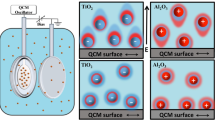
Abstract
In order to investigate the effect of confining surfaces on the structuring of nanoparticles in between, confining surface is modified by either attaching a mica sheet on the silica substrate or physically adsorbing polyelectrolytes on silica surfaces with layer-by-layer technique. In the first case, only the surface potential (or surface charge) is tuned. AFM force measurements show an enhanced amplitude in oscillatory forces while the wavelength and correlation length remain constant with increasing confining surface potential. This is an outcome of reduced particle-wall interaction range, due to the fact that the charged walls release additional counterions accumulated in a thin layer at the wall surfaces and contributed to the Debye length of particle-wall interaction. As a consequence, more particles can be accumulated inside the slit. In the second case, the effect of layer-by-layer modification on the surface potential and the surface roughness are also studied. Experimental findings reveal that the layer-by-layer technique modifies the surface roughness without changing the surface potential of a multilayer with the same outermost layer, by increasing number of constituent layers, ionic strength of the polyelectrolyte solutions, and by selecting an appropriate pair of polyelectrolytes. Wavelength and decay length are not affected by the surface roughness. The corresponding reduction in the oscillatory amplitude and the shift in the phase correlate with the increase in surface roughness. Increasing surface roughness further induces a vanishing of the oscillations and both confining surfaces contribute to the effect of surface roughness on the force reduction. In order to show an oscillatory force, the particles have to show positional correlation over a reasonably long range perpendicular to the surface and the correlation function should be the same over a larger lateral area. This requires that both the particles and the surfaces have a high degree of order or symmetry, otherwise the oscillation does not occur. A roughness of a few nanometers on a single surface, which corresponds to about 10 % of the nanoparticle diameter, is sufficient to eliminate the oscillatory force.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Typically, structural force of confined nanoparticles has a damped oscillatory character as a function of the surface separation [1, 2], reflecting the oscillatory density profile, signifying the formation of layers of nanoparticles parallel to the surfaces. In the previous chapter, the asymptotic behavior of the structural forces has been demonstrated, particularly the wavelength and decay length of the oscillations at large surface separations are governed by the pair structure in the corresponding bulk fluid. This observation is fully consistent with predictions from density functional theory (DFT) [3, 4], according to which the properties of the surfaces should become irrelevant in the asymptotic limit. On the other hand, DFT also predicts that the surface properties (or, more specifically, the interaction between a charged particle and a surface) do influence the amplitude and phase of the oscillations.
The properties of the surface are studied in two aspects in this chapter: the surface potential and the surface roughness. The surface potential is modified by depositing a negatively-charged mica sheet on top of a silicon wafer. To understand the mechanisms of the change in structuring after modifying the surface potential, a grand-canonical Monte Carlo simulation (GCMC) involving confined silica particles, which interact via the DLVO potential, is included [5]. The GCMC results only involving silica ions turn out to be highly sensitive with respect to the actual model for the interaction between a silica particle and the surface(s). In particular, the simulated observations are not reproduced even qualitatively and predicts an opposite behavior as the experimentally observed one when the simplest version of linearized Poisson-Boltzmann (PB) theory [6, 7] is employed, where the exponential decay of the potential is determined by the bulk Debye screening length and the wall potential only comes into play through a prefactor.
To solve this contradiction, a modified fluid-wall (particle-confining surface) potential is introduced, starting from a PB-like theory for a colloidal suspension next to one charged surface [8]. The modification consists of supplementing the bulk Debye screening length appearing in the simplest approach by a contribution from the wall counterions [5]. A similar idea though in a different context is followed in various earlier investigations [9–11]. In these studies, however, the contribution of the wall counterions to the screening parameter in the resulting potential was assumed to be homogeneous. In the present work, at least approximately, the inhomogeneity of the counterion distribution is taken into account, which yields a particle-wall screening length which depends both on the wall counterions (or equivalently, the wall charge) and on the distance between particle and wall. The full fluid-wall potential from the two charged surfaces is then constructed by linear superposition (LSA). The resulting potential is still purely repulsive, but displays a non-monotonic behavior as a function of the wall potential with respect to the degree and range of repulsion. In particular, within the experimentally relevant range of surface potentials, the GCMC results with the new fluid-wall potential model is in qualitative agreement with the experiments.
While oscillatory forces between smooth surfaces are relatively well understood, it is of central importance to understand the effect of surface roughness. Surface roughness is encountered naturally in almost all applicable systems. According to the previous molecular simulation [12, 13] and grand canonical Monte Carlo (GCMC) simulation studies on simple fluids [14–16], the roughness of the confining surfaces is just as important as the nature of the fluid molecules in determining the oscillatory forces. The registry of the confining surface is seen to play an important role on the equilibrium structures formed under confinement and the effect of random roughness reveals a reduction in the oscillatory force amplitude with increasing roughness. At roughness around 33 % of the fluid molecular diameter, the oscillation disappeared.
There are only a few prior experimental studies which have reported the oscillatory forces between surfaces that are no longer atomically smooth. For rough mica surfaces prepared by depositing a compressed Langmuir-Blodgett film of dioctadecyldimethyammonium bromide (DOAB) or adsorbing hexadecyltrimethylammonium bromide (CTAB), the oscillatory force profile of the non-polar liquid octamethylcyclotetrasiloxane measured by surface force apparatus (SFA) showed a reduced range of oscillation (DOAB) or even disappeared altogether (CTAB) [17]. This occurs even if the liquid molecules themselves are perfectly capable of ordering into layers. However, in this scarce experimental study, the influence of surface roughness was not separated from the effect of surface potential. It is not straightforward to study surface roughness since surface potential plays an important role in amplitude and phase of the oscillations [5] and often varies with the roughness.
To address this remaining problem, we apply the layer-by-layer technique in the present study to coat polyelectrolytes on the confining surface, thus modifying the surface properties. The layer-by-layer technique, or consecutive adsorption of oppositely charged polyelectrolytes, has been introduced by Decher et al. [18] This coating is possible because, for many polyelectrolytes, physisorption onto a charged surface is irreversible under mild conditions [19, 20]. Thus, after the first adsorption step the surface can again serve as a substrate for the adsorption of an oppositely charged polyelectrolyte and so on until the desired number of layers is adsorbed. The main features are that the properties of adsorbed polyelectrolyte layers can be easily controlled by the numbers of layers, the ionic strength [21–23], and by the type of polyelectrolyte.
The layer-by-layer technique is applied to a flat silicon wafer with a naturally deposited silica layer on the top, which is used as one confining surface, and to a silica microsphere, when necessary, which is used as the second confining surface. By means of the layer-by-layer physisorption technique, the roughness of the confining surfaces is modified without changing surface potential of a multilayer with the same outermost layer. With increasing number of layers or ionic strength of polyelectrolyte solutions or by selecting appropriate pairs of polyelectrolytes which display similar surface potential, the roughness of the confining surfaces is tuned.
The CP-AFM is then used to measure the oscillatory forces of silica nanoparticle suspensions confined between polyelectrolyte multilayer coated surface(s). The correlation between the amplitude and the phase of the oscillatory force profile of colloidal silica nanoparticles and the roughness of the confining surfaces is investigated. In addition, the effect of surface roughness on the characteristic lengths of the structuring, i.e., the inter-particle distance and the particle correlation length, is studied in this chapter.
5.2 Results and Discussion
5.2.1 Potential of the Confining Surface
In the force experiments two types of substrates are considered: (i) a silicon wafer with a native silica (\({ {SiO}}_{2}\)) top layer, and (ii) a freshly cleaved mica sheet deposited on top of a silicon wafer.Footnote 1 The corresponding surface potentials are \(\psi _\mathrm{S }\approx -80\) mV for silica and \(\psi _\mathrm{S }\approx -160\) mV for mica, respectively. The CP-AFM results for the force-distance curves of 26 nm sized silica particle suspensions, \(F(h)\), involving two different (silica and mica) surfaces are presented in Fig. 5.1. One immediately sees that the larger (absolute) surface potential related to the mica surface leads to a pronounced enhancement of the oscillations as compared to the silica surface. To quantify the effect force curves have been fitted according to Eq. 2.14. Results for the amplitude \(A\), wavelength \(\lambda _{f}\), and correlation length \(\xi _{f}\) are given in Table 5.1.
AFM force curves of 26 nm sized silica particle suspensions confined between a silica micro-sphere (on an AFM cantilever) and a silica (top) and mica (bottom) surface characterized by surface potentials \(\psi _\mathrm{S }\approx -80\) and \(-160\) mV, respectively. The data have been vertically shifted for ease of viewing. The solid lines are fits according to Eq. 2.14. Three concentrations are represented on each surface
The data show that the amplitude \(A\) obtained for the (more strongly charged) mica surface is nearly twice as large as that for silica. On the other hand, the wavelength \(\lambda _{f}\) and correlation length \(\xi _{f}\) of the oscillations remain essentially unaffected. Similar results were previously observed for confined polyelectrolytes [24]. From a conceptual perspective, the constant behavior of \(\lambda \) and \(\xi \) suggests that the characteristic lengths are determined rather by the pair structure among the particles than by their interaction with the wall. Indeed, the experimental observation that the surface potential influences the amplitude but not the characteristic lengths are fully consistent with rigorous predictions from DFT [4].
Clearly, such an enhancement in oscillation amplitude can arise due to various mechanisms, including the possibility that more particles move from the connected bulk reservoir into the slit. Indeed, such a situation has recently been observed in an investigation of charged colloids in a charged wedge [11], where the colloids turn out to accumulate in the cusp due to a localized, attractive region in the interaction potential between a colloid and the walls. Another possible explanation for the present observation is that the increase of wall potential strongly enhances the Coulomb repulsion between the silica particles and the like-charged wall, leading to a stronger layering of particles inside of the slit.
To understand the underlying mechanisms, a grand-canonical Monte Carlo simulation of a coarse-grained model involving confined silica particles, which interact via the DLVO potential, is included as well [5]. Firstly, the GCMC simulation results based on the simplest model for the fluid-wall interaction which neglects the effect of wall counterions on the screening are briefly considered (Eq. 2.23). Corresponding numerical data for the normalized normal pressure \(f(L_{\mathrm {z}} )=P_{\mathrm {zz}} -P_{\mathrm {b}} \) as a function of the wall separation \(L_{\mathrm {z}} \) (same as h used in the AFM force curves) and the (negative) surface potential \(\psi _\mathrm{S }\) are presented in Fig. 5.2.
Dimensionless solvation pressure \(f^{*}(L_{\mathrm {z}} )=P^{*}_{\mathrm {zz}} -P^{*}_{\mathrm{ b}} \) for \(\phi \)=10.5 vol% and various surface potentials \(\psi _{\mathrm{S} }\) as calculated by GCMC simulations involving the simplest model. The values of \(W_{\mathrm{S} }^*=\beta W_\mathrm{S }\) correspond to \(\psi _\mathrm{S }=0, -2.7, -5.4, -10.9\), and \(-27.7\) mV, respectively. The inset shows the corresponding mean silica particle density \(\bar{\rho }^{*}\) as a function of the wall separation
All functions \(f(L_{\mathrm{ z}} )\) display the damped oscillatory behavior, with the oscillations vanishing upon reaching the bulk limit \(L_{\mathrm {z}} \rightarrow \infty \) (i.e., \(P_\mathrm{zz }\rightarrow P_\mathrm{b }\)). More significant in the present context, however, is the fact that the amplitude of \(f(L_{\mathrm {z}} )\) (and thus, the amplitude of the force) decreases monotonically upon increase of \(|\psi _{\mathrm{S} }|\). This clearly contradicts the AFM experimental results. From a theoretical point of view, the behavior of \(f(L_{\mathrm {z}} )\) is a direct consequence of the corresponding behavior of the fluid-wall potential which becomes progressively more repulsive upon increase of \(|\psi _\mathrm{S }|\). Thereby more and more particles are expelled from the slit. This is also reflected by the GCMC results for the mean silica density, \(\bar{\rho }\), plotted in the inset of Fig. 5.2: at fixed \(L_{\mathrm {z}} \), \(\bar{\rho }\) becomes smaller the more negative \(\psi _\mathrm{S }\) is. Indeed, the slit becomes essentially empty at small \(L_{\mathrm {z}} \) already at \(\psi _\mathrm{S }=-27.7\) mV, a wall potential far below that characteristic of a real silica surface.
Having in mind these (obviously wrong) predictions, the corresponding GCMC results based on a new fluid-wall potential, \({ u}_{\mathrm {FS}} (z)\) (Eq. 2.26) is considered in Fig. 5.3, where the screening parameter depends on \(\psi _\mathrm{S }\) and is space-dependent. This is motivated by the release of additional (wall) counterions which accumulate at the walls (Eq. 2.25). Clearly, the dependence of the functions \(f(L_{\mathrm {z}} )\) and \(\bar{\rho }(L_{\mathrm {z}} )\) on \(\psi _\mathrm{S }\) is non-monotonic. The extracted parameters, \(P_{\mathrm {max}} \) (height of the first maximum), \(\theta _\mathrm{f }\) (phase), \(\lambda _{\mathrm {f}} \) (wavelength), and \(\xi _{\mathrm {f}} \) (decay length) as a functions of \(\psi _\mathrm{S }\) were listed in the corresponding paper [5] upon fitting the curves with Eq. 2.21.
GCMC results for a the reduced solvation pressure and b the mean pore density \(\bar{\rho }\) at surface potentials \(\psi _\mathrm{S }=0, -40, -80\;(silica), 120\), and \(-160\) mV (mica). The corresponding bulk concentration is \(\phi \)=10.5 vol%. The solid lines are fit functions obtained from Eq. 2.21. c Shows the resulting structural forces \(F(L_{\mathrm {z}} )/2\pi R\) for \(\psi _\mathrm{S }=-80\) (dashed), \(-120\) (dotted), and \(-160\) mV (dot-dashed). For clarity the curves in (a) are shifted along the \(y\)-axis
When “switching on” the surface potential from \(\psi _\mathrm{S }=0\) up to a value of about \(|\psi _\mathrm{S }|=40\) mV the quantity \(P_{\mathrm {max}} \) first decreases. Upon further increasing \(|\psi _\mathrm{S }|\) towards \(80\) mV and \(160\) mV (which are the experimentally relevant values for silica and mica, respectively), \(P_{\mathrm {max}} \) increase. It is interesting in this context that the value of \(|\psi _\mathrm{S }|=40\) mV where \(P_{\mathrm {max}} \) changes its behavior corresponds to the “reversal point” of the fluid-wall potential. Similar to \(P_{\mathrm {max}} \), an increase of the maximum of the corresponding force-distance curves \(F(L_{\mathrm {z}} )\) obtained by integration of \(f(L_{\mathrm {z}} )\) can be observed (see Fig. 5.3c). Thus, GCMC simulations with a modified fluid-wall potential reproduce, on a qualitative level, the charge-induced enhancement of the oscillations observed in the CP-AFM experiments.
The potential effects on the solvation pressure are mirrored by corresponding effects on the mean density \(\bar{\rho }(L_{\mathrm {z}} )\) of the silica particles, which is plotted in Fig. 5.3b. In particular, within the experimentally relevant range of 80 mV \(\le |\psi _\mathrm{S }|\le 160\) mV, the density at a fixed separation \(L_{\mathrm {z}} \) increases with \(|\psi _\mathrm{S }|\), which is consistent with the enhancement of pressure oscillations. This enhancement of the mean particle density reveal that more particles move into the slit with increasing the surface potential. This is due to the corresponding decrease in the range of particle-wall interaction, resulting from the additional contribution of the wall-counterions into the Debye length. At a given wall separation, the shorter the particle-wall interaction range the more layers of particles can fit into the slit.
The changes in \(P_{\mathrm {max}} \) and \(F_{\mathrm {max}} \) with \(\psi _\mathrm{S }\) are accompanied by the changes in the phase shift, \(\theta _\mathrm{f }\). The latter displays a maximum at \(\psi _\mathrm{S }\approx -40\) mV. In general, the phase shift can be also considered as the depletion zone, which is the separation between the contact layer of particles and the wall. The decrease of \(\theta _\mathrm{f }\) for \(|\psi _\mathrm{S }|\ge 40\) mV is interpreted as a consequence of the corresponding decrease in the range of \(u_{\mathrm {FS}} (z)\). The less the particle-wall interaction range the narrower the depletion zone.
On the other hand, the wavelength \(\lambda _{\mathrm {f}} \) remains essentially constant when \(\psi _\mathrm{S }\) is changed, in agreement to the experimental observations. The correlation length \(\xi _{\mathrm {f}} \) varies only slightly, given the difficulties to obtain accurate values for this quantity (i.e., the large error bars). Nevertheless \(\xi _{\mathrm {f}} \) is judged to remain essentially unaffected as well. This is indeed what one would expect based on theoretical arguments: according to DFT, the precise nature of fluid-wall interactions does influence the amplitude and phase of the (asymptotic) pressure oscillations, but not their wavelength and decay length [4].
5.2.2 Roughness of the Confining Surface
5.2.2.1 Multilayer Characterization
To investigate the effect of roughness of confining surfaces on the ordering of nanoparticles, the silicon wafers and silica microspheres were modified by physisorption of two oppositely charged polyelectrolytes one by one, e.g., PAH and PSS. A layer of PEI was pre-adsorbed onto the silica surface for stabilizing the later adsorption [25].Footnote 2 Polyelectrolyte concentration was kept at \(10^{-2}\) monoM.
Ellipsometry measurements on film thickness as a function of the number of layers of polyelectrolyte adsorbed on the silicon wafer, i.e., si-PEI-PSS-(PAH/PSS)\(_{n}\), either with 0 or 0.1 M NaCl. n is the number of (polycation/polyanion) double layers and N is the number of total layers. \(N=1\) stands for only one layer of PEI, even number of \(N\) stands for PSS as outermost layer, while odd number of N, expect \(N=1\), stands for PAH as outermost layer
In order to ascertain that each polyelectrolyte had been adsorbed successfully onto the surface, ellipsometry measurements were made to characterize the film thickness grown on the silicon wafers. Figure 5.4 shows a regular growth of the thickness of PAH/PSS multilayer obtained from ellipsometry measurements at assembling salt concentration of 0 and 0.1 M NaCl, respectively, confirming the success of consecutive assembling. N refers to the number of total layers. In the salt-free case, the growth of the thickness was linear with an average thickness of 4.6 Å per pair of layers, while at the assembling salt concentration of 0.1 M NaCl, the growth of multilayer film was almost linear with an average thickness of 11 Å per pair of layers. It is obvious that after adding extra NaCl into the polyion solution during multilayer assembling, the thickness increases significantly. Adding salt to polyelectrolyte solution during the multilayer assembling can result in a strong screening of the segment charge on the polyelectrolyte chain and thus cause a coil conformation of the complexes [21–23, 26]. These results are consistent with the layer growth reported for PAH/PSS systems [27].
Zeta-potential measurements were performed on the silica probe assembled by \(10^{-2}\) monoM of PAH/PSS with PEI as the first layer. The zeta-potential changed from \(-55\) to \(+35\) mV after PEI was adsorbed and then oscillated between \(-44\) mV for PSS and \(+41\) mV for PAH (Fig. 5.5). The charge reversal confirmed the success of consecutive assembling in each step. The unchanged zeta-potential of PSS- or PAH-ended multilayers, irrespective of the number of assembled layers, indicates that the surface potential of polyelectrolyte adsorbed surfaces does not change with the number of layers [28–32].
The surface potentials of polyelectrolyte-assembled silicon wafers were determined by measuring the approaching part of the corresponding force curve (in “pure repulsion type” and “repulsion and adhesion type”, see later section “Force profiles in the absence of nanoparticles”) in Milli-Q water. The force curves were fitted with DLVO analysis, in which the electrostatic repulsions were calculated by solving numerically the non-linear Poisson-Boltzmann equation for two parallel surfaces, by assuming constant surface charge or constant surface potential [33].
Generally, fitting with constant surface charge or surface potential is in good agreement with experimental force at larger separations. At small distances the data is better approximated by the constant surface charge model (Fig. 5.6). The surface potential, or the surface charge, hardly changes with increasing number of PAH/PSS multilayer, as long as the multilayer is terminated with the same polyelectrolyte. The average value of surface potentials of PSS terminated multilayers was taken from multiple measurements on the same spot and also on different spots and remained around \(-45\) mV, while the average value of surface charges was around 2.0 mC m\(^{-2}\). No significant difference has been found between the values obtained from curves of “pure repulsion type” and “repulsion and adhesion type”. The calculated surface potential agrees with the zeta-potential study on silica microspheres within the margin of error (Fig. 5.7), indicating that the two methods measure the potential at a similar distance from the surface. A difference only exists for bare silica surfaces, which is probably due to the different cleaning process used for spherical and planar surfaces. The same DLVO analysis was also applied on silicon wafers coated with PAH/PSS multilayer in the presence of 0.1 M NaCl during assembly and on one layer of PAA and HA assembled silicon wafers, i.e. si-PEI-PAA and si-PEI-HA (see Table ). The surface potential of PAH/PSS with 0.1 M NaCl shows no significant difference in comparison to that of the salt-free case. The average value is \(-44\) mV and stays constant with increasing number of layers. The calculated surface potentials of PAA and HA are \(-40\) and \(-42\) mV, respectively, which are similar to the previously reported values for PAA and HA from zeta potential measurements [34–36] and also to the aforementioned surface potential of PSS.
The surface roughness is further analyzed from tapping-mode AFM images in aqueous medium at each step with PSS forming the outermost layer. AFM topography images of PAH/PSS multilayer coated silicon wafers immersed in Milli-Q water with scan size \(2.5\,\upmu \)m \(\times \) \(2.5\,\upmu \)m are shown in Fig. 5.8. With increasing number of adsorbed layers, the contrast on the surfaces becomes more significant, indicating that the surface roughness increases. The roughness was calculated as a root mean square value over each \(1.0\,\upmu \)m \(\times 1.0\,\upmu \)m box in images. The dependency of surface roughness of PAH/PSS multilayer with number of layers is shown in Fig. 5.9. The roughness of PEI-PSS coated silicon wafer is around 12 Å and increases to 21 Å for 10 layers of polyelectrolyte coated silicon wafers. A similar trend of increasing roughness with the number of multilayer up to 10 bilayers has been reported previously [37–39].
Root mean square roughness \(R_{RMS}\) of PAH/PSS multilayer films adsorbed on silicon wafers as a function of the number of layers prepared in 0 and 0.1 M NaCl. The roughness was calculated from \(1.0\,\upmu \mathrm{m}\times 1.0\,\upmu \mathrm{m}\) boxes on the AFM height images (in Milli-Q water, tapping mode)
The roughness increases with increasing ionic strength of the polyelectrolyte solutions, especially after four layers. The roughness of silicon wafer coated with six layers of polyelectrolyte prepared in 0.1 M NaCl is 19 Å, being close to the highest roughness obtained in the salt-free case. An increase in roughness with salt concentration coincides with previous reports [40, 41]. The roughness of PEI-PAA and PEI-HA coated silicon wafers are 35 and 60 Å, respectively. The higher roughness is related to a coil conformation of the polyelectrolytes, or more precisely the charge density of the polyelectrolytes (see Discussion).
5.2.2.2 Force Profiles in the Absence of Nanoparticles
The force profiles were taken as a series of measurements with the same silica microsphere on different spots of the same silicon wafer in Milli-Q water. Normally, 10 curves were acquired at each spot and 100 curves in total on a single surface. Three main types of force curves were observed between confining surfaces, based on the number of polyelectrolyte multilayer adsorbed on the surfaces. Figure 5.10a demonstrates the “pure repulsion type”, in which both approach and retraction branches show only repulsion between the silica microsphere and silicon wafer surface. In Fig. 5.10b, a weak adhesion appears in the retraction branch, while the approach part remains repulsive. This is referred to as the “repulsion and adhesion type”. In Fig. 5.10c, an attraction or no repulsion in the approach branch can also be observed in addition to an adhesion in the retraction branch. This is referred to as the “pure adhesion type”.
Three types of force curves between a silica microsphere and a silicon wafer coated with polyelectrolyte multilayer in Milli-Q water. a Pure repulsion type: complete coverage of polyelectrolyte on the surfaces, no detachment of polyelectrolyte. b Repulsion and adhesion type: complete coverage, polyelectrolyte chains get entangled. c Pure adhesion type: attraction between two oppositely charged patches due to the detachment of polyelectrolyte or incomplete coverage of polyelectrolyte on the surfaces
The reversible transition between “pure repulsion type” to “repulsion and adhesion type” curves can occur in the same experiment. This transition was observed both on the same spot and on the different spots of the silicon wafer. These two types of force curves are most likely observed on surfaces coated with a larger number of polyelectrolyte layers. In the case of only a few adsorbed layers, the “pure adhesion type” curve can be observed occasionally and the transition back to the other two types is irreversible. It is only possible for the samples which show attraction or no repulsion to restore repulsive behavior upon re-dipping the silicon wafers and/or silica microsphere into the last adsorbed polyion solution.
The same repulsive behavior in the approach branch of the “pure repulsion type” and “repulsion and adhesion type” curves and their coexistence in the same experiment indicate that the corresponding polyelectrolytes do not detach from the surface. If some polyelectrolyte chains transferred from one surface to the other, a partial charge reversal would be expected, resulting in a consequent reduction or annihilation of the repulsive force. The surface potential determined using DLVO analysis shows that there is no significant difference between the values obtained from the “pure repulsion type” and “repulsion and adhesion type” curves, which proves that the surface charge remains constant and no detachment occurs. The observed weak adhesion upon retraction therefore does not have an electrostatic origin. Instead, weak adhesion seems to arise when adsorbed polyelectrolyte chains from the opposing surfaces get entangled. The adhesion takes place in the retraction branch, due to the detachment of the silica microsphere from the silicon wafer and to the bridging and extension of the accompanied polyelectrolyte chains [30].
In Fig. 5.10c, an attraction or no repulsion on the approach branch and no spontaneous transition to a repulsive type curve in a measurement indicate that a partial charge reversal indeed occurs at the surfaces. This type of force curve is mostly due to the detachment of polyelectrolyte chains from one surface to other or the incomplete coverage of the surfaces, which results in a strong interaction of the polycation on one surface with the polyion on the opposing one (briefly, the attraction between two oppositely charged patches). This detachment or incomplete coverage mechanism is confirmed by the fact that this type of force only appears for the first few layers of a coated surface and disappears upon increasing the number of layers. For DLVO surface potential determination, the approaching branch of the “pure repulsion type” and “repulsion and adhesion type” curves should be used and not “pure adhesion type” curves.
5.2.2.3 Force Spectroscopy with Nanoparticles
The force curves of 4.0 vol% of 26 nm silica particle suspensions measured between a bare AFM silica microsphere and a silicon wafer assembled with varying number of PAH/PSS multilayer are shown in Fig. 5.11, where N represents the number of total polyelectrolyte layers with PEI as preadsorbed layer and PSS as the outermost layer. It is obvious that as more polyelectrolytes adsorb onto the silicon wafer, the amplitude of the oscillatory force decreases and the position of first maximum (referred to as the “phase”) gradually shifts to larger separations. By fitting the oscillatory force with Eq. 2.14, constant wavelength \(\lambda \) and decay length \(\xi \) are obtained, which remain the same as in the case of bare silicon wafer, indicating that the inter-particle distance and the correlation length, respectively, are not affected by the surface roughness. These observations are consistent with the findings of density function theory (DFT) [4].
Figure 5.12 shows the comparison of force curves of 4.0 vol% of 26 nm silica particle suspensions using a bare silica microsphere, both in the presence and absence of salt during preparation. In the case of one layer of PSS coated silicon wafer, prepared with 0.1 M NaCl, the oscillation has almost the same amplitude as obtained from that without salt during assembling. In contrast to this, the oscillation in the case of \(\mathrm{PEI-PSS-(PAH-PSS) }_{2}\) assembled silicon wafer, with PSS and PAH both prepared in 0.1 M NaCl solution, has a significantly smaller amplitude compared to the oscillation obtained from the silicon wafer coated with same number of layers in absence of salt during the preparation. A phase shift of the experimental curves toward larger separations accompanied the reduction in force oscillation.
Comparison of force curves of 4.0 vol% of 26 nm silica particle suspensions in respect to bare silica microsphere or PAH/PSS multilayer coated silica microsphere on the silicon wafers coated with N number layers of PAH/PSS multilayer. \(N+N\) means that the silicon wafer and silica microsphere both are coated with N number of layers
Reduced oscillatory amplitude is not only observed upon increasing the number of layers or adding salt during assembly, but it also occurs upon assembling the second confining surface, i.e., the silica microsphere, with PAH/PSS multilayer. The additional influence of coating the second confining surfaces on the force profiles of 4.0 vol% of 26 nm silica particle suspensions is shown in Fig. 5.13, where the oscillatory forces between a bare silica microsphere and PAH/PSS multilayer coated silicon wafers are compared with the forces obtained from both surfaces coated with PAH/PSS multilayer. The extra adsorption on the second surface leads to a further reduction in the oscillation amplitude and the oscillation vanishes faster than in the case where just one surface has been modified.
When polyanions PAA and HA were used instead of PSS, the surface forces showed pure monotonic behavior already on silicon wafers coated with only one layer of PAA or HA. Figure 5.14 shows the comparison of surface forces of 4.0 vol% of 26 nm silica particle suspensions on bare silicon wafer and PEI-PAA and PEI-HA coated silicon wafer, using a bare silica microsphere.
5.2.2.4 Force Amplitude Correlation to Surface Roughness
According to previous studies [22, 23, 27], the charge density of the polyelectrolyte plays a very important role in the thickness and roughness of the multilayer. In general, reducing the charge density promotes a coiled polymer chain conformation. The pKa of PAA is about 6.5 [42, 43] which is close to the pH of the assembling solution. Thus the charge density of PAA is assumed to be lower than that of PAH, which has a pKa of 8.8 [43], and of PSS which is a strong polyanion and charged throughout a large pH range. HA has the lowest charge density among these polyelectrolytes. The distance of chargeable groups is 10 Å compared to 2.5 Å in the case of PAH, PSS and PAA. The increased thickness and roughness with increasing ionic strength of the polyelectrolyte solution is also due to the reduced charge density on the polyelectrolyte chains, resulting from the charge screening by the counterions [21, 26]. The layer-by-layer technique can therefore effectively tune the surface roughness with respect to the effective polymer charge.
It’s interesting to note that, although the polymer charge density is different for different types of polyelectrolytes at pH 6.5, the measured zeta potential and/or DLVO-analyzed surface potential do not show a significant difference. This might be due to the fact that the shear plane in zeta potential measurements and/or the constant potential range used in calculation are beyond the length scale which is important for observing difference in the surface potentials. Even if there were local variation in surface charges of the outermost layers, they would be compensated by counterions within length scales shorter than the distance of the shear plane and/or constant surface potential range.
In the present work, the reduction in amplitude and shift in phase of the oscillations occurs in four cases: (1) increasing the number of PAH/PSS multilayer, (2) increasing ionic strength of polyelectrolyte solutions during assembly, (3) replacing the bare silica microsphere with one coated with PAH/PSS multilayer, and (4) by adsorbing a layer of PAA or HA onto the silicon wafer instead of PAH/PSS layers. One can exclude the surface potential as dominant factor for the suppression in oscillation in the present study because our results show no change in potential in the above-mentioned cases. Thus the dominant factor of the force damping and phase shift is the increased surface roughness.
The oscillation of the force profile of nanoparticles vanished around the roughness threshold of 21 Å for a single coated confining surface, shown in Fig. 5.11. The effect of roughness of both confining surfaces contributed to the force profiles. This was confirmed by the significant reduction in the oscillation when the second coated surface was introduced (Fig. 5.13). The significant damping in the oscillation at six layers of polyelectrolyte prepared from 0.1 M NaCl in comparison to the negligible change at two layers, shown in Fig. 5.11, correlated with the significantly increased roughness at six layers and negligibly changed roughness at two layers, shown in Fig. 5.9.
At the roughness threshold, the surface topography presented sufficient difference in varied separation distance to smear out the oscillations and gave instead a pure monotonic behavior. In order to show an oscillatory force, the nanoparticles must be able to show positional correlation over a reasonably long range perpendicular to the surface and the correlation function should be the same over a larger lateral area. This requires that both the nanoparticles and the surfaces have a high degree of order or symmetry, otherwise the oscillation would not occur. A roughness of a few nanometers on a single surface (2.1 nm), which corresponds to about 10 % of the nanoparticle diameter (26 nm), was sufficient to eliminate the oscillatory force.
The reduction of the oscillation amplitude and the phase shift toward larger separations correlate with the results obtained by grand canonical Monte Carlo simulation on a Lennard-Jones fluid confined between two rough surfaces [14]. The roughness-induced reduction in amplitude and change in phase can be modeled as a superposition of oscillatory forces between many smooth surfaces lying at slightly different distances. In addition, the simulation result showed the oscillation vanished at surface roughness around 33 % of the fluid molecular diameter. This is larger than the approximate 10 % of the nanoparticle diameter as the roughness threshold to eliminate the oscillatory force of the silica nanoparticles. The silica nanoparticles supposedly yield greater oscillation amplitude and thus larger roughness threshold, although, the smaller roughness threshold might result from the shear flow in the real experiment and the higher polydispersity of the nanoparticles. The shear flow registered in the real experiments, due to the hydrodynamic friction force that accompanied the approach of two surfaces, would disturb the ordering of nanoparticles in the confinement [44]. The varying surface roughness could also affect the local hydrodynamic friction force [45]. The polydispersity of the silica nanoparticles in contrast to the ideal spherical molecules used in the simulation may be another reason for the reduction in the amplitude [46]. In addition, some other parameters set in the simulation may differ from the ones in the real experiment, such as the surface potential.
Finally, it is worth mentioning that the elasticity of confining surfaces can also influence the ordering of the nanoparticles. Measurements between a rigid silica microsphere and a bubble reveals that the surface elasticity reduces the amplitude of oscillations (see Chap. 6). In the present study, however, the thickness of the multilayer in the most cases was less than 6 nm, thus the effect of surface elasticity was suppressed by the hard silica surface. If there was an effect of surface elasticity, it was manifested as surface force curves that did not increase perpendicularly after the “contact point” but rather deviated from the straight line because of deformation of the surface. This deviation was not observed in the present study even though for the thickest film of 12 nm (PAH/PSS, \(N=10\), at 0.1 M NaCl), indicating that the effect of surface elasticity was negligible.
5.3 Conclusion
The confining surfaces were modified by attaching a mica sheet onto a silica substrate or by physically adsorbing polyelectrolytes onto silica surfaces with the layer-by-layer technique. The enhanced surface potential, or surface charge in the first case results in an increase in the oscillatory force amplitude. The underlying mechanism is the fact that more particles move from the connected bulk reservoir into the slit, indicated by the grand-canonical Monte Carlo simulations with a modified particle-wall potential assuming that the charged walls release additional counterions which accumulated in a thin layer at the surfaces. On the other hand, the wavelength and correlation length which characterize the asymptotic behavior of the oscillation have been shown not to change with the confining surface potential, both by experiment and simulation, in agreement with prediction from density functional theory.
In the second case, the wavelength and correlation length of the oscillation have also been shown to be affected neither by the number of multilayers nor by the pair of the polyelectrolytes. A reduced oscillatory amplitude, however, is observed with increasing the number of multilayers and the ionic strength as well as the charge density of the polyelectrolyte chains. The surface potentials of multilayers have been found not to change with the number of multilayers, ionic strength of the polyelectrolytes solution, or pair of polyelectrolytes (although they change after first layer regarding to the bare silica surface), the reduction in the force amplitude thus correlates with the consequently increased surface roughness. A roughness of a few nanometers on a single surface, which corresponded to about 10 % of the nanoparticle diameter, was sufficient to eliminate the oscillatory force of 26 nm diameter silica nanoparticles.
Notes
- 1.
Reprinted with permission from: Impact of surface charges on the solvation forces in confined colloidal solutions, Stefan Grandner, Yan Zeng, Regine von Klitzing, and Sabine H. L. Klapp, The Journal of Chemical Physics, 2009, 131, 154702. Copyright (2009), American Institute of Physics.
- 2.
Reprinted with permission from: Oscillatory Forces of Nanoparticle Suspensions Confined between Rough Surfaces Modified with Polyelectrolytes via the Layer-by-Layer Technique, Yan Zeng, Regine von Klitzing, Langmuir, 2012, 28, 6313–6321. Copyright (2012) American Chemical Society.
References
Israelachvili, J., & Pashley, R. (1983). Nature, 306, 249–250.
Stubenrauch, C., & von Klitzing, R. (2003). Journal of Physics: Condensed Matter, 15, R1197–R1232.
Evans, R., Henderson, J., Hoyle, D., Parry, A., & Sabeur, Z. (1993). Molecular Physics, 80, 755–775.
Grodon, C., Dijkstra, M., Evans, R., & Roth, R. (2005). Molecular Physics, 103, 3009–3023.
Grandner, S., Zeng, Y., v. Klitzing, R., & Klapp, S. H. L. (2009). Journal of Chemical Physics, 131, 154702.
Hansen, I. R., & McDonald, J. P. (2006). Theory of simple liquids (3rd ed.). Amsterdam: Academic Press.
Hansen, J., & Lowen, H. (2000). Annual Review of Physical Chemistry, 51, 209–242.
Bhattacharjee, S., Elimelech, M., & Borkovec, M. (1998). Croatica Chemica Acta, 71, 883–903.
Denton, A., & Lowen, H. (1998). Thin Solid Films, 330, 7–13.
Allahyarov, E., D’Amico, I., & Lowen, H. (1999). Physical Review E, 60, 3199–3210.
Loewen, H., Haertel, A., Barreira-Fontecha, A., Schoepe, H. J., Allahyarov, E., & Palberg, T. (2008). Journal of Physics: Condensed Matter, 20, 404221.
Curry, J., Zhang, F., Cushman, J., Schoen, M., & Diestler, D. (1994). Journal of Chemical Physics, 101, 10824–10832.
Gao, J., Luedtke, W., & Landman, U. (2000). Tribology Letters, 9, 3–13.
Frink, L., & van Swol, F. (1998). Journal of Chemical Physics, 108, 5588–5598.
Diestler, D., & Schoen, M. (2000). Physical Review E, 62, 6615–6627.
Porcheron, F., Schoen, M., & Fuchs, A. (2002). Journal of Chemical Physics, 116, 5816–5824.
Christenson, H. (1986). Journal of Physical Chemistry, 90, 4–6.
Decher, G. (1997). Science, 277, 1232–1237.
Decher, G., & Schlenoff, J. (2003). Multilayer thin films. Weinheim: Wiley-VCH.
von Klitzing, R. (2006). Physical Chemistry Chemical Physics, 8, 5012–5033.
Kovacevic, D., Van der Burgh, S., de Keizer, A., & Stuart, M. (2002). Langmuir, 18, 5607–5612.
Schlenoff, J., & Dubas, S. (2001). Macromolecules, 34, 592–598.
Steitz, R., Leiner, V., Siebrecht, R., & von Klitzing, R. (2000). Colloids and Surfaces A, 163, 63–70.
Qu, D., Brotons, G., Bosio, V., Fery, A., Salditt, T., Langevin, D., et al. (2007). Colloids and Surfaces A, 303, 97–109.
Lowack, K., & Helm, C. (1998). Macromolecules, 31, 823–833.
Kovacevic, D., van der Burgh, S., de Keizer, A., & Stuart, M. (2003). Journal of Physical Chemistry B, 107, 7998–8002.
Ruths, J., Essler, F., Decher, G., & Riegler, H. (2000). Langmuir, 16, 8871–8878.
Sukhorukov, G., Donath, E., Davis, S., Lichtenfeld, H., Caruso, F., Popov, V., et al. (1998). Polymers for Advanced Technologies, 9, 759–767.
Smith, R., McCormick, M., Barrett, C., Reven, L., & Spiess, H. (2004). Macromolecules, 37, 4830–4838.
Bosio, V., Dubreuil, F., Bogdanovic, G., & Fery, A. (2004). Colloids and Surfaces A, 243, 147–155.
Dejeu, J., Buisson, L., Guth, M. C., Roidor, C., Membrey, F., Charraut, D., et al. (2006). Colloids and Surfaces A, 288, 26–35.
Schwarz, B., & Schonhoff, M. (2002). Langmuir, 18, 2964–2966.
Chan, D., Pashley, R., & White, L. J. (1980). Journal of Colloid and Interface Science, 77, 283–285.
Burke, S., & Barrett, C. (2004). Pure and Applied Chemistry, 76, 1387–1398.
Richert, L., Boulmedais, F., Lavalle, P., Mutterer, J., Ferreux, E., Decher, G., et al. (2004). Biomacromolecules, 5, 284–294.
Burke, S., & Barrett, C. (2005). Biomacromolecules, 6, 1419–1428.
Lowman, G., & Buratto, S. (2002). Thin Solid Films, 405, 135–140.
Soltwedel, O., Ivanova, O., Nestler, P., Mueller, M., Koehler, R., & Helm, C. A. (2010). Macromolecules, 43, 7288–7293.
Ricotti, L., Taccola, S., Bernardeschi, I., Pensabene, V., Dario, P., & Menciassi, A. (2011). Biomedical Materials, 6, 031001.
Dubas, S., & Schlenoff, J. (2001). Macromolecules, 34, 3736–3740.
Losche, M., Schmitt, J., Decher, G., Bouwman, W., & Kjaer, K. (1998). Macromolecules, 31, 8893–8906.
Roma-Luciow, R., Sarraf, L., & Morcellet, M. (2000). Polymer Bulletin, 45, 411–418.
Choi, J., & Rubner, M. (2005). Macromolecules, 38, 116–124.
Kleinschmidt, F., Stutbenrauch, C., Delacotte, J., von Klitzing, R., & Langevin, D. (2009). Journal of Physical Chemistry B, 113, 3972–3980.
Bonaccurso, E., Butt, H., & Craig, V. (2003). Physical Review Letters, 90, 144501.
Piech, M., & Walz, J. (2002). Journal of Colloid and Interface Science, 253, 117–129.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zeng, Y. (2012). Structuring of Nanoparticles Between Modified Solid Surfaces. In: Colloidal Dispersions Under Slit-Pore Confinement. Springer Theses. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-34991-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-34991-1_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-34990-4
Online ISBN: 978-3-642-34991-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)