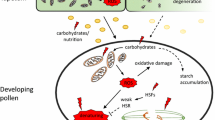
Abstract
Sexual reproduction in plants involves some of the most dramatic developmental changes that plants ever undergo. Meiosis itself brings about enormous ultrastructural changes within the cell, the microspores so formed divide into generative and vegetative cells. These are both contained in the pollen grains that subsequently develop. The development of the pollen grain involves considerable loss of water vapor, so that by anthesis when pollen is finally shed the water content of the pollen may be as low as 9% and rarely more than 60%, depending on the species. Grass pollens normally have a higher water content at maturity than the pollen from most dicotyledonous plants. An exception is the pollen from sea grasses, which, because of the aqueous environment in their habitat, do not suffer the dehydration seen in the land plants.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Banerjee VC, Rowley JR, Alessio ML (1965) Exine plasticity during pollen grain maturation. J Palynol 1: 70–89
Bottomley PA, Rogers HH, Foster TH (1986) NMR imaging shows water distribution and transport in plant root systems in situ. Proc Natl Acad Sci USA 83: 87–89
Boyer JS (1982) Plant productivity and environment. Science 218: 443–448
Boyer JS, Knipling EB (1965) Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proc Natl Acad Sci USA 54: 1044–1052
Cohen JS (ed) (1983) Magnetic resonance in biology, vol 2. Wiley, New York
Dumas C, Knox RB, Gaude T (1984) Pollen recognition:new concepts from electron microscopy and cytochemistry. Int Rev Cytol 90: 239–272
Escaig J, Nicolas G (1976) Cryo-fractures de material biologique realisees a tres basses temperatures en ultravide. C R Acad Sci 283: 1245–1248
Favre-Duchartre M (1963) Structure of cycad pollen. Ann Sci Nat Bot 12th Ser 5: 233–239
Gay G, Kerhoas C, Dumas C (1987) Quality of a stress-sensitive Cucurbita pepo L. pollen. Planta 171: 82–87
Geitler L (1942) State of hydration influences division of sperm cells in pollen. Planta 32: 187–195
Gilissen LJW (1977) The influence of relative humidity on the swelling of pollen grains in vitro. Planta 137: 299–301
Gould GW (1983) Resistance and dormancy of bacterial endospores. In:Hurst A, Gould GW (eds) The bacterial spore, vol 2. Academic Press, New York London, p 173
Heslop-Harrison J (1975) Physiology of the pollen-grain surface. Proc R Soc London Ser B 190: 275–300
Heslop-Harrison J (1978) Cellular recognition systems in plants. Studies in biology, 100, Arnold, London
Heslop-Harrison J (1979 a) Pollen walls as adaptive systems. Ann MO Bot Gard 66: 813–829
Heslop-Harrison J (1979 b) Aspects of the structure, cytochemistry, and germination of the pollen of rye (Secale cereale L.). Ann Bot Suppl 1: 1–47
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymati-cally induced fluorescence, intracellular hydrolysis of fluorescein diacetate. Stain Tech-nol 45: 115–120
Heslop-Harrison J, Heslop-Harrison Y (1980) Cytochemistry and function of the Zwischenkörper in grass pollen. Pollen Spores 22: 5–10
Hinshaw WS (1976) NMR images of whole fruit. J Appl Phys 47: 3709
Jackson JF (1987) DNA repair in pollen — a review. Mutat Res 181: 17–29
Jackson JF, Linskens HF (1978) Evidence for DNA repair after ultraviolet irradiation of Petunia hybrida pollen. Mol Gen Genet 161: 117–120
Jackson JF, Linskens HF (1979) Pollen DNA repair after treatment with the mutagens 4-nitroquinoline-1-oxide, ultraviolet and near-ultraviolet irradiation, and boron dependence of repair. Mol Gen Genet 176: 11–16
Jackson JF, Linskens HF (1980) DNA repair in pollen:range of mutagens inducing repair, effect of replication inhibitors and changes in thymidine nucleotide metabolism, during repair. Mol Gen Genet 180: 517–522
Jackson JF, Linskens HF (1982) Metal ion induced unscheduled DNA synthesis in Petunia pollen. Mol Gen Genet 187: 112–115
Johnson GA, Brown J, Kramer PJ (1987) Magnetic resonance microscopy of changes in water content in stems of transpiring plants. Proc Natl Acad Sci USA 84: 2752–2755
Kaku S, Iwayainove M, Gusta LV (1984) Relationship of nuclear magnetic resonance relaxation time to water content and cold hardiness in flower buds of evergreen azalea. Plant Cell Physiol 25: 875–882
Kerhoas C, Dumas C (1986) Nuclear magnetic resonance and pollen quality. In:Linskens HF, Jackson JF (eds) Modern methods of plant analysis, New Series, vol 2. Springer, Berlin Heidelberg New York, pp 169–190
Kerhoas C, Gay G, Dumas C (1987) A multidisciplinary approach to the study of the plasma membrane of Zea mays pollen during controlled dehydration. Planta 171: 1–10
Knox RB (1984) The pollen grain. In:Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 212–215
Lauterbur PC (1973) Image formation by induced local interactions:examples employing nuclear magnetic resonance. Nature 242: 190–191
Lauterbur PC (1974) Nuclear magnetic imaging of fruits. Pure Appl Chem 40: 149–155
Lee C-H, Mizusawa H, Kapepuda T (1981) Unwinding of double-stranded DNA helix by dehydration. Proc Natl Acad Sci USA 78: 2838–2842
Linskens HF (1982) Pollen collection during a balloon trip. Incompat Newslett 14: 116–121
Mansfield P, Pykett IL (1978) Biological and medical imaging by NMR. J Magn Reson 29: 355–360
Omasa K, Onoe M, Yamada H (1985) Nuclear magnetic imaging of intact plants. Environ Control Biol 23: 99–102
Poddubnaya-Arnoldi VA (1936) Water availability effects division of sperm cells in pollen grains. Planta 25: 502–510
Priestly DA, DeKruijff B (1982) Phospholipid motional characteristics in a dry biological system. A 31P-nuclear magnetic resonance study of hydrating Typha latifolia pollen. Plant Physiol 70: 1075–1078
Richards LA, Oagata G (1958) Thermocouple for vapor pressure measurement in biological and soil systems at high humidity. Science 128: 1089–1090
Saenger W, Hunter WN, Kennard O (1986) DNA conformation is determined by economics in the hydration of phosphate groups. Nature 324: 385–388
Schoper JB, Lambert RJ, Vasilas BL (1986) Maize pollen viability and ear receptivity under water and temperature stress. Crop Sci 26: 1029–1033
Setlow B, Setlow P (1987) Thymine-containing dimers as well as spore photoproducts are found in ultraviolet-irradiated Bacillus subtilis spores that lack small acid-soluble proteins. Proc Natl Acad Sci USA 84: 421–423
Shivanna KR, Heslop-Harrison J (1981) Membrane state and pollen viability. Ann Bot 47: 759–770
Smith KC, Hanawalt PC (1969) Molecular photobiology. Academic Press, New York London
Spanner DC (1951) Thermocouple psychrometer for leaf water potential. J Exp Bot 2: 145–149
Stanley RG, Linskens HF (1974) Pollen biology, biochemistry, management. Springer, Berlin Heidelberg New York
Varghese AJ (1970) 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun 38: 484–490
Vijayaraghavan MR, Bhatia K (1985) Cellular changes during microsporogenesis, vegetative and generative cell formation:a review based on ultrastructure and histochemistry. Int Rev Cytol 96: 263
Vithanage HIMV, Knox RB (1980) Periodicity of pollen development and quantitative cytochemistry of exine and intine enzymes in the grasses Lolium perenne and Phalaris tuberosa. Ann Bot 45: 131–142
Wang AHJ, Fujii S, Boom JH van, Rich A (1983) Right-handed and left-handed double-helical DNA:structural studies. In:Watson JD, Owen D, Brown D (eds) Structure of DNA. Cold Spring Harbor Symp Quant Biol 47: 33–34
Westgate ME, Boyer JS (1986 a) Reproduction at low silk and pollen water potentials in maize. Crop Sci 26: 951–956
Westgate ME, Boyer JS (1986 b) Silk and pollen water potentials in maize. Crop Sci 26: 947–951
Willemse MTM, Reznickov SA (1980) Formation of pollen in the anther of Lilium. Development of the pollen wall. Acta Bot Neerl 29: 127–140
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1989 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Jackson, J.F. (1989). Dehydration and Rehydration During Pollen Development, Pollination, and Fertilization. In: Linskens, HF., Jackson, J.F. (eds) Gases in Plant and Microbial Cells. Modern Methods of Plant Analysis, vol 9. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-83346-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-83346-5_9
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-83348-9
Online ISBN: 978-3-642-83346-5
eBook Packages: Springer Book Archive