure and their neural interconnections, the principles of information coding by individual neurons, and populations of neurons, etc. Therefore, animal studies using nonhuman primates are also of tremendous value as complements to human brain imaging studies, and will continue to be so. In the following sections, we chiefly review the neuroanatomical and neurophysiological studies of the monkey vSI and some relevant neuroimaging studies in humans. Readers may also refer to a related review published recently by another group [9].
2 Representation of Orofacial Structures in Area 3b
The primary somatosensory area (SI) consists of three cytoarchitectonic areas, as shown in Fig. 24.1c: Brodmann areas 3 (3a, 3b), 1, and 2. Area 3 is regarded as the primary somatosensory area in a strict sense and is called the “SI proper”, because this area receives the densest projection from the specific somatosensory relay nuclei of the thalamus. Area 3b chiefly represents the contralateral body surface in a mediolaterally elongated band of the cortex. Along this mediolateral axis, the tail, lower limb (foot), trunk, upper limb (hand), face, and oral structures are represented in a somatotopical manner. Our current knowledge of the neuroanatomy of the vSI is based largely on studies performed by Professor Jon H. Kaas (see review [10]) and those of Professor Edward G. Jones. The representations of the hand and face are separated by a histologically visible border in both New [11, 12] and Old World monkeys [13–15]. This “hand-face border” can be detected as a myelin-light septum in brain sections cut parallel to the cortical surface. Further laterally, myelin-dense ovals were shown to indicate anatomical modules that correspond to representations of different orofacial structures in both New [16, 17] and Old World monkeys [13, 15]. In New World monkeys, the myelin-dense ovals are arranged in a rostrocaudal direction and extend to the ventral frontal lobe. There, the lips or chin, teeth (periodontal receptors), and tongue are represented in a caudorostral sequence. Further rostrally, the ipsilateral side of the teeth and tongue are represented. In Old World monkeys, the lips (cheek mucosa), teeth, and tongue are represented in a mediolateral sequence. Further anterolaterally, the ipsilateral side of the teeth and tongue are represented. The ipsilateral representation is a distinctive feature of oral structures, such as the teeth and tongue and each side of the oral structure is represented in area 3b of both hemispheres. This was confirmed in a wide range of primates: prosimian primates, such as the African galago [10], New World monkeys, such as the squirrel monkey and owl monkey [16, 18], Old World monkeys, such as macaques [15, 19, 20], and humans (see review [21]). Such corepresentation of the contralateral and ipsilateral sides of oral structures may facilitate the convergence of input from functionally related portions of both sides (bilateral integration described in the next section).
Dense neural interconnections between representations of different orofacial structures have been documented in both New [17] and Old World monkeys [15]. For example, a recent neuroanatomical study of macaques [15] demonstrated that the tongue representation received dense projections from regions representing the lower and upper teeth and tissue lining the inside of the cheek and lips. Such interconnections may partly explain the presence of neurons having composite receptive fields covering different orofacial structures (described later). In contrast, the hand representation, located medially to the orofacial representation, provided little to no input to either the face or mouth representations in both New [12] and Old World monkeys [14, 15] and it follows that the anatomical hand-face border mentioned earlier is also regarded as a functional border. Another interesting observation may be the relation between the tongue representation and the gustatory related regions [15]: the tongue representation uniquely received projections from areas in the anterior upper bank of the lateral sulcus and anterior insula that may include the primary gustatory area (area G) and other taste-related areas. Also in that study, the tongue representation was likely to receive an additive projection from the lateral surface of the frontal operculum near the lateral sulcus, although the investigators did not particularly emphasize this. This region may presumably correspond to the precentral extension of area 3, which was also implicated in gustatory function [22, 23].
3 Neuronal Receptive Fields (RFs) as an Indicator of Hierarchical Information Processing in the vSI
Hierarchical information processing in the vSI was summarized in a previous review [24]. As shown in Fig. 24.2, spatial convergence of somesthetic information arising from orofacial structures proceeds across three cytoarchitectonic areas; i.e., areas 3, 1, and 2 [20, 25], in a manner established in the hand representation (see review [26]). Along this rostrocaudal stream, neuronal receptive fields (RFs) become larger and more complex so that the RFs cover functionally related portions of orofacial structures (composite RFs). The patterns of spatial convergence can be classified into three types (Fig. 24.2): bilateral convergence, intermaxillary convergence, and inter-structural convergence. Furthermore, the spatiotemporal integration also proceeds along this stream: the relative incidence of neurons exclusively responsive to light stroking stimuli (movement-specific neurons) increases moving caudally towards area 2 [27]. Of these, the majority responded with directional selectivity, that is, they responded exclusively to stimuli moving in a particular direction. Most of the movement-specific neurons had ordinally uninterrupted RFs and the remaining had composite RFs discretely covering different structures. The relative incidences of neurons with composite RFs in area 2 were significantly higher in movement-specific neurons than in other neurons, suggesting that the spatiotemporal integration for representing moving objects is accompanied by the convergence of inputs from discrete, but functionally related, oral portions. Such a hierarchical scheme in the vSI might be a prerequisite neural process for dexterous orofacial function and oral stereognosis. The spatial convergence found in awake macaque monkeys was indirectly supported in a subsequent human study using functional MRI [28]. Although neuronal RFs could not be studied in humans, the investigators inspected the degree of activation overlaps between the representations of different oral structures, such as the lips, teeth, and tongue. They showed that the overlap in the middle and caudal portions of the postcentral gyrus was significantly greater than in the rostral portion of the postcentral gyrus.
Schematic drawing showing the convergence of somesthetic information across areas of the vSI and adjacent somatosensory-related cortices. Each circle corresponds to a single neuron and its receptive field (RF, drawn in black). Neurons in area 3b are arranged on the left side. Hand, face, and oral structures are represented in a mediolateral sequence. In at least area 3b, the “hand-face border” limits the interconnection between the hand and orofacial representations. Note that actual RF sizes in area 3b neurons are often considerably smaller than depicted here. On moving caudally from area 3b to area 2, neuronal RFs on the hand and orofacial structures become larger and more complex. The patterns of convergence in orofacial neurons can be classified into three types: bilateral convergence, intermaxillary convergence (e.g., upper and lower lips, palate and tongue dorsum), and inter-structural convergence (e.g., tongue tip and anterior teeth). Further caudally in the inferior parietal cortex (area 7b) or laterally in the secondary somatosensory cortex (SII), RFs often cover both the hand and orofacial structures. These neurons in higher-order brain regions are closely related to self-feeding behavior. For further explanation, see the text
The SI receives not only somesthetic inputs arising from the periphery, but also signals from the frontal lobe related to motor command. It is therefore important to determine the activity of vSI neurons during self-generated orofacial movements. The laboratory of Professor B.J. Sessle has played a pioneering role and is making tremendous contributions to this field (for review, see [9]). Their studies on the vSI (chiefly in areas 3b and 1) documented in a trilogy of papers revealed the relations between the neuronal RF properties and neuronal activity during self-generated movements [29–31]. In the first paper [29], monkeys were trained to perform a tongue-protrusion task and biting task. In the tongue protrusion task, a significant alteration of firing rate was observed in ~80 % of tongue RF neurons and 60 % of lip RF neurons. Of note here is that a substantial proportion of neurons did not change their activity during the task, despite apparent orofacial RFs. Moreover, among the task-related neurons, adaptation characteristics of RFs (slowly adapting or rapidly adapting) could not predict the patterns of neuronal activities during the task. For example, task-related neurons with a slowly adapting type of RF did not necessarily fire in a tonic manner during the task: rather, four types of activity patterns; i.e., phasic, tonic, phasic-tonic, and decreased, were detected during the task. In the second paper [30], the monkeys were required to protrude their tongue in each of three directions: the target was positioned at 0°, 30° to the left, or 30° to the right from the midsagittal plane. Again, laterality of a neuronal RF could not predict the preferred tongue-protrusion direction of the neuron. The results of these two papers strongly suggest that neurons with various RF properties are recruited simultaneously even in a simple self-generated orofacial movement. In the third paper [31], electrical or mechanical stimulations were applied to the RFs of each neuron (except to the lingual nerve for tongue RF neurons) when the monkeys were performing the task. Almost all of the neurons tested showed a decrease in evoked activity during the tongue-protrusion task. This finding indicates that disturbing somesthetic inputs arising from the periphery are gated out during self-generated movements. To summarize, the passive properties of neuronal RFs are indeed a reliable indicator for evaluating the flow of sensory information across different brain regions, but those properties alone cannot explain the actual neuronal behavior during self-generated movements.
The bidirectional neural interconnection between the vSI and the ventral primary motor area (vMI) was established in neuroanatomical studies of New [17] and Old World monkeys [15, 32]. In one study on the vMI, most neurons respond to light tactile stimulation rather than stimulation to deep tissues, such as the muscle and joint, which suggests the particular importance of tactile input in motor control [33]. Another important finding in this study was that neurons with RFs on different orofacial structures were intermingled. This may be partly explained by the presence of neurons with composite RFs in the aforementioned vSI and the direct neural projection from the vSI to the vMI. As has been suggested, such complex organization in the vMI may be a prerequisite neural basis for the motor coordination of various structures. The manner of neuroplastic changes in the vMI, as well as vSI, should also be addressed, because such changes permit the acquisition of new motor skills or adaptation to an altered orofacial environment. Studies on this subject were reviewed in detail by Professor B.J. Sessle and colleagues [9, 34].
From the viewpoint of dental pain, vSI neurons that receive input from the tooth-pulp are also important targets of study [35, 36]. In one study, monkeys were trained to detect changes in tooth-pulp stimulus intensity [36]. Some of the neurons examined were implicated in the sensory-discriminative aspect of tooth-pulp sensation, because their discharge rates were correlated with the detection latency of the monkey.
4 Hand-Mouth Motor Coordination in Self-Feeding Behavior
Motor coordination of the hand and mouth is essential for self-feeding behavior in primates. There should be neurons that integrate somesthetic information arising from both of the body parts somewhere in the brain; however, this subject is not currently well studied with regard to the vSI. In addition, at least in area 3b, such convergence is considered to be rare, because of the presence of the hand-face border. An earlier study of the vSI reported that several neurons in areas 3b or 1 had discrete RFs on both the radial hand and the lateral part of the face [37]. Since those neurons had face RFs that were remote from the oral slit, they are unlikely to relate to feeding behavior. Rather, neuronal activity related to hand-mouth coordination during self-feeding was documented in higher order cortical areas other than the vSI (Fig. 24.2). We review those articles for reference purposes, although this may be beyond the scope of the present paper regarding the SI. The laboratory of Professor A. Iriki has made important contributions to this field. In one study, they explored the inferior parietal cortex (area 7b), which is posteriorly adjacent to the vSI, and found “face-hand neurons” [38]. These neurons had discrete RFs on the lower face (mostly around the mouth or in the oral cavity) and hand, of which about half responded to the specific behavior with synergism between the face and hand movements. That is, these neurons responded more strongly when the animal executed face-hand coordinated behavior (e.g., self-feeding behavior) than when the monkey executed identical movements of the face and hand separately. Some neurons in their sample showed an especially strong response when pinching food or holding it with the mouth during self-feeding, which was suggestive of their role in monitoring a sequence of actions or an entire course of behavior. Based on these observations, they assumed that area 7b is related to the integration and construction of actions. In another study, they reported the presence of similar neurons (“hand-mouth neurons”) in the secondary somatosensory area (SII) lying laterally to the vSI in the upper bank of the lateral sulcus [39]. These neurons also showed activity during both food retrieval with the hand and eating. However, the investigators rarely encountered neurons that fired during self-generated feeding but were inactive during passive feeding separated from food retrieval with the hand: the synergism in area 7b neurons was not detected in SII neurons. Another interesting observation from this study was that the hand-mouth neurons showed activity irrespective of the hand used in retrieving food, which suggests the goal-coding nature of these neurons. In other words, it does not matter for these neurons which part of the body is used to bring food to the mouth.
5 Temporal Analysis of Spiking Activities in Neuronal Populations and Individual Neurons
The neural activity (the train of action potentials) of a single neuron is quite variable across trials even when the same stimulus is repeatedly applied. Therefore, simultaneous recording from different single neurons is necessary to analyze the functional neural circuits in a local brain locus or to study the dynamics of information coding by neuronal ensembles. The introduction of such a technique, especially in the past 10–15 years, has led to numerous achievements in various cortical regions. However, there are only a limited number of analogous studies in the vSI. In one study, we evaluated the difference in temporal profiles between spike trains recorded simultaneously from a pair of nearby vSI neurons [40]. A numerical method was adopted that evaluates the difference in the gross temporal profiles over several hundreds of milliseconds or more during sustained tactile stimulation [41]. There were significant temporal discrepancies in the activity of some pairs of putative pyramidal neurons. We speculated that this might help the brain to monitor the time course of stimulation, such as the onset, duration, and offset. Another recent study [42], using a much larger scale of data, examined whether the population activities of neurons in the vSI and vMI could represent the direction of tongue movement. The investigators adopted the mutual information as a measure of the strength in the relationship between spiking activity and direction of tongue protrusion. They showed that the direction of tongue protrusion was accurately predicted on a trial-by-trial basis from the spiking activity of populations of vSI and vMI neurons by using a discrete decoder.
In addition to the population activity of neurons, the temporal structure of spike trains (firing pattern) of individual neurons is an important subject of study. One of the metrics that evaluates firing pattern, the metric of local variation (LvR), is superior in that the temporal structures can be quantified independently of the firing rate [43]. In this study, the investigators clearly showed dissimilarities in firing-pattern across many cortical areas based on LvR scores: spiking patterns are regular in the motor areas, random in the visual areas, and bursty in the prefrontal area. Such numerical analysis is also needed in future studies of the vSI.
The temporal analysis of neural activities could conceivably lead to future improvement of a brain-machine-brain interface (BMBI). This technology, which was developed to restore normal sensorimotor function in the upper limb, consists of two major elements: one is the neural population recording from motor-related areas that permits subjects to move prosthetic arms or a virtual hand on a screen; the other is intracortical microstimulation (ICMS) of the SI giving rise to artificial tactile sensation [44, 45]. Neurophysiological studies of the monkey vSI will play an important role in determining the optimal ICMS conditions in BMBI for orofacial function and contribute to the future treatment of patients suffering from orofacial sensorimotor disorders.
References
Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–44.
Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist. 2011;17:197–208.
Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51:1183–202.
Zarate JM. The neural control of singing. Front Hum Neurosci. 2013;7:237.
Özdemir E, Norton A, Schlauga G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–35.
Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M. The brain of opera singers: experience-dependent changes in functional activation. Cereb Cortex. 2010;20:1144–52.
Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2002;81:743–6.
Quintero A, Ichesco E, Myers C, Schutt R, Gerstner GE. Brain activity and human unilateral chewing: an fMRI study. J Dent Res. 2013;92:136–42.
Avivi-Arber L, Martin R, Lee JC, Sessle BJ. Face sensorimotor cortex and its neuroplasticity related to orofacial sensorimotor functions. Arch Oral Biol. 2011;56:1440–65.
Kaas JH, Qi HX, Iyengar S. Cortical network for representing the teeth and tongue in primates. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:182–90.
Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex. 1998;8:227–36.
Fang PC, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of New World monkeys. J Comp Neurol. 2002;454:310–9.
Qi HX, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp Neurol. 2004;477:172–87.
Manger PR, Woods TM, Muñoz A, Jones EG. Hand/face border as a limiting boundary in the body representation in monkey somatosensory cortex. J Neurosci. 1997;17:6338–51.
Cerkevich CM, Qi HX, Kaas JH. Corticocortical projections to representations of the teeth, tongue, and face in somatosensory area 3b of macaques. J Comp Neurol. 2014;522:546–72.
Jain N, Qi HX, Catania KC, Kaas JH. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. J Comp Neurol. 2001;429:455–68.
Iyengar S, Qi HX, Jain N, Kaas JH. Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of New World monkeys. J Comp Neurol. 2007;501:95–120.
Manger PR, Woods TM, Jones EG. Representation of the face and intraoral structures in area 3b of the squirrel monkey (Saimiri sciureus) somatosensory cortex, with special reference to the ipsilateral representation. J Comp Neurol. 1995;362:597–607.
Manger PR, Woods TM, Jones EG. Representation of face and intra-oral structures in area 3b of macaque monkey somatosensory cortex. J Comp Neurol. 1996;371:513–21.
Toda T, Taoka M. Hierarchical somesthetic processing of tongue inputs in the postcentral somatosensory cortex of conscious macaque monkeys. Exp Brain Res. 2002;147:243–51.
Sakamoto K, Nakata H, Yumoto M, Kakigi R. Somatosensory processing of the tongue in humans. Front Physiol. 2010;1:136.
Ogawa H, Ito S, Nomura T. Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci Res. 1989;6:283–98.
Hirata S, Nakamura T, Ifuku H, Ogawa H. Gustatory coding in the precentral extension of area 3 in Japanese macaque monkeys; comparison with area G. Exp Brain Res. 2005;165:435–46.
Toda T, Hayashi H. Functional organization in the orofacial region of the postcentral somatosensory cortex. J Oral Biosci. 2010;52:365–70.
Toda T, Taoka M. Converging patterns of inputs from oral structures in the postcentral somatosensory cortex of conscious macaque monkeys. Exp Brain Res. 2004;158:43–9.
Iwamura Y. Hierarchical somatosensory processing. Curr Opin Neurobiol. 1998;8:522–8.
Toda T, Taoka M. Hierarchical neural process to detect moving tactile stimuli in the postcentral oral representation of conscious macaque monkeys. J Oral Biosci. 2005;47:253–62.
Miyamoto JJ, Honda M, Saito DN, Okada T, Ono T, Ohyama K, Sadato N. The representation of the human oral area in the somatosensory cortex: a functional MRI study. Cereb Cortex. 2006;16:669–75.
Lin LD, Murray GM, Sessle BJ. Functional properties of single neurons in the primate face primary somatosensory cortex. I. Relations with trained orofacial motor behaviors. J Neurophysiol. 1994;71:2377–90.
Lin LD, Murray GM, Sessle BJ. Functional properties of single neurons in the primate face primary somatosensory cortex. II. Relations with different directions of trained tongue protrusion. J Neurophysiol. 1994;71:2391–400.
Lin LD, Sessle BJ. Functional properties of single neurons in the primate face primary somatosensory cortex. III. Modulation of responses to peripheral stimuli during trained orofacial motor behaviors. J Neurophysiol. 1994;71:2401–13.
Hatanaka N, Tokuno H, Nambu A, Inoue T, Takada M. Input–output organization of jaw movement-related areas in monkey frontal cortex. J Comp Neurol. 2005;492:401–25.
Huang CS, Hiraba H, Sessle BJ. Input–output relationships of the primary face motor cortex in the monkey (Macaca fascicularis). J Neurophysiol. 1989;61:350–62.
Sessle BJ. Face sensorimotor cortex: Its role and neuroplasticity in the control of orofacial movements. Prog Brain Res. 2011;188:71–82.
Biedenbach MA, Van Hassel HJ, Brown AC. Tooth pulp driven neurons in somatosensory cortex of primates: role in pain mechanisms including a review of the literature. Pain. 1979;7:31–50.
Iwata K, Tsuboi Y, Sumino R. Primary somatosensory cortical neuronal activity during monkey’s detection of perceived change in tooth-pulp stimulus intensity. J Neurophysiol. 1998;79:1717–25.
Dreyer DA, Loe PR, Metz CB, Whitsel BL. Representation of head and face in postcentral gyrus of the macaque. J Neurophysiol. 1975;38:714–33.
Yokochi H, Tanaka M, Kumashiro M, Iriki A. Inferior parietal somatosensory neurons coding face-hand coordination in Japanese macaques. Somatosens Mot Res. 2003;20:115–25.
Taoka M, Tanaka M, Hihara S, Ojima H, Iriki A. Neural response to movement of the hand and mouth in the secondary somatosensory cortex of Japanese monkeys during a simple feeding task. Somatosens Mot Res. 2013;30:140–52.
Toda T, Hayashi H. The gross temporal correlation of nearby neuron activity in the macaque postcentral somatosensory cortex representing orofacial structures: with special reference to numerical methods for analysis. J Oral Biosci. 2011;53:170–81.
Fellous JM, Tiesinga PH, Thomas PJ, Sejnowski TJ. Discovering spike patterns in neuronal responses. J Neurosci. 2004;24:2989–3001.
Arce FI, Lee JC, Ross CF, Sessle BJ, Hatsopoulos NG. Directional information from neuronal ensembles in the primate orofacial sensorimotor cortex. J Neurophysiol. 2013;110:1357–69.
Shinomoto S, Kim H, Shimokawa T, Matsuno N, Funahashi S, Shima K, et al. Relating neuronal firing patterns to functional differentiation of cerebral cortex. PLoS Comput Biol. 2009;5:e1000433. doi:10.1371/journal.pcbi.1000433.
O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MA. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479:228–31.
Medina LE, Lebedev MA, O’Doherty JE, Nicolelis MA. Stochastic facilitation of artificial tactile sensation in primates. J Neurosci. 2012;32:14271–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Toda, T., Kudo, Ta. (2015). The Ventral Primary Somatosensory Cortex of the Primate Brain: Innate Neural Interface for Dexterous Orofacial Motor Control. In: Sasaki, K., Suzuki, O., Takahashi, N. (eds) Interface Oral Health Science 2014. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55192-8_24
Download citation
DOI: https://doi.org/10.1007/978-4-431-55192-8_24
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55125-6
Online ISBN: 978-4-431-55192-8
eBook Packages: MedicineMedicine (R0)