Abstract
The mammals and birds have four chambered hearts. The current models show the majority of myocardial cells derive from the first and second heart field, however, little is known regarding how heart field subpopulations contribute to specific regions in the heart. In this study, we revealed that the early Ednra-positive population in the inflow tract region contributes to the chamber myocardium by mouse-chick chimera and CreERT2/loxP lineage tracing system.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
The avian and mammalian heart mainly originates from two distinct embryonic regions: an early differentiating first heart field and a dorsomedially located second heart field. It remains largely unknown when and how these subpopulations of the heart field are established as regions with different fates.
Endothelin-1 (Edn-1) acts on cardiac neural crest cells through endothelin receptor type A (Ednra) and is involved in the normal formation of pharyngeal artery-derived great vessels and ventricular septum [1]. Previously, we identified a distinct cell population defined by the expression of Ednra in the mouse inflow region [2]. These cells are derived from a part of the first heart field, and largely confined to the inflow region at E8.25.
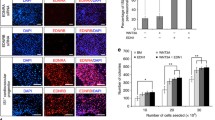
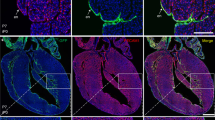
From the expression patterns of β-gal in the Ednra-lacZ knock-in mice, we thought a possibility that this Ednra-positive cell population might move into cardiac chambers from the inflow region. By dye injection and transplantation experiments, we showed that the Ednra-positive cell population moved toward not only the left and right atria but also the left ventricle.
Then, to perform lineage analysis, we have generated an Ednra-CreERT2 mouse line (unpublished). We activated Cre recombinase by tamoxifen at E7.25 and E8.25 and analyzed the distribution of β-gal-labeled cells in the EdnraCreERT2/+;R26R embryo hearts. As a result, the β-gal-labeled cells were found to contribute to the left ventricle and both atria.
To facilitate this lineage analysis, we established a mouse-chick chimera model. When we transplanted the inflow region of the EdnraCreERT2/+;R26R mouse embryos (tamoxifen i.p. at E7.25) to orthotopically into chick embryos, β-gal-positive cells were detected in the right atrium and left ventricles 7 days after transplantation.
From these results, we conclude that the Ednra-positive cell lineage in the early inflow tract certainly contribute to chamber myocardial formation.
References
Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–10.
Asai R, Kurihara Y, Fujisawa K, Sato T, Kawamura Y, Kokubo H, Tonami K, Nishiyama K, Uchijima Y, Miyagawa-Tomita S, Kurihara H. Endothelin receptor type A expression defines a distinct cardiac subdomain within the heart field and is later implicated in chamber myocardium formation. Development. 2010;137:3823–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Asai, R. et al. (2016). Endothelin Receptor Type A-Expressing Cell Population in the Inflow Tract Contributes to Chamber Formation. In: Nakanishi, T., Markwald, R., Baldwin, H., Keller, B., Srivastava, D., Yamagishi, H. (eds) Etiology and Morphogenesis of Congenital Heart Disease. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54628-3_40
Download citation
DOI: https://doi.org/10.1007/978-4-431-54628-3_40
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54627-6
Online ISBN: 978-4-431-54628-3
eBook Packages: MedicineMedicine (R0)