Abstract
A direct approach to 2-(aminomethyl)indoles by copper-catalyzed domino three-component coupling–cyclization of 2-ethynylanilines with a secondary amine and aldehyde has been developed. By use of a cyclic or acyclic secondary amine and aldehyde (paraformaldehyde, aliphatic or aromatic aldehydes) in the presence of 1 mol.% CuBr, 2-ethynylanilines were converted to a variety of substituted 2-(aminomethyl)indoles in good to excellent yields. Utilizing this domino reaction and C–H functionalization at the indole C-3 position, polycyclic indoles were readily synthesized. Construction of benzo[e][1,2]thiazine and indene motifs by the reaction of sulfonamide and malonate congeners is also presented.
This is a preview of subscription content, log in via an institution.
Buying options
Tax calculation will be finalised at checkout
Purchases are for personal use only
Learn about institutional subscriptionsNotes
- 1.
The author considered decreasing of the amount of amine component is important and economical especially when using more valuable amines such as 11 (Scheme 4).
- 2.
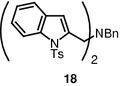
When benzylamine was used instead of a secondary amine, dimeric compound 18 was produced in 82% yield (100 °C, 3 h, then reflux, 1 h).
- 3.
When acetone was used instead of an aldehyde, Mannich-type reaction did not proceed and compound 19 was produced.

References
Gommermann N, Koradin C, Polborn K, Knochel P (2003) Angew Chem Int Ed 42:5763–5766
Gommerman N, Knochel P (2004) Chem Commun 2324–2325
Gommerman N, Knochel P (2005) Chem Commun 4175–4177
Knöpfel TF, Aschwanden P, Ichikawa T, Watanabe T, Carreira EM (2004) Angew Chem Int Ed 43:5971–5973
Aschwanden P, Stephenson CRJ, Carreira EM (2006) Org Lett 8:2437–2440
Espada A, Jiménez C, Debitus C, Riguera R (1993) Tetrahedron Lett 34:7773–7776
Rashid MA, Gustafson KR, Boyd MRJ (2001) Nat Chem 64:1454–1456
Glennon RA, Grella B, Tyacke RJ, Lau A, Westaway J, Hudson AL (2004) Bioorg Med Chem Lett 14:999–1002
Liu C, Masuno MN, MacMillan JB, Molinski TF (2004) Angew Chem Int Ed 43:5941–5945
Sonnenschein RN, Farias JJ, Tenney K, Mooberry SL, Lobkovsky E, Clardy J, Crews P (2004) Org Lett 6:779–782
Kusama H, Takaya J, Iwasawa N (2002) J Am Chem Soc 124:11592–11593
Bandini M, Melloni A, Piccinelli F, Sinisi R, Tommasi S, Umani-Ronchi A (2006) J Am Chem Soc 128:1424–1425
Kuroda N, Takahashi Y, Yoshinaga K, Mukai C (2006) Org Lett 8:1843–1845
Yasuhara A, Sakamoto T (1998) Tetrahedron Lett 39:595–596
Lombardino JG, Wiesman EH (1971) J Med Chem 14:973–977
Lombardino JG, Wiesman EH, McLamore WM (1971) J Med Chem 14:1171–1175
Lombardino JG, Wiesman EH (1972) J Med Chem 15:848–849
Zinnes H, Lindo NA, Sircar JC, Schwartz ML, Shavel J Jr (1973) J Med Chem 16:44–48
Zinnes H, Sircar JC, Lindo N, Schwartz ML, Fabian AC, Shavel J Jr, Kasulanis CF, Genzer JD, Lutomski C, DiPasquale G (1982) J Med Chem 25:12–18
Kwon S-K, Park M-S (1992) Arch Pharm Res 15:251–255
Lazer ES, Miao CK, Cywin CL, Sorcek R, Wong H-C, Meng Z, Potocki I, Hoermann M, Snow RJ, Tschantz MA, Kelly TA, McNeil DW, Coutts SJ, Churchill L, Graham AG, David E, Grob PM, Engel W, Meier H, Trummlitz G (1997) J Med Chem 40:980–989
Lee EB, Kwon SK, Kim SG (1999) Arch Pharm Res 22:44–47
Watanabe H, Mao C-L, Barnish IT, Hauser CR (1969) J Org Chem 34:919–926
Lombardino JG, Kuhla DE (1981) Adv Heterocycl Chem 28:73–126
Motherwell WB, Pennell AMK (1991) J Chem Soc Chem Commun 877–879
Nemazanyi AG, Volovenko YM, Neshchadimenko VV, Babichev FS (1992) Chem Heterocycl Comp 28:220–222
Manjarrez N, Pérez HI, Sorís A, Luna H (1996) Synth Commun 26:585–591
Manjarrez N, Pérez HI, Sorís A, Luna H (1996) Synth Commun 26:1405–1410
Takahashi M, Morimoto T, Isogai K, Tsuchiya S, Mizumoto K (2001) Heterocycles 55:1759–1769
Layman WJ, Greenwood TD, Downey AL, Wolfe JF (2005) J Org Chem 70:9147–9155
Vidal A, Madelmont J-C, Mounetou E (2006) Synthesis 591–593
Aliyenne AO, Kraïem J, Kacem Y, Hassine BB (2008) Tetrahedron Lett 49:1473–1475
Zia-ur-Rehman M, Choudary JA, Elsegood MRJ, Siddiqui HL, Khan KM (2009) Eur J Med Chem 44:1311–1316
Barange DK, Batchu VR, Gorja D, Pattabiraman VR, Tatini LK, Babu JM, Pal M (2007) Tetrahedron 63:1775–1789
Barange DK, Nishad TC, Swamy NK, Bandameedi V, Kumar D, Sreekanth BR, Vyas K, Pal M (2007) J Org Chem 72:8547–8550
Hatano M, Mikami K (2003) J Am Chem Soc 125:4704–4705
Bressy C, Alberico D, Lautens M (2005) J Am Chem Soc 127:13148–13149
Marchal E, Uriac P, Legouin B, Toupet L, van de Weghe P (2007) Tetrahedron 63:9979–9990
Parmentier J-G, Poissonnet G, Goldstein S (2002) Heterocycles 57:465–476
Costa M, Cá ND, Gabriele B, Massera C, Salerno G, Soliani M (2004) J Org Chem 69:2469–2477
Sakai S, Annnaka K, Konakahara T (2006) J Org Chem 71:3653–3655
Arcadi A, Bianchi G, Marinelli F (2004) Synthesis 610–618
Vlasov VM, Terekhova MI, Petrov ES, Sutula VD, Shatenshtein AI (1982) Zhurnal Organicheskoi Khimii 18:1672–1679
Larock RC, Fried CA (1990) J Am Chem Soc 112:5882–5884
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ohta, Y. (2011). Construction of 2-(Aminomethyl)indoles Through Copper-Catalyzed Domino Three-Component Coupling and Cyclization. In: Copper-Catalyzed Multi-Component Reactions. Springer Theses. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-15473-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-15473-7_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-15472-0
Online ISBN: 978-3-642-15473-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)



