Abstract
The significance of the epicardium that covers the heart and the roots of the great arteries should not be underestimated as it is a major component with impact on development, disease, and repair. The epicardium differentiates from the proepicardial organ located at the venous pole (vPEO). The differentiation capacities of the vPEO into epicardium-derived cells (EPDCs) have been extensively described. A hitherto escaped part of the epicardium derives from a second proepicardial organ located at the arterial pole (aPEO) and covers the intrapericardial part of the aorta and pulmonary trunk. In avian and mouse embryos, disturbance of epicardium differentiation causes a spectrum of cardiac anomalies including coronary artery abnormalities, deficient annulus fibrosis with rhythm disturbances, valve malformations, and non-compaction cardiomyopathies. Late in prenatal life the epicardium becomes dormant, losing the activity of many genes.
In human cardiac diseases, both arterial and venous epicardium can be activated again into EPDCs. The epicardial reactivation observed after experimental myocardial infarction and during aneurysm formation of the ascending aorta provides clinical relevance. EPDCs applied for cell therapy demonstrate repair processes synergistic with the resident cardiac progenitor stem cells that probably share an embryonic origin with EPDCs. Future therapeutic strategies might be possible addressing cell autonomous-based and signaling capacities of the adult epicardium.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Origin of the Epicardium
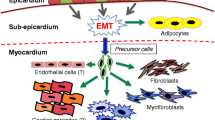
The cardiogenic mesoderm is referred to as first heart field, flanked medially by second heart field (SHF) mesoderm. The addition of SHF-derived cardiac mesoderm enables the formation of all cardiac components. A secondary layer will cover the complete myocardial tube (Fig. 2.1) and the developing roots of the great arteries. At the venous pole, the vPEO develops from which the epicardium (cEP) spreads over the cardiac tube up to the ventriculo-arterial junction [1]. Here, the cEP meets the PEO located at the arterial pole (Fig. 2.2) [2] forming arterial epicardium (aEP) that is continuous with the pericardium. Both PEO structures and the spreading cEP and aEP express Wilms’ tumor-1 suppressor gene (WT-1) among others and harbor endothelial and mesenchymal cells.
Four-chamber view of a mouse heart (ED12.5) immunostained for WT1. Note brown cells lining the pericardial cavity (PC), including the epicardium. The AV groove is indicated (arrows). The left ventricular (LV) wall contains hardly EPDCs, whereas some are present in the RV and in the interventricular septum (IVS). Note the presence of EPDCs at the border of the AV cushion and the interatrial septum (short arrows)
2 Epicardium-Derived Cells (EPDCs)
Epicardial cells lose epithelial contacts by epithelial–mesenchymal transition (EMT) and EPDCs relocate subepicardially [3, 4]. Early EPDCs invade the thin myocardial wall. Proliferation of the myocardium depends on both endocardial- and epicardial-derived signals, including Raldh2 [5] with a spatiotemporal difference between right and left ventricle (Fig. 2.1). The aEP starts EMT slightly later than cEP, and ensuing EPDCs can be detected in the outer layers of the developing great arteries and at the myocardial–endocardial cushion interface (Fig. 2.2). Epicardial heterogeneity with a subset of cells taking part in the initial EMT wave provides the myocardium with the main number of the future interstitial fibroblasts. The next wave of EMT correlates with the formation of the fibrous atrioventricular annulus [6] and contributes to part of the atrioventricular cushion cells. At the ventriculo-arterial junction aEP migrates into the outflow tract forming the future arterial annuli and partake in the semilunar valves [7].
3 Heterogeneity of Epicardial Cells
3.1 The Cardiac Fibroblast
The epicardium is the main source of the interstitial, the fibrous annulus, and the coronary adventitial fibroblasts [7, 8]. The endocardial cells lining the cushions are the other source of the valve fibroblasts [3].
3.2 Arterial Smooth Muscle Cell
After ingrowth of the peritruncal coronary capillary plexus into the aorta, EPDCs surround the main coronary vessels and differentiate into smooth muscle cells (SMC). Differentiation into SMCs is regulated by many genes including FGF, VEGF, Notch, SRF, and PDGFRb and their ligands [8]. Quail–chicken chimera studies demonstrated the timing of EMT and required cell interactions [4].
3.3 Endothelial Cells
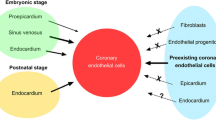
The origin of the coronary endothelium is still under debate. Using a quail vPEO transplanted into the isochronous chick as reviewed in [10] pointed out that coronary ECs do not derive from the coelomic lining but from endothelial cells from the sinus venosus sprouting into the stalk of the vPEO. This is also supported by studies using transgenic mice [11]. We have shown that the sinus venosus-derived endothelial cells express the “arterial” Notch1, underlining the plasticity of these cells [9]. The discussion on the origin of the coronary endothelium is kept alive as specific compartments produce restricted numbers of ECs [12, 13].
3.4 Cardiomyocytes
Conditional reporter mice studies, using WT-1 and Tbx18 as epicardial marker, suggested that a population of EPDCs might differentiate into a myocardial phenotype. However, based on interaction between BMP and FGF, some of the progenitors of cardiomyocytes in the SHF share the same markers with cells in the underlying mesoderm of the SHF and the epicardial population [14]. Other data, including quail–chicken chimera, also do not support EPDC–cardiomyocyte transition [3].
3.5 The Purkinje Fiber
The Purkinje fiber is a specialized cardiomyocyte induced by endothelin produced by endocardium and endothelium. We have postulated an essential interaction between EPDCs and endocardial-/endothelial-derived factors after vPEO tracing and inhibition experiments [15].
4 Congenital and Adult Cardiac Disease
In a large-screen microarray [16], no epicardium-specific gene has been encountered. Therefore, it is challenging to attribute specific cardiac malformations and diseases to epicardial malfunctioning. However, in animal models it is possible to link the epicardium to certain cardiac defects and diseases. We are dealing with complex tissues in which epicardial cells are essential.
4.1 Non-compaction
The most relevant cardiomyopathy resulting from abnormal EPDC function is the primary left ventricular non-compaction cardiomyopathy [4] demonstrating a spongious myocardium usually including the ventricular septum. Differences in amount and timing of LV and RV invasion by EPDCs might account for predilection of the LV. With respect to congenital heart disease, a spongious ventricular septum can be connected with muscular VSDs.
4.2 Conduction System Anomalies
The main components of the conduction system are myocardial in origin. Clinically, it has been hypothesized that the genetically determined long QT syndrome is linked to abnormal Purkinje fiber function. Indirectly, the abnormal formation of the fibrous annulus with persisting accessory pathways can result in reentry tachycardias. PEO inhibition in chick embryos showed defective atrioventricular isolation, delaying the shift from a base-to-apex to an apex-to-base conduction [17].
4.3 Valvulopathies
PEO inhibition can lead to deficient AV valve formation [18, 19]. Furthermore, abnormal differentiation including defective undermining of the valve leaflet is similar to Ebstein’s anomaly of the tricuspid valve as observed in combination with accessory pathways [4]. EPDCs of aEP origin are found in the outflow tract cushions (Fig. 2.2) probably acting via Notch signaling and hence influencing bicuspid aortic valve formation.
4.4 Coronary Vascular Anomalies
Experimental studies disturbing normal coronary development result in a number of malformations that associate human congenital pattern variations with abnormal ventriculo-coronary-arterial communications (fistulae). Fistulae found in avian models with absent coronary arterial orifices in the aorta [20] resembling coronary malformations found in pulmonary atresia without VSD are hypothesized to be a primary coronary vascular disease [21].
5 Cardiovascular Repair
Myocardial infarction . Different approaches have focused on the potential of the adult epicardial cell after myocardial infarction (MI). A c-kit-positive subepicardial population indicates renewed epicardial activity with acquisition of stem cell characteristics [22]. Using a retrovirally induced fluorescent Katushka labeling of dormant epicardium showed EPDCs that migrated into the myocardium and differentiated into a myofibroblast phenotype. The activated epicardium and EPDCs reexpressed WT1 not only in the MI border zone but also in remote areas. Another approach is represented by epicardial cells cocultured with cardiomyocytes in which EPDCs change myocardial alignment and contraction [23]. A direct approach was provided by grafting human adult atrial epicardial cells [24] cultured in vitro from a cobblestone epithelium into spindle shape, thereby acquiring characteristics of mesenchymal stem cells. Injection of these adult human EPDCs into immune-incompetent mice resulted in a marked improvement of cardiac function [24] indicative of repair. Combined injection with adult human cardiomyocyte progenitors (CMPCs) aimed at induction of cardiomyocyte regeneration [25] which showed an additive effect on remodeling, although no new cardiac cell types (endothelial cells, fibroblasts, SMCs, or cardiomyocytes) could be traced to human origin. The capacities, expressed within the normal embryonic state, seem to be preserved in adult life and in disease states.
6 Future Directions and Clinical Applications
Many positive effects of EPDCs either after injection or by stimulation of the dormant native epicardial covering of the heart are due to a paracrine mechanism [22, 24, 25]. These findings bear important potential for drug and cell-based therapeutic approaches to stimulate the native epicardium in repair of the ischemic cardiac wall. An underdeveloped area is the priming of grafts taken from the pericardium for use in cardiac or arterial repair.
References
Vrancken Peeters M-FM, Mentink MMT, Poelmann RE, et al. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat Embryol. 1995;191:503–8.
Perez-Pomares JM, Phelps A, Sedmerova M, et al. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev Dyn. 2003;227:56–68.
Gittenberger-de Groot AC, Vrancken Peeters M-PFM, Mentink MMT, et al. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–52.
Lie-Venema H, van den Akker NMS, Bax NA, et al. Origin, fate, and function of epicardium-derived cells (EPCDs) in normal and abnormal cardiac development. Sci World J. 2007;7:1777–98.
Kikuchi K, Holdway JE, Major RJ, et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell. 2011;20:397–404.
Gittenberger de Groot AC, Winter EM, Bartelings MM, et al. The arterial and cardiac epicardium in development, disease and repair. Differentiation. 2012;84:41–53.
Zhou B, von Gise GA, Ma Q, et al. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–61.
Smith CL, Baek ST, Sung CY, et al. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–26.
Van Den Akker NM, Winkel LC, Nisancioglu MH, et al. PDGF-B signaling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev Dyn. 2008;237:494–503.
Winter EM, Gittenberger-de Groot AC. Cardiovascular development: towards biomedical applicability: epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703.
Merki E, Zamora M, Raya A, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–60.
Katz TC, Singh MK, Degenhardt K, et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 2012;22:639–50.
Tian X, Hu T, Zhang H, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013;23:1075–90.
Kruithof BP, van Wijk B, Somi S, et al. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–22.
Eralp I, Lie-Venema H, Bax NAM, et al. Epicardium-derived cells are important for correct development of the Purkinje fibers in the avian heart. Anat Rec. 2006;288A:1272–80.
Bochmann L, Sarathchandra P, Mori F, et al. Revealing new mouse epicardial cell markers through transcriptomics. PLoS One. 2010;5(e11429):1–13.
Kolditz DP, Wijffels MCEF, Blom NA, et al. Persistence of functional atrioventricular accessory pathways in post-septated embryonic avian hearts: implications for morphogenesis and functional maturation of the cardiac conduction system. Circulation. 2007;115:17–26.
Gittenberger-de Groot AC, Vrancken Peeters M-PFM, Bergwerff M, et al. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 2000;87:969–71.
Briggs LE, Kakarla J, Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84:117–30.
Eralp I, Lie-Venema H, DeRuiter MC, et al. Coronary artery and orifice development is associated with proper timing of epicardial outgrowth and correlated Fas ligand associated apoptosis patterns. Circ Res. 2005;96:526–34.
Gittenberger-de Groot AC, Tennstedt C, Chaoui R, et al. Ventriculo coronary arterial communications (VCAC) and myocardial sinusoids in hearts with pulmonary atresia with intact ventricular septum: two different diseases. Prog Pediatr Cardiol. 2001;13:157–64.
Limana F, Bertolami C, Mangoni A, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(þ) cells: role of the pericardial fluid. J Mol Cell Cardiol. 2010;48:609–18.
Weeke-Klimp A, Bax NA, Bellu AR, et al. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J Mol Cell Cardiol. 2010;49:606–16.
Winter EM, Grauss RW, Hogers B, et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116:917–27.
Winter EM, Van Oorschot AA, Hogers B, et al. A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail. 2009;2:643–53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Gittenberger-de Groot, A.C., Winter, E.M., Goumans, M.J., Bartelings, M.M., Poelmann, R.E. (2016). The Arterial Epicardium: A Developmental Approach to Cardiac Disease and Repair. In: Nakanishi, T., Markwald, R., Baldwin, H., Keller, B., Srivastava, D., Yamagishi, H. (eds) Etiology and Morphogenesis of Congenital Heart Disease. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54628-3_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-54628-3_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54627-6
Online ISBN: 978-4-431-54628-3
eBook Packages: MedicineMedicine (R0)