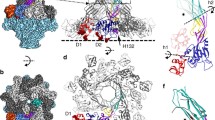
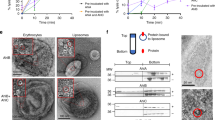
Abstract
Aeromonas hydrophila is a water-borne Gram-negative bacterium associated with gastroenteritis and opportunistic infections.1 The organism secretes a protein toxin called aerolysin that appears to be a major virulence factor of the bacterium.2 The toxin is synthesized as a preproprotein with a typical signal sequence that is removed during transit across the inner membrane of the Aeromonas bacterium.3 The protoxin then appears to fold and dimerize in the periplasm and leaves the cell in a separate step that requires a group of more than 14 genes of the General Secretory Pathway.4,5
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Altwegg M, Geiss HK. Aeromonas as a human pathogen. CRC Crit Rev Microbiol 1989; 16: 253–86.
Chakraborty T, Huhle B, Hof H et al. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated infections. Infect Immun 1987; 55: 2274–80.
Howard SP, Buckley JT. Protein export by a Gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol 1985; 161: 1118–24.
Pugsley AP. The complete general secretory pathway in Gram-negative bacteria. Microbiol Rev 1993; 57: 50–108.
Jiang B, Howard SP. The Aeromonas hydrophila exeE gene, required both for protein secretion and normal outer membrane biogenesis, is a member of a general secretion pathway. Molec Microbiol 1992; 6: 1351–61.
Howard SP, Buckley JT. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry 1982; 21: 1662–67.
Gruber HJ, Wilmsen HU, Cowell S et al. Isolation and reconstitution of the receptor for the hemolytic toxin aerolysin from rat red blood cell membranes. Molec Microbiol 1994; 14: 1093–11.
Parker MW, Buckley JT, Postma JPM et al. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 1994; 367: 292–95.
Howard SP, Garland WJ, Green MJ et al. Nucleotide sequence of the gene for the hole-forming toxin aerolysin of Aeromonas hydrophila. J Bacteriol 1987; 169: 2869–71.
Husslein V, Huhle B, Jarchau T et al. Nucleotide sequence and transcriptional analysis of the aerCaerA region of Aeromonas sobria encoding aerolysin and its regulatory region. Molec Microbiol 1988; 2: 507–17.
Hirono I, Aoki T. Cloning and characterization of three hemolysin genes from Aeromonas salmonicida. Microb Pathog 1993; 15: 269–82.
van der Goot FG, Lakey J, Pattus F et al. Spectroscopic study of the activation and oligomerization of the channel-forming toxin aerolysin: identification of the site of proteolytic activation. Biochemistry 1992; 31: 8566–70.
van der Goot FG, Hardie KR, Parker MW et al. The C-terminal peptide produced upon proteolytic activation of the cytolytic toxin aerolysin is not involved in channel formation. J Biol Chem 1994; 269: 30496–501.
Bernstein FC, Koetzle TF, Williams GJB et al. The Protein Data Bank: a computer based archival file for macromolecular structures. J Mol Biol 1977; 112: 535–42.
Tucker AD, Parker MW, Tsernoglou D et al. Crystallization of a proform of aerolysin, a hole-forming toxin from Aeromonas hydrophila. J Mol Biol 1990; 212: 561–62.
van der Goot FG, Ausio J, Wong KR et al. Dimerization stabilizes the pore-forming toxin aerolysin in solution. J Biol Chem 1993; 268: 18272–79.
van der Goot FG, Pattus F, Parker MW et al. The cytolytic toxin aerolysin, from the soluble form to the transmembrane channel. Toxicology 1994; 87: 19–28.
Argos P. An investigation of protein subunit and domain interfaces. Protein Engng 1988; 2: 101–13.
Bennett MJ, Choe S, Eisenberg D. Domain swapping: entangling alliances between proteins. Proc Natl Acad Sci USA 1994; 91: 3127–31.
Verner K, Schatz, G. Protein translocation across membranes. Science 1988; 241: 1307–13.
Montgomery DW, Don LK, Zukoski CF et al. The effect of zinc and other metals on complement hemolysis of sheep red blood cells in vitro. Proc Soc Exp Biol Med 1974; 145: 263–67.
Avigad LS, Bernheimer AW. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immun 1976; 13: 1378–81.
Wilmsen H-U, Pattus F, Buckley JT. Aerolysin, a hemolysin from Aeromonas hydrophila, forms voltage-gated channels in planar lipid bilayers. J Membr Biol 1990; 115: 71–81.
Wilmsen H-U, Buckley JT, Pattus F. Site-directed mutagenesis at histidines of aerolysin from Aeromonas hydrophila: a lipid planar bilayer study. Molec Microbiol 1991; 5: 2745–51.
Buckley JT, Wilmsen H-U, Lesieur C et al. Protonation of His-132 promotes oligomerization of the channel-forming toxin aerolysin. Biochemistry 1995; in press.
Green MJ, Buckley JT. Site-directed mutagenesis of the hole-forming toxin aerolysin: studies on the role of histidines in receptor binding and oligomerization of the monomer. Biochemistry 1990; 29: 2177–80.
Hardie KR, Schulze A, Parker MW et al. Aeromonas sp. secrete proaerolysin as a folded dimer. Molec Microbiol 1995; in press.
Vyas NK. Atomic features of protein-carbohydrate interactions. Curr Opin Struct Biol 1991; 1: 732–40.
Kozaki S, Kato K, Asao T et al. Activities of Aeromonas hydrophila hemolysins and their interaction with erythrocyte membranes. Infect Immun 1987; 55: 1594–99.
Chakraborty T, Schmid A, Notermans S et al. Aerolysin of Aeromonas sobria: evidence for formation of ion-permeable channels and comparison with alpha-toxin of Staphylococcus aureus. Infect Immun 1990; 58: 2127–32.
Garland WJ, Buckley JT. The cytolytic toxin aerolysin must aggregate to disrupt erythrocytes, and aggregration is stimulated by human glycophorin. Infect Immun 1988; 56: 1249–53.
Howard SP, Buckley JT. Activation of the hole-forming toxin aerolysin by extracellular processing. J Bacteriol 1985; 163: 336–40.
van der Goot FG, Pattus F, Wong KR et al. Oligomerization of the channel-forming toxin aerolysin precedes insertion into lipid bilayers. Biochemistry 1993; 32: 2636–42.
Wilmsen H-U, Leonard K, Tichelaar W et al. The aerolysin membrane channel is formed by heptamerization of the monomer. EMBO J 1992; 11: 2457–63.
Weiss MS, Abele U, Weckesser J et al. Molecular architecture and electrostatic properties of a bacterial porin. Science 1991; 254: 1627–30.
Cowan SW, Schirmer T, Rummel G et al. Crystal structures explain functional properties of two E. coli porins. Nature 1992; 358: 727–33.
Parker MW, Tucker AD, Tsernoglou D et al. Insights into membrane insertion based on studies of colicins. Trends Biochem Soc 1990; 15: 126–29.
Ballard J, Sokolov Y, Yuan W-L et al. Activation and mechanism of Clostridium septicum alpha toxin. Molec Microbiol 1993; 10: 627–34.
Hayashi T, Kamio Y, Hishinuma F et al. Pseudomonas aeruginosa cytotoxin: the nucleotide sequence of the gene and the mechanism of activation of the protoxin. Molec Microbiol 1989; 3: 861–68.
Schmid A, Benz R, Just I et al. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. J Biol Chem 1994; 269: 16706–11.
Milne JC, Collier RJ. pH-dependent permeabilization of the plasma membrane of mammalian cells by anthrax protective antigen. Molec Microbiol 1993; 10: 647–53.
Suzuki C, Nikkuni S. The primary and subunit structure of a novel type of killer toxin produced by a halotolerant yeast, Pichia farinosa. J Biol Chem 1994; 269: 3041–46.
Montecucco C, Papini E, Schiavo G. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett 1994; 346: 92–98.
Arbuthnott JP, Freer JH, Bernheimer AW. Interaction of staphylococcal alpha toxin with artificial and natural membranes. J Bacteriol 1968; 95: 1153–68.
Füssle R, Bhakdi S, Sziegoleit A et al. On the mechanism of membrane damage by Staphylococcus aureus a-toxin. J Cell Biol 1981; 91: 83–94.
Menestrina G. Ionic channels formed by Staphylococcus aureus alpha toxin: voltage dependent inhibition by divalent and trivalent cations. J Membr Biol 1986; 90: 177–90.
Pederzolli C, Cescatti L, Menestrina GJ. Chemical modification of S. aureus a-toxin by diethylpyrocarbonate: role of histidines in its membrane damaging properties. J Membr Biol 1991; 119: 41–52.
Tobkes N, Wallace BA, Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry 1985; 24: 1915–20.
Gouaux JE, Braha O, Hobaugh MR et al. Subunit stoichiometry of staphyloccal a-hemolysin in crystals and on membranes: A heptameric transmembrane pore. Proc Natl Acad Sci USA 1994; 91: 12828–31.
Milne JC, Furlong D, Hanna PC et al. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem 1994; 269: 20607–12.
Morgan PJ, Hyman SC, Byron O et al. Modeling the bacterial protein toxin, pneumolysin, in its monomeric and oligomeric form. J Biol Chem 1994; 269: 5315–20.
Gray GS, Kehoe M. Primary sequence for the a-toxin gene from Staphylococcus aureus Wood 46. Infect Immun 1984; 46: 615–18.
Imagawa T, Dohi Y, Higashi Y. Cloning, nucleotide sequence and expression of a hemolysin gene of Clostridium septicum. FEMS Microbiol Letters 1994; 117: 287–92.
Hunter SEC, Clarke IN, Kelly CD et al. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect Immun 1992; 60: 102–10.
Tweten RK. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from C. perfringens: significant homology with the genes for streptolysin O and perfringolysin. Infect Immun 1988; 56: 3235–40.
Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst 1991; 24: 946–50.
Sibbald PR, Argos P. Scrutineer: a computer program that flexibly seeks and describes motifs and profiles in protein sequence databases. CABIOS 1990; 6: 279–88.
Jones TA, Zou JY, Cowan SW et al. Improved methods for building models in electron density maps and the location of errors in these models. Acta Cryst 1991; A47: 110–19.
Rights and permissions
Copyright information
© 1996 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Parker, M.W., Buckley, J.T., van der Goot, F.G., Tsernoglou, D. (1996). Structure and Assembly of the Channel-Forming Aeromonas Toxin Aerolysin. In: Protein Toxin Structure. Molecular Biology Intelligence Unit. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-22352-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-662-22352-9_5
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-22354-3
Online ISBN: 978-3-662-22352-9
eBook Packages: Springer Book Archive