Abstract
Enantioselective total synthesis of the biologically important indole alkaloids, (+)-lysergol, (+)-isolysergol and (+)-lysergic acid is described. Key features of these total synthesis include: (1) a facile synthesis of a chiral 1,3-amino alcohol via the Pd(0) and In(I)-mediated reductive coupling reaction between l-serine-derived 2-ethynylaziridine and formaldehyde; (2) the Cr(II)/Ni(0)-mediated Nozaki−Hiyama−Kishi (NHK) reaction of an indole-3-acetaldehyde with iodoalkyne; and (3) Pd(0)-catalyzed domino cyclization of an amino allene bearing a bromoindolyl group. This domino cyclization enabled direct construction of the C/D ring system of the ergot alkaloids skeleton as well as the creation of the C5 stereogenic center with transfer of the allenic axial chirality to the central chirality.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
The author planned to develop a new synthetic route to the chiral amino allenes 5 because the synthetic route described in Chap. 4 gave the racemic amino allenes of the type 5 in low diastereoselectivities.
- 2.
The relative configuration of 12 was determined by 1H NOE analysis.

- 3.
The relative configuration of allenic amide 5a was confirmed by comparison with the authentic sample (±)-5a prepared from the known allenic amide (±)-22a, which, in turn, was obtained through an Au-catalyzed Claisen rearrangement of the corresponding propargyl vinyl ether (see Chap. 4).
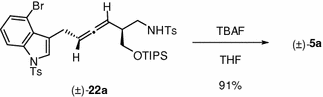
- 4.
The relative configuration of 4a was confirmed by comparison with the authentic sample prepared from the known compound (±)-23a (see Chap. 4).
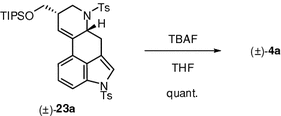
- 5.
The author cannot rule out other factors for rationalization of the observed selectivities. For example, the reactive conformer as depicted in F might have better orbital alignment for anti-addition of the amine nucleophile to the allenic moiety activated by Pd(II) than in epi-F, thus leading to a selective formation of the desired product 4a.
- 6.
The reproducibility of the oxidation reaction was significantly dependent on the purity of the Dess-Martin reagent.
- 7.
Separation of the diastereomer at this step is important for the preparation of lysergic acid (1) in high ee, because the transformation of 25 to 1 accompanying isomerization relies on the chirality at C-5.
- 8.
The optical purity of lysergic acid was confirmed by derivatization to methyl isolysergate 25a and lysergate 25b and their chiral HPLC analyses.

References
Moldvai I, Temesvári-Major E, Incze M, Szentirmay É, Gács-Baitz E, Szántay C (2004) J Org Chem 69:5993–6000
Inoue T, Yokoshima S, Fukuyama T (2009) Heterocycles 79:373–378
Kurosawa T, Isomura M, Tokuyama H, Fukuyama T (2009) Synlett 775–777
Deck JA, Martin SF (2010) Org Lett 12:2610–2613
Weibel J-M, Blanc A, Pale P (2008) Chem Rev 108:3149–3173
Álvarez-Corral M, Muñoz-Dorado M, Rodríguez-García I (2008) Chem Rev 108:3174–3198
Patil NT, Yamamoto Y (2008) Chem Rev 108:3395–3442
Widenhoefer RA, Han X (2006) Eur J Org Chem 4555–4563
Shen HC (2008) Tetrahedron 64:3885–3903
Muzart J (2008) Tetrahedron 64:5815–5849
Widenhoefer RA (2008) Chem Eur J 14:5382–5391
Krause N, Belting V, Deutsch C, Erdsack J, Fan H-T, Gockel B, Hoffmann-Röder A, Morita N, Volz F (2008) Pure Appl Chem 80:1063–1069
Li Z, Brouwer C, He C (2008) Chem Rev 108:3239–3265
Bongers N, Krause N (2008) Angew Chem Int Ed 47:2178–2181
Arredondo VM, McDonald FE, Marks TJ (1998) J Am Chem Soc 120:4871–4872
Arredondo VM, Tian S, McDonald FE, Marks TJ (1999) J Am Chem Soc 121:3633–3639
Ohno H, Kadoh Y, Fujii N, Tanaka T (2006) Org Lett. 8:947–950
Myers AG, Zheng B (1996) J Am Chem Soc 118:4492–4493
Ohno H, Hamaguchi H, Tanaka T (2000) Org Lett 2:2161–2163
Ohno H, Hamaguchi H, Tanaka T (2001) J Org Chem 66:1867–1875
Garner P (1984) Tetrahedron Lett 25:5855–5858
Campbell AD, Raynham TM, Taylor RJK (1998) Synthesis 1707–1709
Ohno H, Hamaguchi H, Ohata M, Kosaka S, Tanaka T (2004) J Am Chem Soc 126:8744–8754
Hofmeister H, Annen K, Laurent H, Wiechert R (1984) Angew Chem Int Ed Engl 23:727–729
Tokuyama H, Miyazaki T, Yokoshima S, Fukuyama T (2003) Synlett 1512–1514
Somei M, Kizu K, Kunimoto M, Yamada F (1985) Chem Pharm Bull 33:3696–3708
Kimura M, Futamata M, Mukai R, Tamaru Y (2005) J Am Chem Soc 127:4592–4593
Harrington PJ, Hegedus LS (1984) J Org Chem 49:2657–2662
Imamoto T, Kusumoto T, Yokoyama M (1982) J Chem Soc Chem Commun 1042–1044
Takita R, Yakura K, Ohshima T, Shibasaki M (2005) J Am Chem Soc 127:13760–13761
Lu G, Li X, Chan WL, Chan ASC (2002) Chem Commun 172
Gao G, Moore D, Xie R-G, Pu L (2002) Org Lett 4:4143–4146
Evans PA, Cui J, Gharpure SJ, Polosukhin A, Zhang H-R (2003) J Am Chem Soc 125:14702–14703
Okude Y, Hirano S, Hiyama T, Nozaki H (1977) J Am Chem Soc 99:3179–3181
Jin H, Uenishi J, Christ WJ, Kishi Y (1986) J Am Chem Soc 108:5644–5646
Takai K, Tagashira M, Kuroda T, Oshima K, Utimoto K, Nozaki H (1986) J Am Chem Soc 108:6048–6050
Wan Z-K, Choi H-W, Kang F-A, Nakajima K, Demeke DK, Kishi Y (2002) Org Lett 4:4431–4434
Choi H-W, Nakajima K, Demeke D, Kang F-A, Jun H-S, Wan Z-KK, Kishi Y (2002) Org Lett 4:4435–4438
Namba K, Kishi Y (2004) Org Lett 6:5031–5033
Midland MM, Tramontano A, Kazubski A, Graham RS, Tsai DJS, Cardinv DB (1984) Tetrahedron 40:1371–1380
Matsumura K, Hashiguchi S, Ikariya T, Noyori R (1997) J Am Chem Soc 119:8738–8739
Corey EJ, Bakshi RK, Shibata S (1987) J Am Chem Soc 109:5551–5553
Corey EJ, Bakshi RK (1990) Tetrahedron Lett 31:611–614
Corey EJ, Helal CJ (1998) Angew Chem Int Ed 37:1986–2012
Stoll A, Hofmann A, Schlientz W (1949) Helv Chim Acta 32:1947–1956
Smith S, Timmis GM (1936) J Chem Soc 1440–1444
Moldvai I, Gács-Baitz E, Temesvári-Major E, Russo L, Pápai I, Rissanen K, Szárics É, Kardos J, Szántay C (2007) Heterocycles 71:1075–1095
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2012 Springer
About this chapter
Cite this chapter
Inuki, S. (2012). Total Synthesis of (+)-Lysergic Acid, (+)-Lysergol, and (+)-Isolysergol. In: Total Synthesis of Bioactive Natural Products by Palladium-Catalyzed Domino Cyclization of Allenes and Related Compounds. Springer Theses. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54043-4_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-54043-4_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54042-7
Online ISBN: 978-4-431-54043-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)





