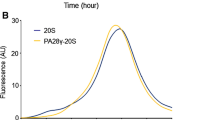
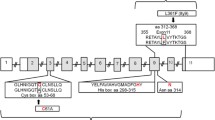
Abstract
In eukaryotes the major non-lysosmal proteolytic system is characterized by a high-molecular-mass ATP-dependent 26S protease (for review see Coux et al. 1996). This complex is composed of the 20S proteasome, forming the proteolytic core, and the 19S regulator PA700, rendering ATP dependence and binding sites for ubiquitinated protein substrates (Rivett 1993; Hochstrasser 1995). The essential 26S enzyme complex is, in collaboration with the ubiquitin signalling pathway, responsible for the selective turnover of cytosolic and nuclear proteins.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ahn JY, Tanahashi N, Akiyama KY, Hisamatsu H, Noda C, Tanaka K, Chung C, Shimbara N, Willy PJ, Mott JD, Slaughter CA, Demartino GN (1995) Primary structures of two homologous subunits of PA28, a gamma interferon inducible protein activator of the 20S proteasome. Febs Lett 366:37–42
Ahn K, Erlander M, Leturcq D, Peterson PA, Früh K, Yang Y (1996) In vivo characterization of the proteasome regulator PA28. J Biol Chem 271:18237–18242
Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski UH, Kloetzel PM (1994) Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J Exp Med 179:901–909
Chu-Ping MS, Laughter CA, DeMartino GN (1992) Purification and characterization of a protein inhibitor of the 20S proteasome (macropain). Biochim Biophys Acta 1119:303–311
Coux O, Tanaka K, Goldberg AL (1996) Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 65:801–847
DeMartino GN, Slaughter CA (1993) Regulatory proteins of the proteasome. Enzyme Protein 47:314–324
Deveraux Q, Ustrell V, Pickart C, Rechsteiner MA (1994) 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem 269:7059–7061
Dick LR, Aldrich C, Jameson SC, Moomaw CR, Pramanik BC, Doyle CK, DeMartino GN, Bevan MJ, Forman JM, Slaughter CA (1994) Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to yield antigenic peptides. J Immunol 15:23884–23894
Dick TP, Ruppert T, Groettrup M, Kloetzel PM, Kuehn L, Koszinowski UH, Stevanovic S, Schild H, Rammensee HG (1996) Coordinated dual cleavages induced by the proteasome regulator PA28 lead to dominant MHC ligands. Cell 86:253–262
Dubiel W, Pratt G, Ferrell K, Rechsteiner M (1992) Purification of an 11S regulator of the multicatalytic proteinase. J Biol Chem 267:22369–22377
Eggers M, Boes-Fabian B, Ruppert T, Kloetzel PM, Koszinowski UH (1995) The cleavage preference of the proteasome governs the yield of antigenic peptides. J Exp Med 182:1865–1870
Fehling HJ, Swat W, Laplace C, Kiihn R, Rajewsky K, Miiller U, von Boehmer H (1994) MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 265:1234–1237
Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL (1995) Inhibition of proteasome activity and subunit specific amino terminal modification of lactacystin. Science 268:726–731
Grant EP, Michalek MT, Goldberg AL, Rock KL (1995) Rate of antigen degradation by the ubiquitin proteasome pathway influences MHC class 1 presentation. J Immunol 155:3750–3758
Gray CW, Slaughter CA, DeMartino GN (1994) PA28 activator protein forms regulatory caps on proteasome stacked rings. J Mol Biol 236:7–15
Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel PM (1995) The interferon-gamma-inducible 11S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. J Biol Chem 270:23808–23815
Groettrup M, Soza A, Eggers M, Kuehn L, Dick TP, Schild H, Rammensee HG, Koszinowski UH, Kloetzel PM (1996a) A role for the proteasome regulator PA28CC in antigen presentation. Nature 381:166–168
Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM (1996b) Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today 17:429–435
Heinemeyer W, Simeon A, Hirsch HH, Schiffer HH, Teichert U, Wolf DH (1991) Lysosomal and non-lysosomal proteolysis in the eukaryotic cell: studies on yeast. Biochem Soc Trans 19:724–725
Hershko A (1996) Mechanisms and regulation of ubiquitin-mediated cyclin degradation. Adv Exp Med Biol 389:221–227
Hilt W, Wolf DH (1996) Proteasomes: destruction as a programme. Trends Biochem Sci 21 196–102
Hilt W, Enenkel C, Gruhler A, Singer T, Wolf DH (1993) The PRE4 gene codes for a subunit of the yeast proteasome necessary for peptidylglutamyl-peptide-hydroly- zing activity. Mutations link the proteasome to stress- and ubiquitin-dependent proteolysis. J Biol Chem 268:3479–3486
Hisamatsu H, Shimbara N, Saito Y, Kristensen P, Hendil KB, Fujiwara T, Takahashi E, Tanahashi N, Tamura T, Ichihara A, Tanaka K (1996) Newly identified pair of proteasomal subunits regulated reciprocally by interferon gamma. J Exp Med 183:1807–1816
Hochstrasser M (1995) Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol 7:215–223
Kania MA, DeMartino GN, Baumeister W, Goldberg AL (1996) The proteasome sub- unit, C2, contains an important site for binding of the PA28 (11S) activator. Eur J Biochem 236:510–516
Kopp F, Dahlmann B, Hendil KB (1993) Evidence indicating that the human proteasome is a complex dimer. J Mol Biol 229:14–19
Kopp F, Kristensen P, Hendil KB, Johnsen A, Sobeck A, Dahlmann B (1995) The human proteasome subunit HsN3 is located in the inner rings of the complex dimer. I Mol Biol 248:264–272
Kuckelkorn U, Frentzel S, Kraft R, Kostka S, Groettrup M, Kloetzel PM (1995) Incorporation of major histocompatibility complex-encoded subunits LMP2 and LMP7 change the quality of the 20S proteasome polypeptide processing products independent of interferon-gamma. Eur J Immunol 25:2605–2611
Kuehn L, Dahlmann B (1996) Reconstitution of proteasome activator PA28 from isolated subunits. Febs Lett 394:183–186
Lowe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R (1995) Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science 268:533–539
Mott JD, Pramanik BC, Moomaw CR, Afendis SJ, DeMartino GN, Slaughter CA (1994) PA28, an activator of the 20 S proteasome, is composed of two nonidentical but homologous subunits. J Biol Chem 269:31466–31471
Niedermann G, Butz S, Ihlenfeldt HG, Grimm R, Lucchiari M, Hoschutzky H, Jung G, Maier B, Eichmann K (1995) Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity 2:289–299
Niedermann G, King G, Butz S, Birsner U, Grimm R, Shabanowitz J, Hunt DF, Eichmann K (1996) The proteolytic fragments generated by vertebrate proteasomes: class I banding peptides. Proc Natl Acad Sci USA 93:8572–8577
Orlowski M, Cardozo C, Michaud C (1993) Evidence for the presence of five distinct proteolytic components in the pituitary multicatalytic proteinase complex. Properties of two components cleaving bonds on the carboxyl side of branched chain and small neutral amino acids. Biochemistry 32:1563–1572
Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, Mengede E, Kloetzel PM, Neefjes J, Koszinowski U, Melief CA (1996) A single residue exchange within a viral CTL epitope alters proteasome mediated degradation resulting in lack of antigen presentation. Immunity 5:115–124
Palombella VJ, Rando OJ, Goldberg AL, Maniatis T (1994) The ubiquitin-proteasome pathway is required for processing the NF-kappaBi precursor protein and the activation of NF-kappaB. Cell 78:773–785
Peters JM, Cejka Z, Harris JR, Kleinschmidt JA, Baumeister W (1993) Structural features of the 26 S proteasome complex. J Mol Biol 234:932–937
Piihler G, Weinkauf S, Bachmann L, Miiller S, Engel A, Hegerl R, Baumeister W (1992) Subunit stoichiometry and three-dimensional arrangement in proteasomes from Thermoplasma acidophilum. Embo J 11:1607–1616
Rivett AJ (1993) Proteasomes: multicatalytic proteinase complexes. Biochem J 291:1–10
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761–771
Rotem-Yehudar R, Groettrup M, Soza AS, Kloetzel P-M, Ehrlich R (1996) LMP-asso- ciated proteolytic activities and TAP-dependent peptide transport for class I MHC molecules are suppressed in cell lines transformed by the highly oncogenic adenovirus 12. J Exp Med 183:499–514
Schmidtke G, Kraft R, Kostka S, Henklein P, Frommel C, Lowe J, Huber R, Kloetzel PM, Schmidt M (1996) Analysis of mammalian 20S proteasome biogenesis: the maturation of beta-subunits is an ordered two-step mechanism involving autoca- talysis. Embo J 15:6887–6898
Seeger M, Ferrell K, Frank R, Dubiel W (1997) HIV Tat inhibits the 20S proteasome and its 11S regulator mediated activation. J Biol Chem (in press)
Seemiiller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W (1995) Proteasome from Thermoplasma acidophilum: a threonine protease. Science 268:579–582
Stein RL, Melandri F, Dick L (1996) Kinetic characterization of the chymotryptic activity of the 20S proteasome. Biochemistry 35:3899–3908
Tamura T, Nagy I, Lupas A, Lottspeich F, Cejka Z, Schoofs G, Tanaka K, Demot R, Baumeister W (1995) The first characterization of a eubacterial proteasome: the 20S complex of Rhodococcus. Curr Biol 5:766–774
Ustrell V, Pratt G, Rechsteiner M (1995) Effects of interferon-gamma on major histocompatibility complex encoded subunits on peptidase activities of human multi- catalytic proteases. Proc Natl Acad Sci USA 92:584–588
Van Kaer L, Ashton-Rickardt P, Geichelberger M, Gaczynska M, Nagashima K, Rock KL, Goldberg AL, Doherty PC, Tonegawa S (1994) Altered peptidase and viral specific T cell response in LMP2 mutant mice. Immunity 1:533–541
Yang Y, Friih K, Ahn K, Peterson PA (1995) In vivo assembly of the proteasomal complexes, implications for antigen processing. J Biol Chem 270:27687–27694
Zwickl P, Grziwa A, Pùhler G, Dahlmann B, Lottspeich F, Baumeister W (1992) Primary structure of the Thermoplasma proteasome and its implications for the structure, function, and evolution of the multicatalytic proteinase. Biochemistry 3:1964–1972
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1998 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Kloetzel, PM. (1998). The Function of Modulators in Proteasome MHC Class I Antigen Processing Activity. In: Eibl, M.M., Huber, C., Peter, H.H., Wahn, U. (eds) Symposium in Immunology VII. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-80466-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-80466-3_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-63360-0
Online ISBN: 978-3-642-80466-3
eBook Packages: Springer Book Archive