Abstract
Interleukin-22 (IL-22), an IL-10 family cytokine, is produced by various leukocytes. The receptor of IL-22, however, is preferentially detected on peripheral tissue epithelial cells. IL-22 functions as a unique messenger from immune system to tissue epithelial cells and to regulate homeostasis of epithelia. IL-22 is able to directly enhance antimicrobial defense mechanisms in epithelial cells and to facilitate epithelial barrier repair and wound healing process. It, therefore, possesses an irreplaceable role in host defense against certain pathogens that specifically invade epithelial cells. In addition, IL-22 can help to preserve the integrity and homeostasis of various epithelial organs during infection or inflammation. The importance of its tissue-protective function is manifested in many inflammatory situations such as inflammatory bowel diseases (IBD) and hepatitis. On the other hand, as a cytokine, IL-22 is capable of induction of proinflammatory responses, especially in synergy with other cytokines. Consequently, IL-22 contributes to pathogenesis of certain inflammatory diseases for example psoriasis.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 IL-22 and IL-22 Receptors
IL-22 was originally cloned from IL-9-treated mouse T cells in 2000 and named IL-TIF (IL-10 related T-cell-derived inducible factor) (Dumoutier et al. 2000a). The human orthologue was subsequently identified with 79 % identity to mouse IL-22 and 25 % identity to human IL-10 (Dumoutier et al. 2000c; Xie et al. 2000). Because of the weak but significant homology with IL-10, IL-22 was classified as a member in the IL-10 cytokine family, along with IL-10, IL-19, IL-20, IL-24, IL-26, IL-28A, IL-28B, and IL-29 (Ouyang et al. 2011). Cytokines from the IL-10 family share 20–30 % amino acid identity and also a weaker homology with cytokines in the interferon (IFN) family. Together, IL-10 and IFN cytokine families form the class II cytokine superfamily.
1.1 Gene and Structure of IL-22
The human IL-22 gene consists of five introns and six exons and is located on chromosome 12q15, adjacent to genes of IFN-γ and IL-26. The isolated cDNA encodes a 179-amino-acid-long protein with a 33-amino-acid signal peptide (Dumoutier et al. 2000b). Sharing structural similarity with other IL-10 family members, IL-22 contains six α-helices (A–F), forming a bundle structure with a compact hydrophobic core inside (Fig. 6.1a). In contrast to IL-10, which forms an intertwined dimer for receptor binding, IL-22 is a monomer (Nagem et al. 2002). IL-22 is heavily glycosylated with three N-linked glycosylation sites (Asn-X-Ser/Thr) at asparagines (Asn) -54, -68 and -97) (Fig. 6.1a) (Xu et al. 2004). However, the biological function of IL-22 is not fully dependent on glycosylation. The Escherichia coli-derived protein has normal biological activity without glycosylation. In fact, comparison of the IL-22 structures purified from Drosophila and E. coli reveals that the glycosylation only causes minor structural changes (Xu et al. 2005). There are four cysteines within IL-22 to create two disulfide linkages (Cys40–Cys130, Cys89–Cys178). The first disulfide bond holds together the N-terminal coil and the DE loop, and the second disulfide bond stabilizes the N-terminus of helix C and the C-terminus of helix F (Fig. 6.1a) (Nagem et al. 2002).
Structure of human interleukin (IL)-22 and IL-22R1. (a) Crystalline structure of IL-22 from IL-22–IL-22R1 complex (Protein Data Bank ID code 3DGC). Six helices (A–F) are colored from the N-terminus (blue) to C-terminus (red). Three glycosylation sites (N54, N68, N97) are labeled with arrowed lines. Two disulfide bonds (C40–C132, C89–C178) are shown with blue double-arrow lines. (b) Complex of IL-22–IL-22R1. IL-22R1 (dark grey) contains D1 and D2 domains. Critical amino acids for IL-22R1 and IL-10R2 binding on IL-22 are labeled
IL-22 interacts with the heterodimeric membrane-spanning receptor complex, IL-22R1 (also known as IL-22Ra1, CRF2-9, and zcytor11), and IL-10R2. Both subunits belong to the class II cytokine receptor or interferon receptor family with signature fibronectin type III domains (Ouyang et al. 2011). The gene of IL-22R1 is located on chromosome 1 and encodes a 574-amino-acid protein that contains an extracellular ligand-binding domain, a membrane-spanning helix, and an intracellular domain (Xie et al. 2000). Il-10r2 is located on chromosome 21 and shares a similar organization with a much shorter cytoplasmic tail (Kotenko et al. 1997). IL-10R2 is a shared common receptor chain for several other IL-10 family cytokines including IL-10, IL-26, IL-28A, IL-28B, and IL-29 (Ouyang et al. 2011). The finding of receptor sharing within the same family is not uncommon, as the common γ-chain is shared by multiple cytokines. Similarly, IL-22R1 can also pair with IL-20R2 to engage the binding of IL-20 and IL-24. Although IL-10R2 is ubiquitously expressed, IL-22R1 is primarily detected on cells with epithelial origin.
1.2 Receptor Binding
As a monomer, IL-22 can form a 1:1 complex with the soluble IL-22R1 extracellular domain in solution. The two tandem fibronectin domains (D1 and D2) of IL-22R1 rotate to an angle to form an L-shape, where the interface comes in contact with IL-22 residues on helix A, the AB loop, and helix F (Fig. 6.1b). Several residues that are critical for IL-22R1 binding are located in helix F (Lys-162, Glu-166, Met-172, Arg-175) and the AB loop (Thr-70), and create either hydrogen bonds or van der Waals interactions between IL-22 and IL-22R1 (Jones et al. 2008). The specific residues in contact surfaces ensure the specificity between IL-22-IL-22R1 interactions and are distinct from other cytokine–receptor complexes in the family. IL-10R2 comes in contact with IL-22/IL-22R1 complex at three distinct surface sites. Helices A and B in IL-22 are predominantly engaged in binding to two of three sites on IL-10R2 whereas the third site interacts with IL-22R1. In contrast to IL-22R1-binding sites where most of the residues locate toward the C-terminus of IL-22, IL-10R2-binding spots on IL-22 are mainly located on the N-terminal end of the helix, including residues Tyr-51, Asn-54, Arg-55, Tyr-114, and Glu-117 (Fig. 6.1b) (Logsdon et al. 2004). The formation of the IL-22–IL-22R1–IL-10R2 complex is sequential. Surface plasmon resonance studies revealed high-affinity binding of IL-22 to IL-22R1 (20 nM) but very weak affinity (120 μM) to IL-10R2 (Logsdon et al. 2004). In fact, IL-10R2 displays a diminished affinity to IL-22 with a substantially increased affinity to the IL-22/IL–22R1 complex (Logsdon et al. 2002).
1.3 IL-22-Binding Protein
A protein that shares 34 % sequence identity with the extracellular region of IL-22R1 was discovered by screening genomic DNA databases and named IL-22-binding protein (IL-22BP, also known as IL-22Ra2, CRF2-10, CRF2-s1, and ZcytoR16). IL-22BP lacks transmembrane and intracellular domains and functions as a naturally occurring IL-22 antagonist (Gruenberg et al. 2001; Kotenko et al. 2001; Logsdon et al. 2002; Weiss et al. 2004; Xu et al. 2001). Soluble receptors or membrane-bound decoy receptors that serve as negative regulators for cytokines have previously been found for other cytokines, such as IL-1 and IL-18, but IL-22BP is the only naturally expressed soluble receptor discovered in the class II cytokine receptor family. IL-22BP is encoded by a distinct gene on chromosome 6, in a proximal distance of the class II cytokine receptor cluster containing Il-20r1 and ifngr1. Although IL-22BP shares weaker homology with other receptors of the IL-10 family cytokine such as IL-10R1 (29 %), it only specifically blocks IL-22 signaling. IL-22BP is expressed in a wide range of tissues including lymphoid and nonlymphoid tissues with three mRNA variants produced by alternative splicing, and the variant-2 with 231 amino acids neutralizes IL-22 activity. IL-22BP structurally resembles IL-22R1 with two disulfide bonds stabilizing D1 and D2 domains, and the interaction interface to IL-22 is located within IL-22 helix A, the AB loop, and helix F. In contrast to IL-22R1, IL-22BP forms five completely different salt bridges or hydrogen bonds, and a hydrophobic cluster with Phe-57, Phe-171, and Met-172 on IL-22. The binding affinity of IL-22BP to IL-22 (~1 pM) is significantly higher than IL-22–IL-22R1 (~nM), illuminating the tight regulation of IL-22BP on IL-22 activity (Jones et al. 2008).
1.4 Signal Transduction
Upon binding to the receptor complex, IL-22 activates Janus Kinase (Jak) and Signal Transducer and Activator of Transcription (Stat) signaling pathways, particularly, phosphorylation of tyrosine kinases Jak1 and Tyk2 (Fig. 6.2). Subsequently, transcription factor Stat3 is activated, and in some cells phosphorylation of Stat1 and Stat5 may also occur (Dumoutier et al. 2000a, 2003; Lejeune et al. 2002; Xie et al. 2000). A serine residue of Stat3 (Ser 727) can be phosphorylated by IL-22 stimulation, and this serine phosphorylation is required to achieve maximal transactivation (Lejeune et al. 2002). In the intracellular tail of the IL-22R1 chain, there are in total eight tyrosine residues that can be phosphorylated by activated Jak1 or Tyk2 and serve as potential docking sites for Stat molecules upon IL-22 stimulation. Interestingly, although these Tyr residuals are necessary for the binding of Stat5 and Stat1 to IL-22R1, they are dispensable for Stat3 activation (Dumoutier et al. 2009). Instead, the coiled-coil domain of Stat3 can bind directly to the C-terminal tail of the IL-22R1 chain (Fig. 6.2). The biological significant of this finding is unclear at present. In vivo, Stat3 is directly activated by IL-22 in epithelia and is necessary in mediating the downstream biological effects of IL-22 (Pickert et al. 2009; Sugimoto et al. 2008; Zheng et al. 2007). In addition to Stat molecules, IL-22 is able to activate key kinases MEK1/2, ERK1/2, JNK, and p38 of MAP kinase pathways, as well as Akt, SOCS-3, and transcription factors NF-κB and AP-1 in different cell types (Andoh et al. 2005; Brand et al. 2006).
IL-22 binds to receptors and activates downstream signaling transduction. The engagement of IL-22 to the IL-22R1–IL-10R2 complex activates Jak/Stat pathways and primarily signals through Stat3. IL-22R can associate with Stat3 in a tyrosine-independent manner. Stat1, Stat5, and MAP kinase pathways can be activated under certain conditions. BP, binding protein
2 Cellular Sources and Regulation of IL-22
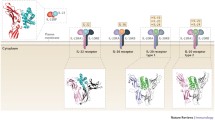
IL-22 is broadly expressed by many types of leukocytes, including CD4+ T cells, CD8+ T cells, and γδ T cells, as well as various innate lymphocytes (ILCs) (Fig. 6.3) (Ouyang et al. 2011; Rutz and Ouyang 2011). Given the essential roles of IL-22 in tissue protection under various homeostatic, infectious, and inflammatory states as discussed herein, these different cellular sources are not redundant for the biological functions of IL-22 in vivo. Especially, the activation signals required for IL-22 production in these cells and homing properties of these cells into different organs are all different.
Various cell types produce IL-22. (a) T-cell subsets secrete IL-22 under different stimulations. CD4 T cells are major producers and are categorized into Th17 and Th22 subgroups based on their cytokine profiles. (b) Innate lymphocytes (ILCs) are innate sources of IL-22. LTi-like and NK-like ILCs, although defined with different surface markers, are able to produce IL-22 under similar conditions
2.1 CD4 T Helper Cells
IL-22 was originally identified as a cytokine produced from activated murine CD4+ T cells and human T cells (Dumoutier et al. 2000a, c; Wolk et al. 2002). In various CD4+ T helper cell subsets, Th1 cells produce a higher level of IL-22 whereas Th2 cells express a very low, but detectable, level of IL-22 (Gurney 2004; Wolk et al. 2002). A new T helper subset, Th17 cells, was described in 2005 (Harrington et al. 2005; Park et al. 2005). Th17 cells, which primarily express the signature cytokine IL-17, are critical mediators of pathogenesis of inflammatory disorders and antibacterial host defense (Ouyang et al. 2008). Shortly after the discovery of Th17 cells, IL-22 was identified as another functionally important cytokine that helped to define this T helper lineage (Chung et al. 2006; Liang et al. 2006; Zheng et al. 2007). IL-22 production from Th17 cells is significantly higher than that from Th1 cells. Transforming growth factor (TGF)-β and IL-6 are necessary and sufficient to induce Th17 cell differentiation from naïve CD4+ T cells (Bettelli et al. 2006; Mangan et al. 2006; Veldhoen et al. 2006). Surprisingly, we noticed three different ‘flavors’ of Th17 cells depending on the concentration of TGF-β present in the culture (Fig. 6.3a) (Zheng et al. 2007). With a high dose of TGF-β, T cells produce only IL-17 but not much IL-22. These IL-17-only-producing T cells have been identified in human ulcerative colitis (UC) patients, and their presence is correlated with active inflammation in the intestine (Broadhurst et al. 2010). A small amount of TGF-β in combination with IL-6 can promote Th17 cells producing both IL-17 and IL-22. IL-6 alone without addition of TGF-β is not able to induce IL-17 production from T cells. Under this condition, however, T cells produce the highest level of IL-22, suggesting TGF-β is differentially required for IL-17 and IL-22 regulation. The IL-22-only-producing CD4+ T helper cells were discovered in human peripheral blood mononuclear cells (PBMC) and are named Th22 cells (Duhen et al. 2009; Eyerich et al. 2009; Trifari et al. 2009). The Th22 cells not only express CCR6, a cell-surface marker for Th17 cells, but also the skin-homing receptors CCR4 and CCR10. Consistently, Th22 cells were found in epidermis of human inflammatory skin disorders, and expressed genes involved in tissue remodeling, suggesting an important role in skin homeostasis. Additional to αβ CD4+ T cells, both CD8+ T cells and γδ T cells produce IL-22 under the stimulation of IL-23 (Fig. 6.3a) (Billerbeck et al. 2010; Ciric et al. 2009; Martin et al. 2009; Sutton et al. 2009; Zheng et al. 2007). Mouse unconventional γδ T cells were found to coexpress IL-22 with IL-17 and IL-21 in response to IL-23 and IL-1β without T-cell-receptor engagement (Billerbeck et al. 2010; Ciric et al. 2009; Martin et al. 2009; Sutton et al. 2009). These cells express CCR6, orphan nuclear receptor RORγt, aryl hydrocarbon receptor (AHR), IL-23R, and receptors for pathogen products, and mediate antibacterial host defense and pathogenesis of experimental autoimmune encephalomyelitis (EAE), a murine model for multiple sclerosis (MS). Invariant natural killer (NK) T cells, another unconventional T-cell type that express both NK and T-cell markers, are capable of releasing IL-22 upon T-cell receptor (TCR) stimulation and protecting liver from inflammatory damage in a murine acute hepatitis model (Goto et al. 2009; Wahl et al. 2009).
2.2 Innate Lymphocytes
NK cells in human PBMC were reported to express a low level of IL-22, suggesting that IL-22 may also be produced by innate leukocytes (Wolk et al. 2002). Indeed, the induction of IL-22 in the colon with Citrobactor rodentium infection remained intact in Rag2-deficient mice, supporting a non-T-cell source of IL-22 (Zheng et al. 2008). A NK-like ILC subset was subsequently discovered by several groups as one of the major innate sources of IL-22 (Fig. 6.3b) (Cella et al. 2009; Satoh-Takayama et al. 2008). The NK-like cells express NK-specific marker NKp46 and an intermediate level of NK1.1, but have low or no expression of stimulatory or inhibitory NK receptors and possess no effector function of NK cells. Moreover, these cells express CD117, CD127, and a high level of RORγt. Developmentally, NKp46+CD3− cells are dependent on RORγt, but not IL-15, which is required for NK cells. They produce abundant IL-22 but little IL-17 in response to commensal microbiota. A similar IL-22-producing counterpart was identified from human mucosa-associated lymphoid tissues such as the tonsil and Peyer’s patches (Cella et al. 2009). Instead of NKp46, the human cells express NKp44, as well as CD56 and CD127 (Cupedo et al. 2009). The NKp44+ cells were named NK-22 because of their ability to produce IL-22 and respond to IL-23. These NK-22 cells are important sources of IL-22 and regulate the epithelial host defense mechanism and maintenance of dynamic equilibrium between microbial flora and immune surveillance. It is noteworthy that IL-22-producing NK-like ILCs are not only associated with mucosal tissues and restricted to NKp46 expression but are also identified in other organs such as skin dermis, suggesting they may broadly participate in host defense through IL-22 production (Dhiman et al. 2009; Hughes et al. 2009; Satoh-Takayama et al. 2009).
In parallel, a previously defined ILC subset, lymphoid tissue inducer (LTi) cells, was noticed to be able to produce IL-22 upon IL-23 stimulation (Fig. 6.3b) (Takatori et al. 2009). Splenocytes from Rag2−/− mice that are lacking both T and NK T cells still produce a large amount of IL-22 in reaction to IL-23, whereas in Rag2−/− common γ−/− splenocytes, IL-22 production is completely abrogated. A group of LTi-like cells, which express CCR6, RORγt, AHR, and IL-23R, are identified as the sources of IL-22 (Takatori et al. 2009; Veldhoen et al. 2008). LTi is essential for the formation of secondary lymphoid organs during embryogenesis (Mebius 2003). Both inhibitor of differentiation 2 (Id2) and RORγt are required for LTi development (Sun et al. 2000; Yokota et al. 1999). The LTi cells phenotypically lack surface markers for T cells, B cells, and myeloid cells, but express lymphotoxin-α (LT-α), LT-β, several chemokine receptors, and cytokine receptors including CD127 and CD117. Additionally, LTi cells express CD4 in the mouse (Spits and Di Santo 2011). Because LTi-like cells are able to secrete IL-22 upon activation and preferentially reside in mucosal-associated lymphoid tissues, they may play essential functions in maintaining mucosal homeostasis (Cupedo et al. 2009; Takatori et al. 2009).
A study suggested that LTi-like cells but not the NK-like cells were the major sources of IL-22 during C. rodentium infection in the colon (Sonnenberg et al. 2011). However, given many common features shared by LTi-like cells and NK-like ILCs, these cells may belong to the same ILC group with slight differences in the expression of certain cell-surface markers. The connection between LTi-like and NK-like cells has been built not only on their functions but also in lineage development (Sawa et al. 2010). The LTi-like cells could upregulate NK cell-surface marker CD56 and acquire a low level of cytolytic activity when culturing under conditions favoring NK cell differentiation (Crellin et al. 2010; Cupedo et al. 2009). Despite a suggestion that NK-like IL22+ cells may come from IL-1β-stimulated immature NK cells, both LTi-like and NK-like IL-22-producing cells remain their RORc expression throughout the culture and represent a stable RORc+ lineage that is functionally and developmentally distinct from conventional NK cells (Crellin et al. 2010; Hughes et al. 2009, 2010; Satoh-Takayama et al. 2010). Although initially IL-22-producing ILCs have been given different names by different groups, such as NK-22, NCR22, and ILC-22, the nomenclature has been made uniform and all IL-22-producing ILCs are grouped into group 3 ILCs (Fig. 6.3b) (Spits et al. 2013).
2.3 Regulation
Similar to other cytokines, the production of IL-22 from leukocytes is tightly controlled (Ouyang et al. 2011; Rutz and Ouyang 2011). The regulation of IL-22 is best studied in CD4+ T helper cells. Because L-22 is a signature cytokine of Th17 cells, many pathways and factors that are essential for Th17 cell development and IL-17 production also control the expression of IL-22 (Korn et al. 2009; Ouyang et al. 2008). IL-6, IL-21, and IL-23 can all activate Stat3 in T cells. All three cytokines play critical roles in regulation of Th17 development and IL-17 and IL-22 production. The downstream transcription factor Stat3 thus also has an indispensable function in induction of both IL-17 and IL-22 from T cells (Yang et al. 2007). Other pro-inflammatory cytokines including IL-1β and tumor necrosis factor (TNF)-α can augment IL-17 and IL-22 expression. As discussed earlier, IL-23 not only induces IL-22 production from T-cell subsets but also is indispensable for IL-22 expression from ILCs, especially in vivo (Zheng et al. 2007, 2008). The orphan nuclear receptors RORc in human, RORγt, and RORα in mouse are considered as master transcription factors for Th17 lineage formation, and these factors also participate in regulation of IL-22 production in Th17 cells (Ivanov et al. 2006; Volpe et al. 2009; Yang et al. 2008). The AHR, a ligand-dependent transcription factor sensing xenobiotic metabolites, promotes Th17 cell differentiation and drives IL-22 expression (Quintana et al. 2008; Veldhoen et al. 2008). AHR ligand stimulation of human PBMC strongly upregulates IL-22 production in Th17 cells, and Notch signaling enhances IL-22 secretion indirectly through activating the AHR (Alam et al. 2010; Brembilla et al. 2011; Ramirez et al. 2010).
Several pathways, including TGF-β, inducible costimulator (ICOS), and IL-27, negatively regulate IL-22 production in T cells (Liu and Rohowsky-Kochan 2011; Paulos et al. 2010; Rutz et al. 2011; Volpe et al. 2009; Zheng et al. 2007). We recently identified c-Maf as a potential downstream transcription repressor that mediated the suppression of IL-22 in T cells by TGF-β. Because TGF-β is required for IL-17 induction in T cells, it controls the balance of IL-17 and IL-22 in T cells. IL-22 shares similar biological functions with IL-17 for induction of antimicrobial responses, chemokines, and other pro-inflammatory genes from epithelial cells. During pathogen invasion, coexpression of IL-22 and IL-17 may enhance the host defense mechanism through the synergistic effects of both cytokines (Aujla et al. 2008). Consistently, in inflammatory diseases such as psoriasis and rheumatoid arthritis, Th17 cells coexpress IL-17 and IL-22 in inflamed tissues and may contribute to the pathogenesis (Pene et al. 2008). On the other hand, IL-22 has strong tissue-protective functions (Zenewicz et al. 2008; Zheng et al. 2007). As discussed herein, IL-22-only-producing Th22 cells may have distinct biological roles from Th17 cells in both infectious and autoimmune diseases.
3 Biological Functions of IL-22
Carrying the name of “interleukin,” IL-22 was first searched within the lymphopoietic system for its biological function. However, from freshly isolated human monocytes, T cells, NK cells, and B cells, there was no detectable IL-22R1 expression even with activation (Wolk et al. 2004). Consistently, IL-22 induced neither any cytokine production from PBMC nor upregulation of activation markers. IL-22 is secreted by various leukocyte subsets, whereas the expression of IL-22R1 is restricted to epithelial cells and the cells originated from epithelium (Aggarwal et al. 2001; Dumoutier et al. 2000c). Therefore, IL-22 is a key cytokine to mediate the crosstalk between immune systems and peripheral tissues. In contrast to IL-10, which performs primarily regulatory functions during inflammation, IL-22 specifically upregulates the production of antimicrobial peptides, pro-inflammatory chemokines, and cytokines, and directly stimulates epithelial cell proliferation and the self-repair process (Ouyang et al. 2011). IL-22 can elicit three major biological functions from various epithelial tissues: (1) antimicrobial host defense mechanisms, (2) tissue-protective effects, and (3) pro-inflammatory functions.
3.1 Epithelial Host Defense
3.1.1 IL-22 Induces the Production of Antimicrobial Peptides from Epithelial Cells
To enhance innate defense, IL-22 drives the production of a broad spectrum of antimicrobial proteins from epithelial cells in tissues that are directly exposed to external pathogens. IL-22 acts on skin keratinocytes and induces S100 family proteins containing S100A7 (psoriasis), S100A8, and S100A9, and defensins including β-defensin 2 and β-defensin 3 (Liang et al. 2006; Sa et al. 2007; Wolk et al. 2004, 2006). In the lung, bronchial epithelial cells preferentially produce β-defensin 2, S100A7, S100A12 (calgranulin), and lipocalin-2 upon stimulation of IL-22 (Aujla et al. 2008). IL-22 can also stimulate colonic mucosal epithelium to express S100A8, S100A9, RegIIIβ, RegIIIγ, haptoglobin, SAA3, and lactotransferrin (Zheng et al. 2008). Furthermore, IL-22 was reported to induce hepatic lipopolysaccharide (LPS)-binding protein (LBP) to the serum concentration known to neutralize LPS, a major outer membrane component of gram-negative bacteria (Wolk et al. 2007). All these antimicrobial peptides exert essential roles in host defense (Kolls et al. 2008).
3.1.2 Role of IL-22 in Epithelial Defense Against Bacterial Infection
It has been speculated since its discovery that IL-22 might have an important role in host defense (Aggarwal et al. 2001; Dumoutier et al. 2000c; Wolk et al. 2004). Early effort with in vivo infection of an intracellular pathogen, Listeria monocytogenes, indicates that IL-22 is not essential for both the innate and adaptive immunity against the infection (Zenewicz et al. 2007). Because IL-22R1 is preferentially expressed on various epithelial cells and IL-22 induces the expression of many antimicrobial peptides (Sa et al. 2007), we hypothesized that the major function of IL-22 may be in control of extracellular bacteria, especially on the mucosal surface. Previously we have demonstrated that IL-22 is a downstream cytokine of IL-23 that can induce the expression of IL-22 from various lymphocytes (Zheng et al. 2007). A study published at the same time from Weaver’s group established an indispensable role of IL-23 in host defense against C. rodentium, a gram-negative bacterium that infects murine colon epithelial cells and induces transient infectious colitis (Mangan et al. 2006). We speculated that IL-22 could also contribute significantly in this model of mucosal infection. Indeed, IL-22 expression is augmented very quickly in the colon in an IL-23-dependent manner upon C. rodentium infection (Zheng et al. 2008). IL-22 is both necessary and sufficient for controlling systemic bacteria dissemination and mortality during the early stage of the infection. Mice deficient in IL-22 succumb within the second week after infection. IL-22 can directly induce many antimicrobial peptides including the Reg family and S100 family of peptides from intestinal epithelial cells (IECs). The induction of Reg family peptides such as RegIIIβ and RegIIIγ by C. rodentium is abolished in the IL-22-deficient mice. Recombinant RegIIIγ can partially rescue the IL-22 deficient mice, suggesting RegIIIγ is one of the downstream host defense molecules induced by IL-22. Reg family peptides can directly kill gram + bacteria and induce aggregation of gram − bacteria (Cash et al. 2006; Iovanna et al. 1991).
More interestingly, in this model, ILCs, but not T cells, are the major early IL-22 producing cells in the intestine (Cella et al. 2009; Satoh-Takayama et al. 2008; Zheng et al. 2008). T cells, however, are still required for full control of C. rodentium infection, because the development of the anti-C. rodentium IgG response that eventually clears the bacteria from colon and CD4 T cells is required for this response (Bry and Brenner 2004; Maaser et al. 2004). Furthermore, a second wave of IL-22 production is also detected after C. rodentium inoculation in mice (Basu et al. 2012), which provides more complete host protection in the early stage of infection. This second wave of IL-22 is produced by Th22 cells in an IL-6-dependent, but IL-23-independent, manner.
In addition to controlling invading pathogens in the intestine, IL-22 can help to maintain the homeostasis of the mucosal barrier and commensal bacteria. A recent study shows that depletion of IL-22-producing ILCs leads to peripheral dissemination of commensal bacteria, especially Alcaligenes species, and systemic inflammation (Sonnenberg et al. 2012). Exogenous IL-22 is able to prevent the systemic dissemination of commensal bacteria and control the inflammation. Furthermore, IL-22 controls segmented filamentous bacteria (SFB) (Ivanov et al. 2009; Upadhyay et al. 2012), which initiate and augment Th17 responses in the mouse small intestine. IL-22 not only reduces the amount of SFB detected in the small intestine, also regulates host defense against bacterial infections in other organs, for example, Klebsiella pneumoniae infection in the lung (Aujla et al. 2008). In this model, IL-22 cooperates with IL-17 to induce the expression of proinflammatory cytokines and chemokines, as well as lipocalin-2, that kills K. pneumoniae in the lung.
In summary, IL-22 is one of the essential cytokines that regulate epithelial defense function and protect epithelial integrity. The functions of IL-22 in host defense can be categorized in several areas. First, IL-22 directly induces innate antimicrobial mechanisms via promoting the expression of bactericidal peptides. Second, IL-22 preserves the epithelial integrity and promotes the survival of epithelial cells during infection and inflammation. Third, IL-22 also augments wound-healing responses and acts on stem cells to promote recovery of the epithelial organs from damage caused by infection. Finally, IL-22 can synergize with other pro-inflammatory cytokines to induce the expression of chemokines and cytokines that recruit and activate other leukocytes.
3.1.3 Potential Functions of IL-22 in Viral Infection
The type III interferon (IFN) (or IFN-λ family) cytokines, IL-28A, IL-28B, and IL-29 (also called IFN-λ2, IFN-λ3, and IFN-λ1, respectively), exert important antiviral functions (Ouyang et al. 2011). Interestingly, these cytokines structurally are much more closely related to IL-22 than type I IFNs. In addition, type III IFNs share the same IL-10R2 chain with IL-22 as a common receptor subunit. It is tempting, therefore, to speculate a role of IL-22 in antiviral responses. However, although type III IFNs elicit a typical IFN-stimulated gene factor 3 (ISGF3) signal pathway in antiviral response, IL-22 has not been reported to directly induce ISGF3 complex and antiviral activities in cells. It is still possible that IL-22 can enhance or synergize with IFNs during antiviral responses.
Alternatively, as IL-22 promotes tissue-protective functions, it may help to alleviate tissue damage caused by viral infection. IL-22 can also enhance antibacterial activity from the tissue and reduce the chance of secondary bacterial infections after viral invasion. Recent studies with various viral infection models support these hypotheses. In a chronic lymphocytic choriomeningitis virus (LCMV) infection model with clone 13, IL-7 limited organ pathology and damage (Pellegrini et al. 2011) . One of the downstream factors induced by IL-7 in this model is IL-22, which provides an important cytoprotective function and reduces the severity of hepatitis during infection. Consistently, IL-22 is elevated in the liver during chronic hepatitis B virus infection in humans (Feng et al. 2012a). The increased expression of IL-22 in the liver is correlated with the grade of the inflammation and the proliferation of liver progenitor cells. Both in vitro and in vivo, IL-22 is able to promote the proliferation of liver progenitor cells, suggesting a role of IL-22 in liver protection during hepatitis B virus (HBV) infection. A similar tissue protection role of IL-22 is also observed in the lung during influenza infection (Kumar et al. 2013; Paget et al. 2012). IL-22-deficient mice infected with influenza develop much more severe damage of the tracheal epithelial cells than that in wild-type (WT) mice. IL-22 knockout mice also do not recover their body weight lost during the infection. In the gut, the major cellular sources of IL-22 are T cells and innate lymphoid cells. In patients with human immunodeficiency virus (HIV) infection, there is a significant reduction of IL-22-producing T helper subsets, which is correlated with compromised epithelial integrity and increased bacterial translocation (Kim et al. 2012). Long-term antiretroviral therapy is able to increase IL-22-producing T cells and reverse the tissue damage in the gut. These data support an important role of IL-22 in preventing HIV-induced mucosal immunopathogenesis in the gut.
All together these data support a critical function of IL-22 in protecting various epithelial tissues from virus- and inflammation-caused destruction. Further studies are needed to investigate whether there is a direct role of IL-22 in controlling certain viral infections in epithelial cells. On the other hand, IL-22 can synergize with other cytokines to enhance inflammation under certain conditions. It has been reported that IL-22-deficient mice are more resistant to the lethal West Nile virus (WNV) encephalitis. In this model, IL-22 promotes chemokine pathways to recruit neutrophils into the central nervous system (CNS) (Wang et al. 2012).
3.1.4 Role of IL-22 in Yeast Infection
Indispensable functional roles of Th17 and IL-17 in host defense against yeast infection have been established (Ouyang et al. 2008). Patients with mutations in either IL-17 receptor or IL-17F develop chronic mucocutaneous candidiasis (CMC) (Puel et al. 2011). Interestingly, in patients with CMC, their PBMCs not only produce less IL-17A but also less IL-22 upon stimulation with Candida albicans (Eyerich et al. 2008). In addition, antibodies against IL-17A, IL-17F, and IL-22 have been observed from patients with autoimmune polyendocrine syndrome type I (APS-I)/autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) (Kisand et al. 2010; Puel et al. 2010). Dampened IL-22 response as well as IL-17 response is considered to associate with CMC in these patients. However, a direct causal relationship remains to be established (Zelante et al. 2011).
The role of IL-22 in preclinical yeast infection has been examined in recent years. During infection of oropharyngeal candidiasis, IL-22 seems to be dispensable though Th17 and IL-17 pathways are required for host defense (Conti et al. 2009). Similarly, IL-22 is not required for optimal host defense when skin is infected with C. albicans (Kagami et al. 2010a). However, IL-22 is required to prevent C. albicans from disseminating into stomach and kidney when the yeast is infected introgastrically (De Luca et al. 2010). In this case, IL-22 induces various antimicrobial peptides such as S100A8 and RegIII-γ. Furthermore, during Aspergillus fumigatus infection in the lung, IL-22 is induced through a Dectin-1- and IL-23-dependent pathway and contributes to the early innate host defense in the lung (Gessner et al. 2012). These data support that IL-22, similar to IL-17, participates in host defense of yeast infection.
3.2 Maintenance of Epithelial Homeostasis
The antimicrobial functions elicited by IL-22 help to prevent tissue damage from invading pathogens. In addition, IL-22 directly promotes tissue protection and regeneration. First, IL-22 has anti-apoptotic functions and prevents cell damage and cell death caused by inflammation (Cella et al. 2009; Radaeva et al. 2004; Zenewicz et al. 2007). Second, IL-22 promotes wound repair through increasing cell migration and proliferation of keratinocytes, fibroblasts, and IECs (Boniface et al. 2005; Brand et al. 2006; McGee et al. 2013). Stat3 activation by IL-22 is essential for epithelial protection and mucosal wound healing in murine colitis triggered by chemical epithelial damage, inflammatory T cells, or bacterial infection (Pickert et al. 2009; Sugimoto et al. 2008; Zenewicz et al. 2008; Zheng et al. 2008). Third, IL-22 helps to maintain mucosal barrier functions through the restoration of a mucus layer and tight junction. Mucus-associated proteins (MUC), structural components of mucus, form a static external barrier along the epithelial cell surface and are critical for epithelial protection and the wound-healing process (Ho et al. 2006; Van der Sluis et al. 2006). MUC1, MUC3, MUC10 and MUC13 were upregulated from colonic epithelial cells upon IL-22 stimulation (Sugimoto et al. 2008). IL-22 mediates enterocyte homeostasis and restores expression and redistribution of tight junction protein zonula occludens (OZ)-1 (Kim et al. 2012; Klatt et al. 2012). Finally, IL-22 is able to act on epithelial stem cells and progenitor cells and promote tissue regeneration. Given these unique functions of IL-22 in tissue protection, IL-22 may contribute to regulation of the pathogenesis in many diseases.
3.2.1 Tissue-Protective Role of IL-22 in Inflammation
The IL-22 receptor is expressed on many other cells with epithelial origins including hepatocytes in the liver and acinar cells in the pancreas. IL-22 plays an indispensable role in protecting these cells during inflammation. IL-22 expression is induced in liver during concanavalin A (ConA)-induced hepatitis. Blocking the IL-22 pathway by a neutralizing antibody or using IL-22-deficient mice exacerbates hepatitis as indicated by increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Radaeva et al. 2004; Zenewicz et al. 2007). Overexpression of IL-22 can protect liver from injury caused by an agonist antibody targeting Fas, ConA, or carbon tetrachloride (Pan et al. 2004). In addition, treatment with exogenous IL-22 reduces alcoholic liver injury in mice receiving chronic-binge ethanol feeding (Ki et al. 2010). In these models, IL-22 exerts anti-apoptotic effects, induces the expression of antioxidative products, and promotes the proliferation of the hepatocytes. More interestingly, IL-22 is also involved in liver regeneration. The expression of IL-22R on hepatocytes is increased after a partial hepatectomy in mice. Blocking the IL-22 pathway in this case reduces the proliferation of hepatocytes (Ren et al. 2010). Pancreas is another organ that is targeted by IL-22. IL-22R1 is expressed at the highest level in pancreas compared to other human tissues, and acinar cells are the predominant IL-22R1+ cell population (Aggarwal et al. 2001). Both freshly isolated murine acinar cells and a transformed cell line were able to respond to IL-22 and upregulate pancreatitis-associated protein (PAP1)/regenerating gene (Reg)2 and osteopontin (OPN). Moreover, the induction was immediately observed after in vivo IL-22 administration in an animal model. PAP/Reg proteins belong to the C-type lectin (calcium-dependent lectin) gene superfamily, which also includes RegIII genes discussed previously (Zhang et al. 2003). They are not expressed in normal pancreas but are greatly elevated during pancreatic inflammation and directly stimulate β-cell proliferation, facilitate pancreatic islet regeneration, and ameliorate diabetic syndrome (Keim et al. 1984, 1992; Orelle et al. 1992; Watanabe et al. 1994). A report has showed that both β-cells and α-cells may express some level of IL-22R1, but there is no direct evidence to show that they respond to IL-22 (Shioya et al. 2008). In a cerulein-induced chronic pancreatitis model, exogenous IL-22 is able to reduce the release of digestive enzymes, pancreatic cell death, and inflammation, suggesting that IL-22 may have therapeutic value in this disease (Feng et al. 2012b).
IL-22R1 expression was found in airway epithelial cells, indicating IL-22 as a regulator in lung inflammation (Besnard et al. 2011). In asthmatic patients, and patients with interstitial lung diseases such as idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), and pulmonary sarcoidosis, IL-22 expression is altered comparing to healthy lungs, suggesting IL-22 has biological function in pulmonary diseases (Whittington et al. 2004). As already discussed, IL-22 is essential for maintenance of lung integrity during bacterial, fungal, and viral infection (Aujla et al. 2008; Gessner et al. 2012; Kumar et al. 2013) and promotes both the pro-inflammatory antimicrobial responses and tissue-protective functions in the lung. Interestingly, dependent on the environmental factors, the functions of IL-22 can be beneficial or detrimental to the lung. In a bleomycin-induced lung inflammation model, IL-22 exerts a protective function when the IL-17A pathway is blocked. However, in the presence of the IL-17 pathway, IL-22 can synergize with IL-17 to amplify the inflammatory response (Sonnenberg et al. 2010). On the other hand, there is evidence that IL-22 limits the Th2 inflammation in an allergic airway model (Takahashi et al. 2011; Taube et al. 2011), represses IL-25 production from lung epithelial cells, and reduces eosinophil infiltration in the airway (Takahashi et al. 2011).
In addition to its role in promoting liver regeneration, IL-22 also facilitates the regeneration of thymus (Dudakov et al. 2012). IL-22-deficient mice displayed significant delayed thymic regeneration after sublethal total body irradiation. Furthermore, IL-22 functions in maintaining intestinal stem cells (ISCs), which reside within intestinal crypts and could generate the entire crypt–villus structure (Barker et al. 2007; Hanash et al. 2012; Medema and Vermeulen 2011; Simons and Clevers 2011). In a murine graft-versus-host disease model, blocking the IL-22 pathway results in the loss of the ISCs and the increase of crypt apoptosis. Although the IL-22 receptor is not expressed on leukocytes, a role of IL-22 in retaining lymphoid structure has been revealed recently. In the murine C. rodentium infection model, IL-22 is downstream of the lymphoid toxin pathway. IL-22 is required for the maintenance of the isolated lymphoid follicles and colonic patches in the colon during infection (Ota et al. 2011). In this case, IL-22 does not directly act on leukocytes; rather, it helps to preserve the epithelial architecture and chemokines that required for the organization of the lymphoid follicles.
3.2.2 IL-22 May Be Beneficial for IBD Through Its Roles Both in Tissue Protection and Antimicrobial Functions
Inflammatory bowel diseases can be classified into either ulcerative colitis (UC) or Crohn’s disease (CD), both of which manifest chronic inflammation in the gastrointestinal tract. Although UC mainly affects only the colon with continuous superficial inflammation, CD involves the entire intestinal track with patchy, sometimes transmural inflammation. Both environmental factors and genetic predispositions contribute to the pathogenesis of the IBD, although the exact triggers of the disease remain unclear. Recent genome-wide association studies (GWAS) in IBD and data from preclinical animal models suggest that maintenance of the homeostasis of the intestinal microflora and mucosal epithelial barrier may be important.
The IL-22 pathway is not linked with IBD in GWAS analysis, but significantly increased expression of IL-22 is reported in inflamed intestinal mucosa biopsies from IBD patients, and the level of IL-22 is correlated with disease severity (Andoh et al. 2005; Brand et al. 2006; Schmechel et al. 2008). As discussed, IL-23 is an essential upstream cytokine that induces the expression of IL-22 in the intestine. Both IL-23R and IL-12/IL-23p40 subunit are associated with IBD genetically. Interestingly, there is a correlation between serum IL-22 levels and IL-23R minor alleles in CD, in which lower IL-22 expression is linked with protective single nucleotide polymorphisms (SNPs) of IL-23 (Schmechel et al. 2008). As discussed previously, IL-22 effectively induces or synergizes the secretion of pro-inflammatory cytokines and chemokines (IL-6, IL-8, IL-11, leukemia inhibitory factor), pro-angiogenic mediators (MMP-1, MMP-3, MMP-10), growth factor (amphiregulin), and immune suppressor (IL-10, SOCS3) in colon (Andoh et al. 2005; Brand et al. 2006; Nagalakshmi et al. 2004). IL-22 facilitates IFN-γ for inducible nitric oxide synthase (iNOS) production in human colon carcinoma cells, and iNOS is associated with inflammation and immune activation (Ziesche et al. 2007).
IL-22 is upregulated and has a beneficial role in several preclinical colitis models (Neufert et al. 2010; Pickert et al. 2009; Sugimoto et al. 2008; Zenewicz et al. 2008). Although IL-22 does not directly repress inflammatory responses, it may help to dampen the symptoms in IBD in several ways (Fig. 6.4). First, IL-22 induces the production of antimicrobial peptides, such as Reg family of C-type letins, defensins, and cathelicidins, from intestinal epithelial cells and Paneth cells (Pickert et al. 2009; Sugimoto et al. 2008; Zenewicz et al. 2008; Zheng et al. 2008). Second, IL-22 preserves the integrity of the mucosal epithelial layers. IL-22 not only may reduce epithelial apoptosis but also helps to retain the tight junctions in epithelial cells during infection and inflammation (Cella et al. 2009; Kim et al. 2012). IL-22 also promotes epithelial proliferation and triggers wound-healing responses (Brand et al. 2006; Neufert et al. 2010; Pickert et al. 2009). In addition, IL-22 can direct act on intestinal epithelial stem cells and enhance the regeneration (Hanash et al. 2012). Third, IL-22 augments the production of MUC proteins, which can sequester luminal flora from directly interacting with epithelial cells (Sugimoto et al. 2008). Finally, IL-22 stimulates chemokine production from epithelial cells to boost leukocyte infiltration.
IL-22 protects intestinal epithelia in inflammatory bowel disease (IBD). IL-22 restores epithelial integrity through four major biological functions: promoting antimicrobial peptide secretion from epithelial cells; directly stimulating epithelial cell survival, proliferation, migration, and regeneration; increasing the production of mucous proteins by goblet cells, and triggering leukocyte infiltration via activating the chemokine production from epithelial cells
Based on its functions, IL-22 may offer certain therapeutic value in IBD, especially when epithelial damage and changes of luminal flora are major triggers of intestinal inflammation. As IL-22 does not suppress inflammation directly, additional anti-inflammatory treatments may be needed to cooperate with the effects of IL-22. In fact, IL-22 by itself can exacerbate intestinal inflammation under certain conditions. In colitis induced by transferring CD45Rblow CD4+ memory cells that are deficient in IL-10 pathway, IL-22 amplifies the intestinal inflammation (Kamanaka et al. 2011). A similar pathogenic role of IL-22 is again revealed in a small intestinal inflammation model triggered by infection with Toxoplasma gondii (Munoz et al. 2009).
3.2.3 Role of IL-22 in Oncogenesis
Stat3 is a known oncogene and has an important role in many different types of cancer (Hodge et al. 2005). Cytokines, such as IL-6, that activate Stat3, have been connected with tumorigenesis under chronic inflammatory conditions (Naugler et al. 2007). Because IL-22 directly targets epithelial cells and induces Stat3 activation, it is possible that IL-22 may promote certain epithelial-derived tumors. Under homeostatic states, IL-22 can induce epithelial proliferation and elicit wound-healing responses. However, its pro-proliferative activity is in general much weaker than that of some of the other epithelial growth factors such as EGF and KGF (Sa et al. 2007).
Earlier study with IL-22 overexpressed in a murine carcinoma cell line, colon 26 cells, demonstrated that the growth and metastasis of the tumor in syngeneic mice is not different from the control parental cells (Nagakawa et al. 2004). The survival of the mice that are intraperitoneally inoculated with IL-22-expressing colon 26 cells is significantly prolonged. The reason of this extended survival of the mice is unclear.
The first important information regarding a potential role of IL-22 in tumorigenesis comes from the study with IL-22 transgenic mice. Gao and colleagues generated an IL-22 transgenic (Tg) mouse strain in which IL-22 is under the control of a albumin promoter (Park et al. 2011). Although IL-22 is specifically expressed in the liver, IL-22 is readily detectable in the serum. Different from the previous IL-22 Tg mice that die prenatally, these albumin-driven IL-22 Tg mice developed normally and survived more than 2 years. In these mice, the authors did not find any spontaneously developed tumors. Interestingly, IL-22 Tg mice are more susceptible to diethylnitrosamine, a liver carcinogen that induces hepatocarcinoma, supporting a role of IL-22 in promoting the growth of existing cancers. IL-22 is overexpressed in adipose tissue under the control of aP2 promoter in a Tg line (Wang et al. 2011). Although no obvious metabolic phenotypes are observed in these Tg mice, all Tg mice developed spontaneous liposarcomas after feeding with a high-fat diet (HFD). Interestingly, none of the wild-type (WT) mice fed with HFD or IL-22 Tg mice fed with a normal diet develop similar tumors. In both cases, it is unclear what the local IL-22 concentration is in the targeted tissue, which may be significantly higher than normal endogenous level of IL-22.
The potential role of physiological IL-22 level in tumorigenesis has been recently examined in colon cancer models using IL-22, IL-22BP knockout mice, and IL-22 neutralizing antibody (Huber et al. 2012). In the carcinogen-induced, colitis-associated colon cancer model, IL-22BP knockout mice have increased tumor burden, which is dependent on IL-22. Similar results are also observed in the APCmin/+ model of colon cancer. These data again support a role of IL-22 in promoting existing colon cancer. On the other hand, the development of colon cancer is associated with colitis, and IL-22 can alleviate colitis in preclinical models. IL-22 may prevent the initiation of colon cancer by repression of colitis. Indeed, anti-IL-22 treatment can increase tumor burden if treated early in the model, supporting a protective role of IL-22 in colon tumorigenesis at the early stage.
Although IL-22 promotes the existing colon tumors at the late stage, the long-term outcome of these tumors has not been examined. Stat3 was reported to have a dual role in the development of colon cancer in APCmin/+ mice carrying IEC-specific deletion of Stat3 (Musteanu et al. 2010). In the absence of Stat3, the number of intestinal epithelial adenoma is reduced at an early stage, suggesting the Stat3 pathway may play a role in promoting tumor growth. Interesting, at the later stage, there is significantly increased tumor burden in both small intestine and colon without Stat3. Loss of Stat3 not only enhances the tumor progression and invasiveness but also significantly reduces the survival of APCmin/+ mice. Together, the data suggest that activated Stat3 may help control the progression of intestinal cancers. It is unclear whether there is a similar role of IL-22, given its function in activation of Stat3.
3.3 Inflammation and Autoimmune Diseases
Similar to many other cytokines including IL-6, TNF-α, and IL-1β, IL-22 is able to promote inflammatory responses. Three major functions of IL-22 contribute to the inflammatory cascade. First, many of the antimicrobial peptides such as S100 family proteins, induced by IL-22, can recruit and activate leukocytes. Second, IL-22 stimulates the expression of a large group of chemokines from epithelial cells. Third, IL-22 can directly act on liver and other organs to trigger the release of acute-phase proteins, whose plasma concentration changes in response to inflammation to restrain infection, damp local inflammation, and rebuild tissue homeostasis (Baumann and Gauldie 1994; Steel and Whitehead 1994). As discussed earlier, however, despite these pro-inflammatory effects, the net functional outcome of IL-22 in inflammation and infection could be an alleviated inflammatory response (Radaeva et al. 2004; Sugimoto et al. 2008; Zenewicz et al. 2007, 2008; Zheng et al. 2008). Under other conditions, IL-22 elicits strong pro-inflammatory effects and contributes to the pathogenesis of many inflammatory diseases (Kamanaka et al. 2011; Munoz et al. 2009; Zheng et al. 2007). These contradictory effects of IL-22 are likely caused by the shifted balance between the tissue-protective effects and pro-inflammatory functions of IL-22. Thus, pro-inflammatory functions of IL-22 are largely dictated by the context of tissue microenvironment, type of infection, or other pro-inflammatory mediators.
3.3.1 IL-22 Induces Expression of Chemokines and Triggers Acute-Phase Reaction
Despite the minimal effect of IL-22 on cytokine secretion, IL-22 strongly upregulates many chemokines in various organs and contributes to immune regulation by recruiting leukocyte infiltration. IL-22 directly upregulates CXCL10 (IP-10), CCL2 (MCP-1), IL-8, and CXCL1 in hepatocytes, resulting in the recruitment of monocytes, effector T cells, NK cells, and neutrophils (Donnelly et al. 2004; Liang et al. 2010). Similarly, chemoattractants IL-8, CXCL1, CXCL2 (MIP2-a), CXCL3 (MIP2-b), CXCL6, and CCL7 were positively regulated by IL-22 in human colonic subepithelial myofibroblasts and were elevated in inflamed colonic lesions of IBD patients (Andoh et al. 2005; Brand et al. 2006). There was an even greater variety of chemokines induced by IL-22 in human keratinocytes including IL-8, CXCL1, CXCL7, CXCL9 (MIG), CXCL10, CXCL11 (I-TAC), CCL2, CCL5 (RANTES), CCL20 (MIP3-a), and CCL26 (Eotaxin-3). All these chemokines not only activate chemotaxis in various leukocyte populations (monocytes, macrophages, dendritic cells, activated T cells, eosinophils, basophils, and neutrophils), but also stimulate other processes such as mitogenesis, synthesis of extracellular matrix, glucose metabolism, and phagocytosis (Eyerich et al. 2009; Sa et al. 2007). Similar observations were found in pulmonary diseases and rheumatoid arthritis (RA) (Aujla et al. 2008; Besnard et al. 2011; Ikeuchi et al. 2005). In preclinical studies, the infiltration of leukocytes into inflammation sites was significantly reduced in IL-22-deficient animals or those treated with IL-22-neutralizing antibody.
The liver is the predominant organ of pro-inflammatory response. IL-22 was initially characterized as a hepatocyte-stimulating factor for its ability to upregulate acute-phase proteins, such as serum amyloid A (SAA), a1-antichymotrypsin, and haptoglobin in several hepatoma cell lines (Dumoutier et al. 2000c). In addition, the production of both fibrinogen and LBP by hepatocytes was enhanced after IL-22 administration (Liang et al. 2010; Wolk et al. 2007). With sustained exposure to IL-22 delivered by the adenoviral system or liver-specific transgene expression, circulating blood cells were altered with increased blood platelets and decreased red blood cells. Furthermore, body weight loss, thymic atrophy, and renal proximal tubule metabolic activity associated with acute inflammation were also detected (Liang et al. 2010). Besides acting on the vital organs, IL-22 is expressed in RA synovial tissues and mononuclear cells in synovial fluid, and contributes to joint inflammation primarily through directly promoting fibroblast proliferation and monocyte chemoattractant protein 1 (MCP-1) (Ikeuchi et al. 2005).
3.3.2 Pathogenic Role of IL-22 in Psoriasis
The possible link between IL-22 and psoriasis was first revealed by studying the closely related family member IL-20 (Blumberg et al. 2001). Overexpressing IL-20 in Tg mice results in abnormal skin phenotypes, including wrinkled and shiny skin with a thickened epidermis. Histological analysis further identifies epidermal hyperplasia and compact stratum corneum, consistent with some of the histological features observed in psoriatic skin, in these mice. Interestingly, similar phenotypes are also observed in IL-22 and IL-24 Tg mice, suggesting common biological functions of this group of cytokines in the skin (He and Liang 2010; Wolk et al. 2009). IL-22 receptors are highly expressed by keratinocytes (Sa et al. 2007). Detailed in vitro studies with human primary epidermis demonstrate that IL-22 as well as other family members including IL-19, IL-20, and IL-24 can directly induce many downstream biological features observed in psoriatic skin. For example, IL-22 activates p-STAT3 in human keratinocytes and induces the expression of several pro-inflammatory proteins including members of S100 family of calcium-binding proteins, β-defensins, and matrix metalloproteinases (MMP) (Boniface et al. 2005; Ma et al. 2008; Sa et al. 2007; Wolk et al. 2004). Specifically, S100A7, S100A8, and S100A9 are produced promptly when normal human epidermal keratinocytes are treated with IL-22, and they function as chemoattractants of innate immune cells (Roth et al. 2003; Watson et al. 1998). β-Defensin 2 and -3 are induced by IL-22 in human primary keratinocytes, and contribute to inflammation through activation and degranulation of mast cells and CCR6-mediated chemotaxis for immature DCs and CD4 memory T cells (Niyonsaba et al. 2001; Yang et al. 1999). MMPs, also called matrixins, have an essential role in cell mobility and tissue remodeling (Nagase and Woessner 1999). In addition, IL-22 promotes epidermal keratinocyte proliferation and abnormal differentiation similar to those observed in psoriatic skin. Among all its family members, IL-22 appears to be the most potent in induction of these biological effects from keratinocytes (Sa et al. 2007).
In humans, elevated IL-22 has been detected in lesional skin and serum from psoriasis patients (Boniface et al. 2005, 2007; Kagami et al. 2010b; Pene et al. 2008; Wolk et al. 2004). In addition, IL-22-producing T helper subsets, including Th17 and Th22 cells, have been isolated from psoriatic skin. Th17 cells produce both IL-22 and IL-17. IL-17 also exerts essential pathogenic functions in psoriasis. It induces many similar genes as those by IL-22 from keratinocytes (Liang et al. 2006; Tohyama et al. 2009). Blocking the IL-17 pathway ameliorates skin inflammation in many preclinical psoriatic models (van der Fits et al. 2009). More importantly, in clinic, blocking the antibody targeting IL-17 or the IL-17 receptor results in very impressive efficacy in treatment of psoriasis (Hueber et al. 2010; Leonardi et al. 2012; Papp et al. 2012). IL-22 is able to synergize with IL-17 and amplify inflammatory responses in the skin.
Human genetic studies in psoriasis also support an important role of IL-22 in the pathogenesis. Both IL-12/IL-23 p40 subunit and IL-23 receptor have been linked to psoriasis in GWAS (Capon et al. 2007). A protective SNP identified in IL-23R leads to reduced IL-23R expression and decreased IL-22 production upon IL-23 stimulation in T cells (Pidasheva et al. 2011). IL-23 has important functions in Th17 cell development in vivo (McGeachy and Cua 2007) and is indispensable for IL-22 induction from both T cells and ILCs (Ouyang et al. 2011). In clinic, the antibody-neutralizing IL-12/IL-23 p40 subunit succeeds in treatment of psoriasis (Krueger et al. 2007). The efficacy of anti-IL-23 could result partly from the block of IL-22 induction by IL-23. Indeed, in a preclinical mouse model, IL-23 is able to directly induce skin inflammation and acanthosis, and these phenotypes are alleviated in IL-22-deficient mice (Zheng et al. 2007). Blocking IL-22 is efficacious in several other preclinical models of skin inflammation (Ma et al. 2008; Van Belle et al. 2012).
Another protein, Act1, is identified to associate with psoriasis by three independent GWAS (Ellinghaus et al. 2010; Huffmeier et al. 2010; Strange et al. 2010). Act1 is an important adaptor molecule recruited to the receptor of IL-17 and is essential for the function of IL-17 (Chang et al. 2006; Qian et al. 2007). The variant of Act1 identified in psoriasis replaces asparagine for the aspartic acid at the position 10. This loss-of-function mutation results in abolished IL-17-dependent functions. Given an important pathogenic function of IL-17 as already discussed, it is paradoxical that this Act1 mutation is associated with increased risk of development of psoriasis. A recent study, however, revealed that in the mouse this mutation leads to a significantly increased production of IL-22 from T cells (Wang et al. 2013). In addition, these mice spontaneously develop skin inflammation that can be blocked by IL-22-neutralizing antibody.
Taken together, these data support an important role of IL-22 in the pathogenesis of psoriasis. However, it is unclear whether blocking IL-22 by itself will provide sufficient therapeutic benefit in psoriasis. First, several other IL-10 family members, IL-19, IL-20, and IL-24 are also elevated in human psoriatic skin and can induce similar downstream biological effects in the skin. Second, although IL-22 induces many unique patterns and genes from keratinocytes in psoriasis, it is a relative weak cytokine in promoting inflammatory responses in comparison with other cytokines such as IL-17 and TNF-α. IL-22 usually synergizes with these cytokines to further amplify inflammation. Further clinical studies may be necessary to fully assess the role of IL-22 in psoriasis.
References
Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL (2001) Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res 21:1047–1053
Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K (2010) Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 107:5943–5948
Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A et al (2005) Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129:969–984
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K et al (2008) IL-22 mediates mucosal host defense against gram-negative bacterial pneumonia. Nat Med 14:275–281
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature (Lond) 449:1003–1007
Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT (2012) Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37:1061–1075
Baumann H, Gauldie J (1994) The acute phase response. Immunol Today 15:74–80
Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F et al (2011) Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med 183:1153–1163
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature (Lond) 441:235–238
Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B et al (2010) Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA 107:3006–3011
Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A et al (2001) Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104:9–19
Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F (2005) IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 174:3695–3702
Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H et al (2007) A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol 150:407–415
Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A et al (2006) IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 290:G827–G838
Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, Chizzolini C (2011) In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS One 6:e18741
Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P (2010) IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2:60ra88
Bry L, Brenner MB (2004) Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol 172:433–441
Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, Timms K, Gutin A, Abkevic V, Burden AD et al (2007) Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet 122:201–206
Cash HL, Whitham CV, Behrendt CL, Hooper LV (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130
Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature (Lond) 457:722–725
Chang SH, Park H, Dong C (2006) Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281:35603–35607
Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C (2006) Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16:902–907
Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A (2009) IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol 182:5296–5305
Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW et al (2009) Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311
Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H (2010) Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med 207:281–290
Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H (2009) Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 10:66–74
De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F et al (2010) IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3:361–373
Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R (2009) IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 183:6639–6645
Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H (2004) The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol 76:314–321
Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ et al (2012) Interleukin-22 drives endogenous thymic regeneration in mice. Science 336:91–95
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10:857–863
Dumoutier L, Louahed J, Renauld JC (2000a) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164:1814–1819
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC (2000b) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 1:488–494
Dumoutier L, Van Roost E, Colau D, Renauld JC (2000c) Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA 97:10144–10149
Dumoutier L, Lejeune D, Hor S, Fickenscher H, Renauld JC (2003) Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription (STAT)1, STAT2 and STAT3. Biochem J 370:391–396
Dumoutier L, de Meester C, Tavernier J, Renauld JC (2009) New activation modus of STAT3: a tyrosine-less region of the interleukin-22 receptor recruits STAT3 by interacting with its coiled-coil domain. J Biol Chem 284:26377–26384
Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, Belouchi M, Fournier H, Reinhard C, Ding J et al (2010) Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet 42:991–995
Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C (2008) Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol 128:2640–2645
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H et al (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119:3573–3585
Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, Gao B (2012a) Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 143(188–198):e187
Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, Zheng M, Zakhari S, Kolls JK, Gao B (2012b) Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci 8:249–257
Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C (2012) Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun 80:410–417
Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T (2009) Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol 254:81–84
Gruenberg BH, Schoenemeyer A, Weiss B, Toschi L, Kunz S, Wolk K, Asadullah K, Sabat R (2001) A novel, soluble homologue of the human IL-10 receptor with preferential expression in placenta. Genes Immun 2:329–334
Gurney AL (2004) IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol 4:669–677
Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW et al (2012) Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37:339–350
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132
He M, Liang P (2010) IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J Immunol 184:1793–1798
Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, Corredor J, Polk DB, Shekels LL (2006) Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology 131:1501–1517
Hodge DR, Hurt EM, Farrar WL (2005) The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512
Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr, Murphy AJ et al (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature (Lond) 491:259–263
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P et al (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2:52ra72
Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, Juneblad K, Apel M, McManus R, Ho P et al (2010) Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 42:996–999
Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA (2009) Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood 113:4008–4010
Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L et al (2010) Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 32:803–814
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y (2005) Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum 52:1037–1046
Iovanna J, Orelle B, Keim V, Dagorn JC (1991) Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem 266:24664–24669
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV et al (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498
Jones BC, Logsdon NJ, Walter MR (2008) Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 16:1333–1344
Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A (2010a) IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 185:5453–5462
Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A (2010b) Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 130:1373–1383
Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W Jr, Wan YY, Nakae S, Iwakura Y, Hao L, Flavell RA (2011) Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med 208:1027–1040
Keim V, Rohr G, Stockert HG, Haberich FJ (1984) An additional secretory protein in the rat pancreas. Digestion 29:242–249
Keim V, Iovanna JL, Orelle B, Verdier JM, Busing M, Hopt U, Dagorn JC (1992) A novel exocrine protein associated with pancreas transplantation in humans. Gastroenterology 103:248–254
Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B (2010) Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52:1291–1300
Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY et al (2012) A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol 5:670–680
Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N et al (2010) Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207:299–308
Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL et al (2012) Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 5:646–657
Kolls JK, McCray PB Jr, Chan YR (2008) Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol 8:829–835
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517
Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S (1997) Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J 16:5894–5903
Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S (2001) Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol 166:7096–7103
Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M (2007) A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 356:580–592
Kumar P, Thakar MS, Ouyang W, Malarkannan S (2013) IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 6:69–82
Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC (2002) Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277:33676–33682
Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S (2012) Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366:1190–1199
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279
Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL et al (2010) IL-22 induces an acute-phase response. J Immunol 185:5531–5538
Liu H, Rohowsky-Kochan C (2011) Interleukin-27-mediated suppression of human Th17 cells is associated with activation of STAT1 and suppressor of cytokine signaling protein 1. J Interferon Cytokine Res 31:459–469
Logsdon NJ, Jones BC, Josephson K, Cook J, Walter MR (2002) Comparison of interleukin-22 and interleukin-10 soluble receptor complexes. J Interferon Cytokine Res 22:1099–1112
Logsdon NJ, Jones BC, Allman JC, Izotova L, Schwartz B, Pestka S, Walter MR (2004) The IL-10R2 binding hot spot on IL-22 is located on the N-terminal helix and is dependent on N-linked glycosylation. J Mol Biol 342:503–514
Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M et al (2008) IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 118:597–607
Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L (2004) Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun 72:3315–3324
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature (Lond) 441:231–234
Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M (2009) Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31:321–330
McGeachy MJ, Cua DJ (2007) The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol 19:372–376
McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA, Horsley V (2013) IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol 133(5):1321–1329
Mebius RE (2003) Organogenesis of lymphoid tissues. Nat Rev Immunol 3:292–303
Medema JP, Vermeulen L (2011) Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature (Lond) 474:318–326
Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K et al (2009) Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrix metalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med 206:3047–3059
Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L et al (2010) Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology 138(1003–1011):e1001–e1005
Nagakawa H, Shimozato O, Yu L, Takiguchi Y, Tatsumi K, Kuriyama T, Tagawa M (2004) Expression of interleukin-22 in murine carcinoma cells did not influence tumour growth in vivo but did improve survival of the inoculated hosts. Scand J Immunol 60:449–454
Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R (2004) Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol 4:679–691
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Nagem RA, Colau D, Dumoutier L, Renauld JC, Ogata C, Polikarpov I (2002) Crystal structure of recombinant human interleukin-22. Structure 10:1051–1062
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317:121–124
Neufert C, Pickert G, Zheng Y, Wittkopf N, Warntjen M, Nikolaev A, Ouyang W, Neurath MF, Becker C (2010) Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle 9:652–655
Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I (2001) Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol 31:1066–1075
Orelle B, Keim V, Masciotra L, Dagorn JC, Iovanna JL (1992) Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J Clin Invest 90:2284–2291
Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W (2011) IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol 12:941–948
Ouyang W, Kolls JK, Zheng Y (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454–467
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109
Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P et al (2012) Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem 287:8816–8829
Pan H, Hong F, Radaeva S, Gao B (2004) Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol 1:43–49
Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S (2012) Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366:1181–1189
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS et al (2011) In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 54:252–261
Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH (2010) The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med 2:55ra78
Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M et al (2011) IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144:601–613
Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, Moles JP, Danger Y, Ravon E, Lesaux S et al (2008) Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 180:7423–7430
Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S et al (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206:1465–1472
Pidasheva S, Trifari S, Phillips A, Hackney JA, Ma Y, Smith A, Sohn SJ, Spits H, Little RD, Behrens TW et al (2011) Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One 6:e25038
Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J et al (2010) Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207:291–297
Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M et al (2011) Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332:65–68
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J et al (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71
Radaeva S, Sun R, Pan HN, Hong F, Gao B (2004) Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39:1332–1342
Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, Saurat JH, Roosnek E, Chizzolini C (2010) Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol 40:2450–2459
Ren X, Hu B, Colletti LM (2010) IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol 298:G74–G80
Roth J, Vogl T, Sorg C, Sunderkotter C (2003) Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 24:155–158
Rutz S, Ouyang W (2011) Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol 23:605–612
Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W (2011) Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol 12:1238–1245
Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178:2229–2240
Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O et al (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29:958–970
Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, Vosshenrich CA, Di Santo JP (2009) The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol 183:6579–6587
Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP (2010) IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med 207:273–280
Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G (2010) Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330:665–669
Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, Lohse P, Goke B, Brand S (2008) Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis 14:204–212
Shioya M, Andoh A, Kakinoki S, Nishida A, Fujiyama Y (2008) Interleukin 22 receptor 1 expression in pancreas islets. Pancreas 36:197–199
Simons BD, Clevers H (2011) Stem cell self-renewal in intestinal crypt. Exp Cell Res 317:2719–2724
Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D (2010) Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 207:1293–1305
Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D (2011) CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122–134
Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM et al (2012) Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321–1325
Spits H, Di Santo JP (2011) The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12:21–27
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE et al (2013) Innate lymphoid cells: a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149
Steel DM, Whitehead AS (1994) The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today 15:81–88
Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG et al (2010) A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 42:985–990
Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118:534–544
Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR (2000) Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 288:2369–2373
Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341
Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, Tokoyoda K, Renauld JC, Iwamoto I, Nakayama T, Nakajima H (2011) IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol 128(1067–1076):e1061–e1066
Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206:35–41
Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D et al (2011) IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One 6:e21799
Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, Hirakawa S, Tokumaru S, Okazaki H, Sayama K, Hashimoto K (2009) IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur J Immunol 39:2779–2788
Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10:864–871
Upadhyay V, Poroyko V, Kim TJ, Devkota S, Fu S, Liu D, Tumanov AV, Koroleva EP, Deng L, Nagler C et al (2012) Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol 13:947–953
Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, Renauld JC, Dumoutier L (2012) IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol 188:462–469
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845
Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109
Volpe E, Touzot M, Servant N, Marloie-Provost MA, Hupe P, Barillot E, Soumelis V (2009) Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood 114:3610–3614
Wahl C, Wegenka UM, Leithauser F, Schirmbeck R, Reimann J (2009) IL-22-dependent attenuation of T cell-dependent (ConA) hepatitis in herpes virus entry mediator deficiency. J Immunol 182:4521–4528
Wang Z, Yang L, Jiang Y, Ling ZQ, Li Z, Cheng Y, Huang H, Wang L, Pan Y, Yan X, Chen Y (2011) High fat diet induces formation of spontaneous liposarcoma in mouse adipose tissue with overexpression of interleukin 22. PLoS One 6:e23737
Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR et al (2012) IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One 7:e44153
Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, Liu C, Herjan T, Misra S, Carman JA et al (2013) The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol 14:72–81
Watanabe T, Yonemura Y, Yonekura H, Suzuki Y, Miyashita H, Sugiyama K, Moriizumi S, Unno M, Tanaka O, Kondo H et al (1994) Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc Natl Acad Sci USA 91:3589–3592
Watson PH, Leygue ER, Murphy LC (1998) Psoriasin (S100A7). Int J Biochem Cell Biol 30:567–571
Weiss B, Wolk K, Grunberg BH, Volk HD, Sterry W, Asadullah K, Sabat R (2004) Cloning of murine IL-22 receptor alpha 2 and comparison with its human counterpart. Genes Immun 5:330–336
Whittington HA, Armstrong L, Uppington KM, Millar AB (2004) Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol 31:220–226
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168:5397–5402
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21:241–254
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36:1309–1323
Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R (2007) IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 178:5973–5981
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S et al (2009) IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med 87:523–536
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275:31335–31339
Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, Dillon SR, Gao Z, Gilbert T, Madden K et al (2001) A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci USA 98:9511–9516
Xu T, Logsdon NJ, Walter MR (2004) Crystallization and X-ray diffraction analysis of insect-cell-derived IL-22. Acta Crystallogr D Biol Crystallogr 60:1295–1298
Xu T, Logsdon NJ, Walter MR (2005) Structure of insect-cell-derived IL-22. Acta Crystallogr D Biol Crystallogr 61:942–950
Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528
Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282:9358–9363
Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS et al (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28:29–39
Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P (1999) Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature (Lond) 397:702–706
Zelante T, Iannitti R, De Luca A, Romani L (2011) IL-22 in antifungal immunity. Eur J Immunol 41:270–275
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27:647–659
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA (2008) Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29:947–957
Zhang YW, Ding LS, Lai MD (2003) Reg gene family and human diseases. World J Gastroenterol 9:2635–2641
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature (Lond) 445:648–651
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289
Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H (2007) The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem 282:16006–16015
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Wang, X., Ouyang, W. (2014). Interleukin-22: A Bridge Between Epithelial Innate Host Defense and Immune Cells. In: Yoshimoto, T., Yoshimoto, T. (eds) Cytokine Frontiers. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54442-5_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54442-5_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54441-8
Online ISBN: 978-4-431-54442-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)