Abstract
Some decades ago it was assumed that students do not have any preconceptions or knowledge in chemistry: good preparation for a chemistry lesson only had to decide, which new terms were to be introduced in which order and with which experiments or models. Empirical studies, however, showed that learners have preconceptions for many topics and that these preconceptions do not match today’s scientific concepts. For that reason a first basic question of chemistry education is: which preconceptions exist for which topics and how can we effect conceptual change? Often the preconceptions are simply called “false” – without considering that students make correct observations and create individual mental models on the base of their observations. Therefore, these conceptions should better be called: Conceptions of everyday life Primary or prescientific conceptions Student precomprehension or preconception Misconcepts or misconceptions
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Strube W (1976) Der historische Weg der Chemie. Grundstoffindustrie, Leipzig
Reuber R, Wellens H, Gruss K (1972) Chemikon – Chemie in Übersichten. Umschau, Frankfurt
Lockemann G (1950) Geschichte der Chemie. de Gruyter, Berlin
Bugge G (1955) Das Buch der Grossen Chemiker. Band 1. Chemie, Weinheim
Dijksterhuis FJ (1956) Die Mechanisierung des Weltbildes. Springer, Berlin
Lasswitz K (1890) Geschichte der Atomistik. Bände 1 und 2. Voss, Hamburg
Barke H-D (1995) Strukturorientierter Chemieunterricht und Teilchenverknüpfungsregeln. Chemie in der Schule 42:49
Driver R (1985) Children’s ideas in science. University Press, Philadelphia
Pfundt H (1975) Ursprüngliche Vorstellungen der Schüler für chemische Vorgänge. MNU 28:157
Münch R et al (1982) Luft und Gewicht. NiU-P/C 30:429
Weerda J (1981) Zur Entwicklung des Gasbegriffs beim Kinde. NiU-P/C 29:90
Pfundt H (1981) Das atom – Letztes Teilungsstück oder Erster Aufbaustein. Chimdid 7:75
Novick S, Nussbaum J (1981) Pupils’ understanding of the particulate nature of matter. Sci Ed 65:187
Barke H-D (1987) Irgendwas muß doch da sein – der Horror vacui in den Schülervorstellungen vom Aufbau der Gase. GDCh-Mitteilungsblatt Nr. 10, Frankfurt
Ausubel DP (1974) Psychologie des Unterrichts. Weinheim, Beltz
Piaget J, Inhelder B (1971) Die Entwicklung des raeumlichen Denkens beim Kinde. Klett, Stuttgart
Pfundt H (1975) Urspruengliche Erklaerungen der Schueler für chemische Vorgaenge. MNU 28:157
Duit R (1996) Lernen als Konzeptwechsel im naturwissenschaftlichen Unterricht. In: Lernen in den Naturwissenschaften. Kiel, IPN
Temechegn E, Sileshi Y (2004) Concept cartoons as a strategy in learning, teaching and assessment in chemistry. Addis Ababa, Ethiopia, University Print
Taber K (2002) Chemical misconceptions – prevention, diagnosis and cure, vol 1, 2. Royal Society of Chemistry, London
Barke H-D (2009) Misconceptions in chemistry – addressing perceptions in chemical education. Springer, Heidelberg, New York
Petermann K, Friedrich J, Oetken M (2008) Das an Schuelervorstellungen orientierte Unterrichts-verfahren. Chemkon 15:110
Barke H-D, Doerfler T (2009) Das an Schuelervorstellungen orientierte Unterrichtsverfahren: beispiel neutralisation. Chemkon 16:141
Barke H-D, Strehle N, Roelleke R (2007) Das Ion im Chemieunterricht – noch Vorstellungen von gestern ? MNU 60:366
Sileshi Y (2007) The particulate nature of matter. Diagnosis of misconceptions and their remedy in chemical education. Schueling, Muenster
Johnstone AH (1997) Chemistry teaching – science or alchemy? J Chem Educ 74:268
Mahaffy P (2004) The future shape of chemistry education. Chem Educ: Res Pract 5:229
Vygotsky LS (1978) Mind and society: the development of higher mental processes. Harvard University Press, Cambridge
Shulman LS (1986) Those who understand: knowledge growth in teaching. Educ Res 15:4
Becker H-J (1988) Verbraucherfragen im RIAS-Telefonstudio: Gegenstand fachdidaktischer Forschung? Chim did 14:69
Further Reading
Andersen B (1990) Pupils conceptions of matter and its transformation (Age 12–16). Stud Sci Educ 18:53–85
Benson DL, Wittrock MC, Baur ME (1993) Students’ preconceptions of the nature of gases. J Res Sci Teach 30:587–597
Ben-Zvi R, Eylon BR, Silberstein J (1986) Is an atom of copper malleable? J Chem Educ 63:64–66
Boo HK (1998) Students’ understanding of chemical bonds and the energetics of chemical reactions. J Res Sci Teach 35:569–581
Cakmakci G, Leach J, Donnelly J (2006) Students’ ideas about reaction rate and its relationship with concentration or pressure. Int J Sci Educ 28:1795–1815
Carson EM, Watson JR (1999) Undergraduate students’ understanding of enthalpy change. Univ Chem Educ 3:46–51, Available from http://www.rsc.org/images/Vol_3_No2_tcm18-7037.pdf
Chandrasegaran AL, Treagust DF, Mocerino M (2007) The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students’ ability to describe and explain chemical reactions using multiple levels of representation. Chem Educ Res Pract 8:293–307, http://www.rsc.org/images/Chandrasegaran%20final_tcm18-94351.pdf
Driver R, Leach J, Scott P, Wood-Robinson C (1994) Young people’s understanding of science concepts: implications of cross-age studies for curriculum planning. Stud Sci Educ 24:75–100
Horton C (2001) Student preconceptions and misconceptions in chemistry. [Online] Accessed May 27, 2004 from http://daisley.net/hellevator/misconceptions/misconceptions.pdf
Ivarsson J, Schoultz J, Säljö R (2002) Map reading versus mind reading: revisiting children’s understanding of the shape of the earth. In: Limón M, Mason L (eds) Reconsidering conceptual change. Issues in theory and practice. Kluwer, Dordrecht, pp 77–99
Johnson P (1998) Children’s understanding of changes of state involving the gas state, Part l: Boiling water and the particle theory. Int J Sci Educ 20(5):567–583
Johnson P (1998) Children’s understanding of changes of state involving the gas state, Part 2: Evaporation and condensation below boiling point. Int J Sci Educ 20(6):695–709
Kind V (2004) Beyond appearance: students’ misconceptions about chemical ideas (2nd Edn). School of Education Durham University, Durham
Nakhleh MB (1993) Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual thinkers in chemistry. J Chem Educ 70:52–55
Nakhleh MB, Mitchell RC (1993) Conceptual learning versus problem solving: there is a difference. J Chem Educ 70:190–192
Othman J, Treagust DF, Chandrasegaran AL (2007) An investigation of the relationship between students’ conceptions of the particulate nature of matter and their understanding of chemical bonding. Int J Sci Educ 30:1531–1550
Özmen H, Ayas A (2003) Students’ difficulties in understanding conservation of matter in open and closed-system chemical reactions. Chem Educ Res Pract 4:279–290
Read JR (2004) Children’s misconceptions and conceptual change in science education. Available from http://acell.chem.usyd.edu.au/Conceptual-Change.cfm
Sewell A (2002) Constructivism and student misconceptions: why every teacher needs to know about them. Aust Sci Teach J 48(4):24–28
Singer JE, Tal R, Wu H (2003) Students’ understanding of the particulate nature of matter. School Sci Math 103(1):28–44
Sneider CI, Ohadi MM (1998) Unraveling students’ misconceptions about the Earth’s shape and gravity. Sci Educ 82:265–284
Stavy R (1988) Children’s conception of gas. Int J Sci Educ 10(5):553–560
Stavy R (1990) Children’s conception of changes in the state of matter: from liquid (or solid) to gas. J Res Sci Teach 27(3):247–266
Taber K (1997) Students understanding of ionic bonding: molecular versus electrostatic framework. School Sci Rev 78(285):85–95
Taber K (2002) Chemical misconceptions-prevention, diagnosis and eure Volume I: theoretical background. Royal Society of Chemistry, London
Taber KS (1996) Chlorine is an oxide, heat causes molecules to melt, and sodium reacts badly in chlorine: a survey of the background knowledge of one A-level chemistry class. Sch Sci Rev 78:39–48
Treagust DF (1988) Development and use of diagnostic tests to evaluate students’ misconceptions in science. Int J Sci Educ 10:159–169
Zoller U (1990) Students understandings and misconceptions in College Freshman chemistry (general and organic). J Res Sci Teach 27(10):1053–1065
Author information
Authors and Affiliations
Corresponding author
Appendices
Problems and Exercises
-
P1.1.
Some students’ misconceptions are also conceptions from scientists of past centuries. Give three examples and show parallels between historic conceptions and preconceptions of students today. Explain the scientific bases of the given examples.
-
P1.2.
In explanations of natural processes or laboratory phenomena, children often reason in a “magical-animistic” way. Name three examples for such reasoning. Explain the scientific bases and suggest corrected explanations that are suitable for children.
-
P1.3.
Chemical compounds are often described by sentences like “they contain the elements” or they “consist of elements” (“water consists of hydrogen and oxygen”). Which problems hide behind such comments? Suggest expressions that are correct from your point of view. Consider Dalton’s atomic model and propose correct expressions from that point of view as well.
-
P1.4.
Concerning the structure of matter terms like “continuum” and “discontinuum” on one hand, “preformed and nonpreformed particles” or the “horror vacui” on the other hand are used in chemical education. Explain these discussions. Which teaching ideas, which experiments and models do you suggest for teaching these issues?
-
P1.5.
Experiments are convincing instruments to make students realize their misconceptions and to motivate them to reduce or to change their misconceptions in favor of scientific ideas. Describe three examples of misconceptions and the experimental procedure to outline the reduction of these mistakes.
Experiments
-
E1.1.
Torricelli’s Experiment on the Existence of the Vacuum
Problem: Air pressure is neither palpable nor concrete for scientists of past centuries or today’s students, even when the weather forecast gives the current air pressure in millibar or hectopascal every day. Torricelli’s historic experiment is a good opportunity to illustrate the balance of a 20-km-air column and a 760-mm-mercury column. On the basis of this experiment students are able to understand a mercury barometer, and so the air pressure.
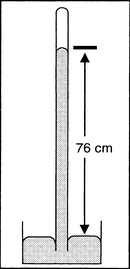
Material: Safety bowl, small crystallizing dish, glass tube (90 cm, sealed on one end), pipette, small funnel (to fit into the opening of the glass tube), folding ruler; mercury (T).
Procedure: The crystallizing dish in the safety bowl is to be filled with mercury up to 3 cm. The glass tube – over the safety bowl – is to be totally filled with mercury with the help of the funnel and the pipette, sealed with the index finger and opened under mercury in the crystallizing dish. The height of the metal column is to be measured with the ruler. The glass tube is to be put into an inclined position and back into an upright position.
Observation: The mercury column sinks – depending on current air pressure – to a height of 750–770 mm. In the inclined position the glass tube is totally filled with mercury, the mercury column sinks to the metered value again in the upright position.
Disposal: The mercury is to be put back into the container. If part of the metal remains in the safety bowl it is to be picked up with the mercury tongs. The glass tube is to be sealed with a stopper and stored in the cabinets for further experiments.
-
E1.2.
Reaction of Copper Oxide and Hydrogen
Problem: This experiment exemplarily shows that elements can be obtained from a compound: they are not “gone away” after their reaction. Redish-brown, shiny copper can be obtained from the reaction of black copper oxide with hydrogen – although copper cannot be seen in the black substance. It is possible to discuss “bearer of properties” versus “new substances.” Simultaneous oxidation and reduction can be illustrated as well as redox reactions in terms of oxygen transfer.
Material: Combustion tube with plug and outlet tube, porcelain ship, test tube; black copper oxide (Xn), hydrogen (F+), cobalt chloride paper.
Procedure: Hydrogen is to be passed through the combustion tube, which contains a porcelain ship filled with copper oxide. The hydrogen has to be ignited on the outlet tube after a negative test of hydrogen–oxygen mixtures. The copper oxide is to be heated with a burner. As soon as the reaction sets in, recognizable by the formation of metallic copper, the burner can be switched off. After the reaction the tube has to be cooled off in the hydrogen stream – otherwise the copper reacts back to copper oxide. Then the hydrogen stream can be stopped.
Observation: By the glowing substance red brown copper appears, drops of a liquid can be observed in the outlet tube; these drops can be identified as water with cobalt chloride paper.
Disposal: The copper can be used for further experiments or it is being oxidized in air to reuse copper oxide for the next experiment.
-
E1.3.
Mass Change During Acetone Evaporation
Problem: Students often understand the evaporation as a disappearance of the substance. The evaporation and the well-known mass decrease can be observed on a balance in a first step, in a second step the vapor can be collected in a gas syringe to show that a gaseous substance remains, that acetone only changes from the liquid state of matter to the gaseous state.
Material: Analytical balance with display, watch glass, Erlenmeyer flask with glass beads and drilled stopper, gas syringe; acetone (F) or ether (F+).
Procedure: Put some drops of acetone or ether on the watch glass and weigh it on the balance. Wait 1 min. Put some drops into the Erlenmeyer flask with glass beads, attach the gas syringe, shake the Erlenmeyer flask, and observe the moving piston.
Observation: The balance shows a decreasing mass until the liquid has evaporated. The gas syringe fills bit by bit until the gas volume is constant.
-
E1.4.
Dissolution of Alkaline Metals in Water
Problem: The popular reaction of lithium or sodium and water leads students to the assumption that the metals “disappear irretrievably.” One has to show to students that the metals and water react to new substances: on the one hand a gas is formed (hydrogen) and acid–base indicators switch their colors indicating an alkaline reaction. On the other hand white sodium hydroxide or lithium hydroxide can be obtained after the evaporation of water from the solution. From these white solids one could obtain the pure metals by electrolysis of the melts.
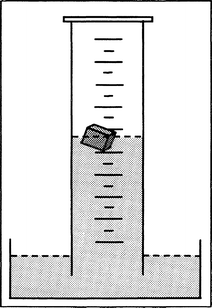
Material: Big glass bowl, plain cylinder with cover glass, test tubes, beaker, tweezers, knife, burner, filter paper; sodium (F/C), lithium (F/C), phenolphthalein solution in ethanol (F), universal indicator solution (F).
Procedure: (a) Fill the glass bowl half-full with water and place it on the overhead projector. Put a piece of sodium on the water surface and observe the sodium ball. Repeat the experiment a couple of times.
(b) Fill the cylinder with water and open it under water with help of the cover glass. Taking tweezers push a piece of lithium (not sodium!) into the water under the cylinder and observe it. After the reaction invert the plain cylinder and ignite the formed gas.
(c) Test both solutions in a test tube with each indicator solution. Let the water from a part of the solution evaporate in the beaker.
Observation: In the bowl, the moving sodium piece, a fizzing sound, and gas formation can be observed during the reaction. In the cylinder the colorless gas is collected and ignited: a red flame can be observed for a short time. The indicators change their colors: phenolphthalein from colorless to red, universal indicator from red to blue. After evaporating the water solid white substance remains in the beaker.
Disposal: Put remains of sodium or lithium metal in ethanol and wait for their dissolution. Dilute all solutions so that they can be disposed off down the drain.
-
E1.5.
Comparison of Petrol and a Solution of Fat in Petrol
Problem: Students think that the fat disappears when grease spots are being removed from clothes by petrol: “the spots are gone irretrievable.” Students have to understand that spot removing is nothing else but the dissolution of fat in petrol and the evaporation afterwards, after the evaporation the fat remains in the cloth.
Material: Petrol ether (F/XN/N), solution of olive oil in petrol ether, filter paper.
Procedure: Put some drops of petrol ether on one filter paper and some drops of the fat solution on another filter paper at the same time. Observe both papers for a minute.
Observation: The spot of the pure dissolvent shrinks and evaporates residue free, the spot of the fat solution also shrinks, but the fat remains.
-
E1.6.
Burning Metals on a Balance
Problem: Due to everyday experience students believe that substances loose mass and become lighter during combustion – like in the familiar combustion of alcohol, wood, paper, or candles. Metal combustion shows that due to the bound part of oxygen in the solid metal oxide instead of a mass decrease there actually happens a mass increase. This can be demonstrated in experiments on a balance. The masses of candles during combustion will be observed later (E1.7).
Material: Beam balance, digital balance, porcelain crucible with cover, tripod and clay triangle, crucible tongs; iron wool, magnesium ribbon (F).
Procedure: (a) Iron wool that hangs on one side of the balanced beam is to be ignited; blow gently if necessary to accelerate the reaction and to see the metal glowing.
(b) A strip of 10 cm magnesium ribbon is to be weighed in the porcelain crucible. The crucible is to be heated with a roaring flame until the magnesium ignites. To catch the white smoke of magnesium oxide the crucible is to be covered and opened and covered again several times during the reaction. The cooled crucible is to be weighed again.
Observation: The balance arm with the red-glowing iron wool sinks: formation of black iron oxide. The magnesium forms a white combustion product, while it glows brightly: magnesium oxide. The balance shows a bigger mass after the combustion. A green substance appears, when the white combustion product is being broken up: magnesium nitride.
-
E1.7.
Burning Candle on a Balance
Problem: Students are able to understand the mass increase during the combustion of metals and the formation of solid metal oxides. But they will argue that this does not apply to spirits, paper, or candles. To convince students that the mass increase also applies to these substances, a candle can be burnt on a balance and the invisible gaseous combustion products caught with special chemicals. Carbon dioxide and water vapor can be bound with soda lime, a mixture of sodium hydroxide and calcium oxide. The combustion gases are being absorbed in the device for this experiment (see picture). Due to the bound oxygen in the invisible gases, their mass is bigger than the burnt part of the candle before.
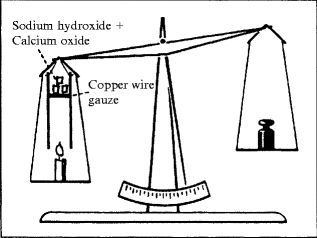
Material: Candle or tea light, beam balance, jar with soda lime (C) or sodium hydroxide (C).
Procedure: The upper part of the jar is to be filled loosely with soda lime so that the formed gas can flow through the pieces. The pans are to be balanced and the candle is to be lit. If smoke development occurs in the jar (the absorbent is packed too tightly!), the experiment has to be started again.
Observation: The balance pan with the burning candle sinks.
-
E1.8.
Conservation of Matter by Burning Iron Wool and Matches
Problem: The previous experiments have shown the increasing of mass due to bonding of oxygen in combustion products – for these experiments an open apparatus was chosen. If one takes a closed apparatus, weighs all educts first and later all reaction products, one will see that masses are not changing, but are conserved. Lomonossov and Lavoisier were the first to have found this law of conservation of mass in the eighteenth century. Dalton explained it in 1808 by combining the idea of elements with the idea of atoms, and stating that no atom can be created nor destroyed, instead atoms are rearranged in every chemical reaction.
Material: Digital scales, test tubes, balloons, burner; iron wool, matches.
Procedure: (a) A test tube is filled with iron wool, closed with a balloon and weighed on the balance. After heating the test tube with the burner and observing the iron wool, the test tube is weighed again once it cooled down to room temperature.
(b) A test tube is filled with five to eight matches, closed with a balloon and weighed on the balance. After heating the test tube close to the match heads with the burner and observing the matches, the test tube is weighed again after cooling it down to room temperature.
Observation: The balloon gets bigger and bigger; the iron wool glows red and black iron oxide is formed. The match heads are burning with a bright flame. In both cases the mass is the same before and after the reaction.
-
E1.9.
Conservation of Matter by Carbon Combustion
Problem: The experiments with iron wool and matches show that combustion products like solid iron oxide or the wooden remnants of the matches remain. In the case of carbon or charcoal nothing will remain and young students may think: “if the carbon is totally gone there is nothing to be weighed, the law of conservation of mass cannot be valid.” So it seems very important that the gaseous combustion product carbon dioxide must be shown and weighed to convince students that the law is valid even in this case. The next experiment (E1.10) will also show that gases have specific densities, that the density of carbon dioxide is higher than that of air.
Material: Digital scales, 2 L round bottom flask, balloon, burner, gas syringe with 20 cm glass tube, test tube; small pieces of charcoal, oxygen, lime water.
Procedure: The flask is filled with oxygen and 3–5 small pieces of charcoal, then closed with a balloon and weighed exactly. With the burner the pieces of carbon are heated from outside until they ignite, then the flask is turned around and around to show the glowing carbon pieces. After complete combustion the flask has to be cooled down and weighed again. The flask can be opened, with the syringe 100 mL of the gas is taken out of the flask and bubbled through 1–2 mL of lime water in the test tube.
Observation: The balloon gets bigger and bigger, and the carbon pieces glow very bright and disappear totally. The gas produces a milky precipitation in lime water: carbon dioxide.
-
E1.10.
Density of Air and Carbon Dioxide
Problem: Students are well aware of the existence of air around them, but not aware of air as a space-filling substance with special properties and measurable density. This density should be measured and compared to the density of carbon dioxide. The density of air can also be discussed in the context of air pressure (see E1.1).
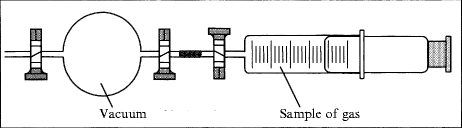
Material: Digital balance, 250-mL glass ball with valve, gas syringe, water jet pump, hose; air, carbon dioxide.
Procedure: The gas syringe is to be filled with 100 ml of air exactly and closed. The glass ball with valve is to be evacuated by the water jet pump and weighed exactly. The gas syringe is to be attached to the glass ball, and the air inside the gas syringe is transferred into it by opening the valve. The glass ball is to be weighed again. The density of air can be derived from the mass difference and the given volume. The experiment is to be repeated with carbon dioxide, both measured densities are compared.
Observation: 100 mL of air weigh 0.13 g; 100 mL of carbon dioxide weigh 0.20 g. The densities are 1.3 g/L for air (tabular value: 1.29) and 2.0 g/L for carbon dioxide (1.97).
Note: The experiment can also be run with the help of an empty plastic bottle with plug and valve. The bottle is to be weighed exactly with plug and valve. 100 mL of gas are to be pumped into the bottle with the help of the gas syringe. The bottle is to be weighed again.
-
E1.11.
Properties of Hydrogen and Other Colorless Gases
Problem: Students usually call colorless gases “air,” in everyday language they do not differentiate between air, oxygen, nitrogen, or carbon dioxide. Some colorless gases should therefore be introduced with some easy detection reactions, which show the different properties of these gases. Emphasis should be laid on hydrogen reactions, since the properties of hydrogen and the safety rules are especially new to students.
Material: Five gas cylinders with cover glass, wooden splint, balloon, combustion spoon, glass tube, beaker, empty tin can with a concentric hole (diameter: 1 mm); air, oxygen, nitrogen, carbon dioxide, butane (F+), lime water (Xi), hydrogen (F+).
Procedure: The first mentioned five gases are being filled into the cylinders by air displacement, and the gas jars are to be covered and labeled. A burning splint is to be held in every cylinder first, later only a glowing splint. For the distinction of nitrogen and carbon dioxide, a small amount of lime water is added to both jars and shaken. The jar with butane can be taken and poured out in the presence of a burning splint.
Observation: In air the burning splint is not changing its flame. In oxygen the splint burns very bright and even a glowing splint ignites (test for oxygen). The flame and the glowing splint go out in nitrogen and also in carbon dioxide, but in carbon dioxide a white milky substance appears from the colorless lime water (test for carbon dioxide). Butane ignites and burns with a calmly yellow flame. When pouring butane out of the cylinder it ignites and a big flame appears.
Procedure of Hydrogen Experiments
-
(a)
A balloon is to be filled with hydrogen, sealed and released
-
(b)
A candle is fixed on a 1-m-long glass tube and ignited, and the burning candle is to be put near the balloon until the reaction sets in (caution: bang)
-
(c)
Hydrogen streaming out of the steel container is to be ignited at a glass tube: a nearly invisible flame appears
-
(d)
A dry beaker with the opening down is to be held above the flame, the formed water vapor condenses and a layer of water can be seen inside the beaker
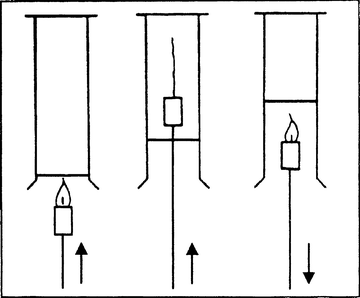
-
(e)
Hydrogen is to be filled in an upside-down gas jar by air displacement. A burning candle, which is fixed on a glass tube, is to be held inside the gas jar, the candle is to be taken out and held inside again (see picture)
-
(f)
A gas jar is to be filled with hydrogen upside-down and put on another gas jar of the same size which contains air. Both gases are being mixed by turning the gas jars. The gas jars are to be separated with cover glasses, the gas mixtures to be ignited with a burning splint (caution: bang)
-
(g)
The empty tin can with a concentric hole is to be put on a flat surface with the opening on the bottom and to be filled with hydrogen by air displacement. The gas is to be ignited at the hole and observed exactly (caution: very loud bang after about 20 s)
Observation: (a) The balloon rises, (b) the balloon burns with a loud bang, (c) the pure hydrogen burns calmly, (d) water vapor condenses in the beaker to form liquid water, (e) the candle goes out in the jar, but ignites again, when it is taken out of the jar, (f) the mixture of hydrogen and air (oxyhydrogen gas) burns very fast and forms water steam with a loud bang, (g) first hydrogen burns calmly (a sheet of paper can be put above the hole: it ignites), a buzzing sound can be heard after about 20 s and shortly after a loud bang (prepare students for that bang!).
-
(a)
-
E1.12.
Composition of Air
Problem: The students use the terms “good air” and “stale air” in their everyday language, but they do not think about the oxygen content. For this reason it is important to run experiments demonstrating the composition of air. It has to be explained to the students why a pure metal or phosphorous is being used: both are reacting with oxygen to form solid oxides, they take the oxygen out of the air and release other gases of the air mixture.
Material: Two 100 mL gas syringes, combustion tube, glass bowl, small gas cylinder with cover glass, splint, bell jar, combustion spoon with plug, ruler; iron wool, red phosphorus (F).

Procedure: (a) A combustion apparatus is to be built (see picture). The trapped air with a volume of 100 mL is to be lead over the heated iron wool a couple of times. The volume of the remaining gas is to be measured. The remaining gas is to be collected in the small gas jar pneumatically and tested with the burning splint.
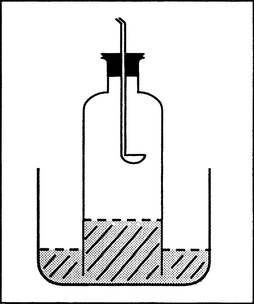
(b) A bell jar stands in the water of the glass bowl (picture). A spatula tip of phosphorus is to be put into the combustion spoon and ignited; the spoon is to be fixed inside the bell jar. The rising water level can be observed in the gas jar, and the remaining part of gas can be estimated.
Observation: (a) Iron wool glows and forms a black product, the gas volume decreases to 80 mL, and the remaining gas smothers a burning splint.
(b) Phosphorus burns for some time, a white smoke forms, the flame goes out, the water level in the bell jar rises, and the volume of the remaining gas is about 80% from before.
-
E1.13.
Carbon from Carbon Dioxide
Problem: Students may accept that metals can be obtained from the corresponding metal oxides. But they cannot imagine that carbon in form of a black solid can be obtained from the colorless gas carbon dioxide. The reaction of carbon dioxide with burning magnesium should be shown to convince students.
Material: Glass cylinder with cover, crucible tongs, burner; magnesium ribbon (F), carbon dioxide, sand.
Procedure: Some sand is to be put on the bottom of the gas jar for protection, and it is filled with carbon dioxide by air displacement and to be covered. With the crucible tongs a 10-cm-long ribbon of magnesium is to be ignited in the flame of the burner and put deep into the gas jar.
Observation: The flame does not go out and it burns with a crackling sound. Black spots can be observed on the inside of the gas jar and they prove to be soot when wiping them off with a finger.
-
E1.14.
Condensation of Butane under Pressure
Problem: Students know butane lighters and the term “liquid gas,” maybe they already observed the fluid and the gaseous butane phases in a transparent lighter. Despite such observations the term “liquid gas” remains as a misconception and one has to point out that an equilibrium exists between liquid and gaseous butane in a butane lighter or in a cartridge of camping gas. This can be demonstrated with the following experiment. The experiment also shows – besides some properties of butane gas – that the movement of butane particles must take a bigger volume than in the liquid, that there is no “butane,” “air,” or “other material” between the particles in a portion of gas (see also the next experiment E1.15).
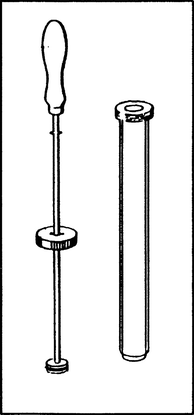
Material: Gas liquefaction pump, camping gas cartridge, hose; butane (F+) from the camping gas cartridge.
Procedure: The pump is to be opened and filled totally with butane by air displacement (get the hose deep into the cylinder and take it out slowly). The piston is being attached, pushed firmly into the cylinder of the pump and locked. The locking mechanism is being loosened and the liquid butane is being observed. This process can be repeated many times.
Observation: A big drop of liquid butane forms, when the gas is being compressed, the gas volume decreases to one-tenth. After the locking mechanism is being loosened, the piston moves out of the cylinder to the same extent as before, the drop disappears and cools down the bottom of the cylinder: the gas volume is the same as at the beginning.
-
E1.15.
Evaporation and Condensation of Ethanol
Problem: Young students accept that matter consists of smallest particles: ethanol particles exist in ethanol, water particles exist in water. When they have to interpret small particles in a gas and are asked about the space between the particles, they mostly think of “air” or “other material.” To demonstrate that there is nothing between those particles, ethanol is evaporated first to show the big volume compared with the liquid volume and then it is cooled down and condensed to the same very little volume as before: no air or other material is observed. The bigger volume of ethanol vapor should be interpreted by the higher movement of the ethanol particles and bigger distances between the particles.
Material: Beaker (2 L), electric heater, balloon, pipette, tweezers; ethanol.
Procedure: 1 L of water is heated in the beaker until it keeps boiling (at 100°C at normal pressure). 1–2 mL of ethanol are given into the balloon, closed by a knot and thrown into the boiling water (ethanol boils at 79°C). After some time the balloon is taken out of the water and thrown again into the boiling water.
Observation: The balloon increases to a volume of about 200–300 mL, after cooling down the same small volume of 1–2 mL as before is to be observed. Throwing the balloon again into the boiling water, the same big volume appears as before.
Some More Concept Cartoons for Diagnosis and Challenge of Misconceptions







Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Barke, HD., Harsch, G., Schmid, S. (2012). Learners Ideas and Misconceptions. In: Essentials of Chemical Education. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21756-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-642-21756-2_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21755-5
Online ISBN: 978-3-642-21756-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)









