Abstract
The ductus arteriosus (DA) is a fetal vessel bypassing the still nonfunctional lungs. Closure of the DA at birth is essential for the transition from a fetal to a neonatal circulation. This closing process begins with a physiological contraction followed by definitive anatomical closure. The latter process starts already before birth by development of intimal thickening followed after birth by degeneration of the inner media, including cytolytic necrosis and apoptosis. The DA will remain patent when there is insufficient maturation in prematurely born babies or when there is a structural abnormality as seen in persistent DA (PDA). The histological changes during normal DA closure resemble the features seen in the premature ageing vessels in children with the Hutchinson progeria syndrome. The latter syndrome is caused by a mutation in the lamin A/C gene resulting in accumulation of the progerin splice variant. We studied human DA biopsies from the fetal to the neonatal period to investigate whether lamin A/C and progerin might be involved in the DA closure process. The results show an increase in the intima and inner media of progerin in the normal neonatal DA, while expression of lamin A/C is diminished. In the non-closing aorta, the fetal DA and the PDA, no or hardly any progerin expression was found. We postulate that the lamin A/C to progerin balance is important during normal anatomical closure of the DA presenting a unique case of physiological premature vascular ageing.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
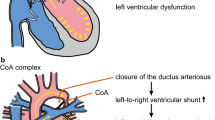
The ductus arteriosus (DA) is a fetal vessel that connects the pulmonary trunk to the distal aortic arch. The patent DA is functional before birth directing oxygen-rich blood from the placenta to the systemic circulation bypassing the still immature lung vascular bed. After birth, the DA contracts, and this physiological closure is followed by definitive anatomical closure. Ultimately, the DA will be remodeled into a fibrous strand [1, 2]. The physiological closure is regulated by many substances in which prostaglandins play an important role [3]. The onset of anatomical closure in the human fetus starts already in the second trimester of gestation with blebbing of the endothelium and formation of intimal thickening [2]. The degenerative changes in the media, in which cytolytic necrosis and apoptosis play an important role [4], start immediately after birth. By then, marked intimal thickening supports the closure of the DA upon contraction. This maturation process of ductal closure (Fig. 34.1) follows a temporo-spatial pattern. Upon premature delivery of the baby, the DA closure process may not be effective already resulting in an immature patent DA. Usually, this resolves spontaneously within a few days to weeks after birth. The process might be enhanced by providing prostaglandin inhibitors like indomethacin [2]. Another reason for non-closure resulting in a persistent DA (PDA) may be structural abnormalities, in most cases based on an elastin deposition problem [3]. The histological features of normal DA closure resemble atherosclerotic changes in the vessel wall albeit without the atheroma component [5]. The mature DA vessel wall structure is highly similar to the premature ageing histopathology seen in vessels of children with the Hutchinson-Gilford syndrome (HGPS) [6]. This syndrome is based on a genetic defect in the lamin A (LMNA) gene which leads to pathological accumulation of the splice variant progerin [7, 8]. Increased progerin expression has also been reported during normal vascular ageing [9, 10]. Based on the observed similarities, we investigated the role of an LMNA/progerin balance during normal perinatal closure of the DA.
2 Material and Methods
Following aortic arch reconstruction and surgical closure of the DA, a biopsy specimen of human neonatal DA (n = 16) and adjoining descending aorta (n = 2) was obtained. The fetal DA (one of 14 weeks’ and one of 18 weeks’ gestation) was acquired from postmortem fetal specimen after legal or spontaneous abortion. One biopsy specimen was obtained from a 2-year-old child with PDA. Details on sectioning, fixation, and immunohistochemistry have been described as well as the technique for RNA isolation and RT-PCR reactions [11].
3 Results
The RT-PCR studies of the aorta and neonatal DA revealed that next to lamin A also progerin was detected. Lamin A was seen both in the aorta and neonatal DA, while low levels of progerin were only seen in the neonatal DA [11]. The elastin expression showed clearly that the DA is a muscular artery, while the aorta is an elastic artery (Fig. 34.2a, d, e, j). Immunohistochemistry of the tissue sections revealed alpha smooth muscle actin to be present in all cases studied both in the intima (when thickened) and in the media. The expression level of alpha smooth muscle actin became less in the cases of a normal closing neonatal DA where inner media degeneration was seen with developing cytolytic necrosis (Fig. 34.2a, b). Furthermore, the expression of lamin A/C was found in all DA and the aorta with exception of the 14-week fetal DA. There was a clear difference with regard to progerin expression (Fig. 34.3). This was only found in the normal closing neonatal DA. If the latter was already fully developed including apoptosis and loss of nuclei, the progerin expression was diminished. This shows that progerin expression precedes apoptosis and degeneration. Progerin was not detected in the fetal 18-week DA, being also absent in the non-closing PDA and aorta.
Transverse histological sections of the ductus arteriosus (DA) and the aorta (AO). (a) Overview of the DA wall with a resorcin-fuchsin staining (RF) showing the morphology of a muscular artery with an internal elastic membrane (IEL) (a, d) between the intima (I) and the media (m). In the inner media, there is cytolytic necrosis (CN), which is better appreciated by the alpha smooth muscle actin loss (actin) in the smooth muscle cells (b). The endothelial cells (ECs) are negative for actin, also in the AO (f, g). (e) Overview of the elastic wall of the AO with a regular manifestation of elastic lamellae (j) and marked actin staining in the smooth muscle cells (k). There is no CN in the aorta. (c, h, i, l) In both the DA and AO lamin A/C, positive cells are found. ECs are negative for lamin A/C. Adventitia: A
(a) Overview of the intima (I) and media (M) of a 10-day-old neonatal ductus arteriosus (DA) with faint cytoplasmic and strong nuclear and perinuclear expression of progerin in the vascular smooth muscle cells of the media (c) and intima (d). The endothelial cells (ECs) are negative (b, d). (e) In the aortic wall (AO), no regions with strong progerin expression are found (g, h). ECs are also negative (f, h). IEL internal elastic lamina. Bars = 30 μm
4 Discussion
Normal ductal closure is regulated by many molecular pathways [3]. In our study, we introduced for the first time a role for lamina A/C and progerin [11]. This observation was triggered by the histopathology found in the vessel wall of young adolescents with the HGPS [8], in which premature atherosclerosis was observed. The physiological development of prenatal intimal thickening and the development of cytolytic necrosis in the DA showing contractions after birth mimic the degenerative changes seen in the HGPS. Alterations in the farnesylation process can be the cause of accumulation of progerin at the nuclear membrane. This process is associated with the initiation of apoptosis in these progerin-expressing cells [12], being characteristic for degeneration in the DA media [13]. The cause for progerin increase in the closing DA needs further investigation, but it is known that alternative splicing is affected by oxidative stress [14] which is activated during normal DA closure [15]. Accumulation of reactive oxygen species has also been observed during vascular ageing [10] in which an increase of progerin has been described in the smooth mus cle cells of the coronary vessels of elderly persons as well as a gradual increase of progerin-expressing cells in other vessels with upclimbing age [7, 10]. It is noteworthy that the non-closing PDA, although only one case has been studied, and the aorta did not show progerin increase in the neonatal stage. This aspect needs further study as in families with bicuspid aortic valve (BAV) a correlation with PDA [16] has been reported. Unpublished data from our group show a diminished progerin expression in the dilated ascending aorta in BAV as compared to dilation of the aorta in tricuspid valves. The latter shows an increase in progerin indicative of advanced ageing.
Potentially, ductal closure based on increased progerin expression could lead to fetal demise in HGPS; this has, however, not been reported.
5 Future Directions and Clinical Applications
Further research is necessary to elucidate the role of the lamin A/C to progerin balance in vascular pathology, ageing, and the unique observation in selective DA closure at birth.
Clinical applications might be related to the use of farnesyltransferase inhibitors which may prevent onset and progression induced by the accumulation of progerin [17]. It cannot be excluded that inhibition of physiological progerin expression might prevent anatomical closure of the DA, which can be relevant in ductus-dependent congenital heart disease.
References
Gittenberger-de Groot AC. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977;39:610–8.
Gittenberger-de Groot AC, van Ertbruggen I, Moulaert AJMG, et al. The ductus arteriosus in the preterm infant: histological and clinical observation. J Pediatr. 1980;96:88–93.
Bokenkamp R, DeRuiter MC, van Munsteren C, et al. Insights into the pathogenesis and genetic background of patency of the ductus arteriosus. Neonatology. 2009;98:6–17.
Gittenberger-de Groot AC, Strengers JLM. Histopathology of the arterial duct (ductus arteriosus) with and without treatment with prostaglandin E1. Int J Cardiol. 1988;19:153–66.
Slomp J, van Munsteren JC, Poelmann RE, et al. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis. 1992;93:25–39.
Goldman RD, Shumaker DK, Erdos MR, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8.
Olive M, Harten I, Mitchell R, Beers JK, Djabali K, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–9.
Stehbens WE, Wakefield SJ, Gilbert-Barness E, et al. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol. 1999;8:29–39.
McClintock D, Ratner D, Lokuge M, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2:e1269.
Ragnauth CD, Warren DT, Liu Y, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–10.
Bökenkamp R, Raz V, Venema A, et al. Differential temporal and spatial progerin expression during closure of the ductus arteriosus in neonates. PLoS One. 2011;6:e23975.
Bridger JM, Kill IR. Aging of Hutchinson-Gilford progeria syndrome fibroblasts is characterised by hyperproliferation and increased apoptosis. Exp Gerontol. 2004;39:717–24.
Tananari Y, Maeno Y, Takagishi T, et al. Role of apoptosis in the closure of neonatal ductus arteriosus. Jpn Circ J. 2000;64:684–8.
Kajimoto H, Hashimoto K, Bonnet SN, et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria derived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation. 2007;115:1777–88.
Slomp J, Gittenberger-de Groot AC, Glukhova MA, et al. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol. 1997;17:1003–9.
Glancy DL, Wegmann M, Dhurandhar RW. Aortic dissection and patent ductus arteriosus in three generations. Am J Cardiol. 2001;87:813–5.
Capell BC, Olive M, Erdos MR, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci U S A. 2008;105:15902–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Gittenberger-de Groot, A.C. et al. (2016). Progerin Expression during Normal Closure of the Human Ductus Arteriosus: A Case of Premature Ageing?. In: Nakanishi, T., Markwald, R., Baldwin, H., Keller, B., Srivastava, D., Yamagishi, H. (eds) Etiology and Morphogenesis of Congenital Heart Disease. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54628-3_34
Download citation
DOI: https://doi.org/10.1007/978-4-431-54628-3_34
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54627-6
Online ISBN: 978-4-431-54628-3
eBook Packages: MedicineMedicine (R0)