Abstract
The severe acute respiratory syndrome (SARS) is a newly identified infectious disease caused by a novel zoonotic coronavirus (SARS-CoV) with unknown animal reservoirs. The risk of SARS reemergence in humans remains high due to the large animal reservoirs of SARS-CoV-like coronavirus and the genome instability of RNA coronaviruses. An epidemic in 2003 affected more than 8,000 patients in 29 countries, with 10% mortality.
SARS infection is transmitted by air droplets. Clinical and laboratory manifestations include fever, chills, rigor, myalgia, malaise, diarrhea, cough, dyspnoea, pneumonia, lymphopenia, neutrophilia, thrombocytopenia, and elevated serum lactate dehydrogenase, alanine aminotransferase, and creatine kinase activities. Health care workers are a high-risk group, and advanced age is strongly associated with disease severity. Treatment has been empirical, and there is no licensed SARS vaccine for humans so far. However, presence of long-lived neutralizing antibodies and memory T- and B-lymphocytes in convalescent SARS patients raises hope for active immunization. Furthermore, results from preclinical SARS vaccines expressing spike protein to elicit neutralizing antibodies and cellular responses that are protective in mouse and nonhuman primate models are encouraging.
Very little is known of the early events in viral clearance and the onset of innate and inflammatory responses during the SARS infection. Regulation of the innate immune response is associated with the development of adaptive immunity and disease severity in SARS infection. Notably, SARS-CoV has evolved evasive strategies to suppress antiviral type I interferon responses in infected cells. In addition, inflammatory responses are characterized by upregulation of proinflammatory cytokines/chemokines such as IL-6, IP-10, and MCP-1 in tissues and serum, and massive infiltrations of inflammatory cells such as macrophages in infected tissues.
Due to the lack of animal models that mimic the clinical manifestations of human SARS infection for mechanistic study and vaccine evaluation, development of a safe prophylactic SARS vaccine for human use remains a huge challenge. This chapter is written to summarize and highlight the latest clinical, serological, and immunological parameters relevant to the pathogenesis and protective immunity of SARS infection in humans.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Severe Acute Respiratory Syndrome
- Severe Acute Respiratory Syndrome
- Severe Acute Respiratory Syndrome Patient
- Venezuelan Equine Encephalitis Virus
- Modify Vaccinia Ankara
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The severe acute respiratory syndrome (SARS) is a newly identified infectious disease caused by a novel zoonotic coronavirus (SARS-CoV) with unknown animal reservoirs. SARS emerged first in the Guangdong province of China in late 2002 and had spread to 29 countries by July 2003. During the 2002 and 2003 outbreak, more than 8,000 cases with 9.6% mortality were reported from 29 countries (WHO, http//www.who.int.csr/sars/country/table2003_09_23/en/). Health care workers are the most vulnerable group, and advanced age (>60) is strongly associated with disease severity (reviewed in Cheng et al. 2007; Zhao 2007). SARS is transmitted by air droplets, and transmission efficiency is associated with severely ill patients and those with rapid clinical deterioration. The mean incubation period is 4.6 days with a variance of 15.9 days, and the infectious period is 7 days after illness onset. Clinical diagnosis of SARS is made by X-ray and CT radiography, and rapid definitive laboratory diagnosis is mainly by virus isolation and RT-PCR from respiratory and/or stool specimens.
The clinical spectrum of the outcome of SARS infection is highly variable, from mild flu-like symptoms to severe pneumonia (Tsang et al. 2003; Lee et al. 2003; Poutanen et al. 2003; Drosten et al. 2003; Ksiazek et al. 2003; Peiris et al. 2003). Typically, the disease is manifested by high fever (>38°C), chills, rigor, myalgia, malaise, diarrhea, cough, dyspnea, pneumonia, and rapidly progressing radiographic changes. Upper respiratory tract symptoms are not prominent, but watery diarrhea is common in most patients. About 10–15% of patients fail to respond to treatment and may progress to acute respiratory distress syndrome (ARDS), which is the major cause of death among fatal SARS cases.
The respiratory system, especially the lower lung, is the major site of SARS-CoV replication, although the virus can also be found in urine and fecal samples from SARS patients (Peiris et al. 2003). In autopsy samples, SARS-CoV can be detected in intestine, liver, kidney, brain, spleen, and lymph nodes, as well as lung samples, by immunohistochemical and in-situ-hybridization techniques (Nicholls et al. 2003, 2006; To et al. 2004; Gu et al. 2005). In the lungs, diffuse alveolar damage (DAD) with fibrosis is the prominent pathological feature of SARS infection, suggestive of active tissue injury and repair. Other pathological changes include massive infiltrations of inflammatory cells, predominantly macrophages, and enlargement of pneumocytes in the lungs. Lymphoid depletion is also common in the spleen and other lymphoid organs.
SARS-CoV is the largest RNA virus and is classified in group 2 of the Coronavirus family (Nicholls et al. 2006; Marra et al. 2003; Stadler et al. 2003). The virion is roughly 90–120 nm in diameter. It is an enveloped positive-strand RNA virus and contains a lipid bilayer surrounding a helical nucleocapsid structure that protects the genome. The genome size is about 30 kb in length and encodes 15 open reading frames (Orfs). Major Orfs encode four structural proteins: spike (S) protein (180/90 kDa), envelope (E) protein (8 kDa), membrane (M) protein (23 kDa), and nucleocapsid (N) protein (50–60 kDa). Replicase proteins encoded in the 5′-most two-thirds of the SARS-CoV genome are essential for polyprotein processing, replicase complex formation, and efficient virus replication. M protein and other Orfs, such as Orf 3a, Orf 3b, Orf 6 and Orf 7a, are important in apoptosis and antagonism of type I interferon responses in infected cells (Chan et al. 2007; Law et al. 2005b; Tan et al. 2004; Freundt et al. 2009). N protein is essential in the formation of the helical nucleocapsid during virion assembly. Recently, N protein was found to modulate TGF-β signaling to block apoptosis of SARS-CoV-infected host cells, with important implications in tissue fibrosis (Zhao et al. 2008).
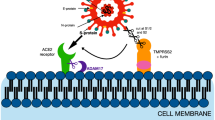
Entry of SARS-CoV into the host target cell is mediated by surface spike protein, which binds and fuses with the host cell. The mechanism of membrane fusion of SARS-CoV is very similar to that of class I fusion proteins, such as HIV gp160 and influenza hemagglutinin (reviewed in Kielian and Rey (2006)). The N-terminal half of the S protein (S1) contains the receptor-binding domain (RBD), and the C-terminal half (S2) is the membrane-anchored subunit that encodes a putative fusion peptide and two heptad repeat regions (HR1 and HR2) (Liu et al. 2004; Bosch et al. 2004). Entry of SARS-CoV into the host target cell involves receptor binding and a conformational change in the S protein, followed by cathepsin L-mediated proteolysis within endosomes (Simmons et al. 2005; Tripet et al. 2004; Bosch et al. 2003; Hofmann and Pohlmann 2004).
The functional receptor for the spike protein of SARS-CoV is a metallopeptidase, angiotensin-converting enzyme 2 (ACE2) (Li et al. 2003). It is expressed on human lung alveolar epithelial cells (type I and type II pneumocytes), enterocytes of the small intestine, brush borders of the proximal tubular cells of the kidney, endothelial cells of arteries and veins, and arterial smooth muscle cells in several organs (Hamming et al. 2004). SARS-CoV infection of ACE2-expressing cells is dependent on the activity of the proteolytic enzyme cathepsin L, which is pH-sensitive (Simmons et al. 2005; Huang et al. 2006). Hence, tissue tropism and viral replication of SARS-CoV in these organs could be explained by the distribution of the ACE2 receptor and cathepsin L activity in tissues. In addition, SARS infection could be enhanced by C-type lectins such as DC-SIGN and L-SIGN (Yang et al. 2004a; Jeffers et al. 2004). It is interesting to note that another newly identified human coronavirus, NL63, which only causes minor colds, also uses ACE2 as a receptor, and the infection is independent of cathepsin L (Hofmann et al. 2005; Huang et al. 2006). The protective function of ACE2 in acute lung injury is regulated by the balance of angiotensin and angiotensin-converting enzyme (ACE) in the renin–angiotensin pathway. Tissue injury caused by angiotensin II could be negatively regulated by ACE2 (Imai et al. 2005). In an animal model of acute lung injury, acute lung failure could be induced by injection of the SARS S protein, which downregulates ACE2; moreover, such effect could be attenuated by blocking the renin–angiotensin pathway (Kuba et al. 2005).
Although a huge global public health initiative by the WHO successfully contained the SARS outbreak in 2003, the risk of reemergence of SARS in humans remains high due to the large number of animal reservoirs that harbor SARS-CoV-like coronavirus. Phylogenetic study has shown that SARS-CoV is a zoonotic virus that crossed the species barrier and evolved in palm civets and humans, with the observation that the SARS-CoV became increasingly virulent following human-to-human transmission (reviewed in Zhao (2007)). However, failure to isolate SARS-CoV from wild or farmed civets from nonepidemic areas argued against civets being the natural reservoir of the virus (Shi and Hu 2008). Recent sequence analysis of SARS-CoV-like viruses from other wild animals suggests that wild bats are the natural reservoir of SARS-CoV (Lau et al. 2005; Li et al. 2005). If this is the case, it would be difficult to control the further spread of this virus to the human population, as bats are asymptomatic carriers of many human pathogens, such as Hendra and Nipha paramyxoviruses (Murray et al. 1995; Chua et al. 2000). Given the highly infectious properties and complex public health impact of SARS infection, there is an urgent need to develop effective treatments and prophylactic vaccines to control and prevent any future SARS outbreak.
So far, there has been no consensus regarding whether any treatment, especially the use of steroids and convalescent plasma therapy, could benefit SARS patients (Stockman et al. 2006). Moreover, the role played by the host immunity against SARS-CoV in viral clearance or tissue damage during the disease progression is not clear. High initial viral load is shown to be independently associated with severity of the disease and may be influenced by host immune responses (Chu et al. 2004). However, recent studies have suggested that type I IFN plays a key role in the switch from innate to adaptive immunity during the acute phase of SARS; indeed, patients with poor outcomes showed type I IFN-mediated immunopathological events and deficient adaptive immune responses (Cameron et al. 2007).
Several studies have shown that most recovered SARS patients have higher and sustainable antibody responses compared to those observed in fatal cases (Temperton et al. 2005; Ho et al. 2005). Taken together, this suggests that antibody responses are likely to play an important role in determining the ultimate disease outcome of SARS infection. Several forms of possible vaccines, such as attenuated or inactivated SARS-CoV, DNA, and viral vector-based vaccines have been evaluated in a number of animal models, including nonhuman primates (Roberts et al. 2008). Neutralizing antibodies to S protein are the major components of protective immunity (Yang et al. 2004b; Zhu et al. 2007). However, these animal models, including nonhuman primates, lack the severe clinical features observed in humans (Hogan 2006). Hence, it is difficult to evaluate whether these vaccines will prevent the disease in humans. Progress is further complicated by concerns over the safety of coronavirus vaccines; indeed, immune-mediated enhancement of pathology has been reported in other animal coronavirus infections (reviewed in Perlman and Dandekar (2005)) as well as in animals vaccinated with modified vaccine virus encoding SARS-CoV S protein (Weingartl et al. 2004). In addition, some variants of SARS-CoV are resistant to antibody neutralization, and the infection is enhanced by the antibodies (Yang et al. 2005b; Kam et al. 2007). As the inflammatory response is strongly implicated in the pathogenesis of SARS, a full understanding of the mechanism of protective immunity against SARS-CoV in humans is critical in the development of a safe prophylactic vaccine for use in humans.
2 Immune Responses
Host responses to viral infection include both immune activation and programmed cell death. Resistance to many viral infections is predominantly mediated by adaptive immunity comprising both humoral and cell-mediated immunity (reviewed in Zinkernagel and Hengartner (2004)). The antiviral effect of humoral immunity is mediated by the generation of neutralizing antibodies capable of blocking virus entry/infection of the target cells. The effector components of cell-mediated immunity, CD4 and CD8 T cells, mediate their antiviral effect through the secretion of cytokines/effector molecules and the killing of virus-infected target cells. Recently, there has been increasing evidence that the development of the adaptive immunity is governed by the initial interaction between invading microorganisms and the innate immune system. SARS-CoV virus has evolved a way to evade innate antiviral type I interferon responses of host cells in order to prolong viral replication and survival (reviewed in Frieman and Baric (2008)). Recent studies have also suggested that dysregulated type I and II interferon responses may culminate in a failure of the switch from hyperinnate immunity to protective adaptive immune responses in SARS patients with a poor outcome (Cameron et al. 2007).
3 Innate Immunity
Proteomic analysis of plasma from SARS patients shows activation of innate immune responses by SARS-CoV, including increased acute phase proteins such as serum amyloid A and MBL (Chen et al. 2004). MBL could bind to SARS-CoV and inhibit virus infectivity in vitro by blocking the carbohydrate-recognition domains of the S protein (Ip et al. 2005). Moreover, low MBL serum levels and haplotypes associated with MBL deficiency have been observed in SARS patients. Reduction of NK (CD3−CD56+CD16+) cells and CD158b+ NK cells is detected during the course of SARS infection, and such reduction is associated with disease severity (Anonymous 2004; He et al. 2005). However, the significance of these changes in SARS pathogenesis is not clear. In a C57BL/6 mouse model of SARS infection, NK and NKT cells are not required for viral clearance in beige and CD1−/− mice (Glass et al. 2004).
A drastic elevation of cytokines and chemokines (known as a cytokine storm) in the tissues and serum is a classical feature in acute SARS infection (Ng et al. 2004b; Wong et al. 2004; Zhang et al. 2004; Cheung et al. 2005; Law et al. 2005a; Huang et al. 2005; Tseng et al. 2005; Li et al. 2008). Although there is considerable variation in the literature with regards to the stage of sample collection and method of measurement, the general pattern is the same: during the first two weeks of illness onset, levels of IL1β, IL-6, IL-12, IFN-γ, IL-8, IP-10, and MCP-1 are markedly upregulated; a subsequent reduction in the level of these cytokines is associated with recovery from SARS pneumonia. These cytokines are predominantly proinflammatory cytokines and represent mainly the innate immune responses such as monocytes/macrophages and NK cells. The predictive value of cytokines in disease outcome is still limited. IL-6 has been strongly associated with radiographic score, and increased Th2 cytokines are observed in patients with a fatal outcome (Chien et al. 2006; Li et al. 2008). The presence of immunosuppressive molecules such as IL-10, TGF-β, and prostaglandin E2 (PGE2) in the serum of SARS patients may provide an alternative explanation for prolonged and severe clinical outcomes (Huang et al. 2005; Tseng et al. 2005; Li et al. 2008). Furthermore, dysregulation of type I and II interferon responses was associated with defective adaptive immunity and a severe disease outcome for SARS patients (Cameron et al. 2007).
The cellular source of proinflammatory cytokines/chemokines is identified by immunocytochemical methods and is validated by an in vitro infection model. IP-10 is detected in the infected pneumocytes and alveolar macrophages of infected lungs by immunohistochemical methods (Jiang et al. 2005). Under in vitro conditions, SARS-CoV infection could induce monocytes/macrophages, dendritic cells, and lung epithelial cells such as A549 to produce IL-1β, IL-6, IL8, IP-10, and MCP-1 (Cheung et al. 2005; Law et al. 2005a; Yen et al. 2006). Among all the primary cells tested, only type II pneumocytes could support SARS-CoV replication and release of viral particles in vitro (Mossel et al. 2008). Infections of purified monocytes, monocyte-derived macrophages/dendritic cells, and peripheral blood mononuclear cells (PBMC) by SARS-CoV are all abortive, without the production of mature viral particles, although production of chemokines is not impaired (Yilla et al. 2005; Cheung et al. 2005).
The role of the cytokine storm in the pathogenesis of SARS or similar human H5N1 infections is very complex, and the circular cause and consequence relationship of cytokines makes the interpretation very difficult. Attempts have been made to elucidate the mechanism with both in vitro and in vivo models. Using a polarized lung epithelial cell line, Calu-3, supernatants from the apical and basolateral domains of infected epithelial cells are potent in suppressing the phagocytic functions of macrophages, the maturation of dendritic cells, and T-cell priming by dendritic cells (Yoshikawa et al. 2008). Moreover, this inhibition is caused by IL-6 and IL-8 from SARS-infected epithelial cells (Yoshikawa et al. 2009). Recently, oxidized phospholipids (OxPC) resulting from severe inflammation have been detected in infected lung samples from patients with fatal SARS and H5N1 infections. These oxidized phospholipids are shown to stimulate IL-6 production from lung macrophages via the TLR4–TRIF–TRAF6 pathway in vitro (Sheahan et al. 2008) and in an animal model of acute lung injury. Furthermore, such stimulation occurred independently of MyD88, an adaptor protein in the classical pathway, which is triggered by lipo-polysaccharide (LPS) (Imai et al. 2008). However, in a separate study using a recombinant mouse-adapted SARS-CoV, mice deficient in MyD88 were defective in inflammatory cell recruitment and more susceptible to lethal SARS-CoV (rMA15) infection (Sheahan et al. 2008). Compared to wild-type mice, MyD88−/− mice have a more enhanced pulmonary pathology, and most die by day 6 postinfection. The pathology is associated with increased viral loads, reduction of proinflammatory cytokines, and mononuclear infiltrates in the infected lungs. These data suggest that innate and inflammatory responses mediated by MyD88 are important for protective immunity in lethal mouse-adapted SARS (rMA15) infection. As excessive inflammatory cytokines have been implicated in severe lung disease such as SARS and H5N1 infection, the importance of the pathogenic or protective role of proinflammatory cytokines and chemokines in disease progression needs to be clarified for future treatment strategies.
4 Viral Evasive Strategies
Inhibition of antiviral type I interferons (IFN) is a characteristic of SARS infection, as well as other group 2 coronaviruses, such as mouse hepatitis virus (reviewed in Frieman and Baric (2008)). It has been found that production of type I IFN is impaired in cells infected with SARS-CoV, but pretreatment of cells with IFN prevents growth of SARS-CoV (Spiegel et al. 2005; Cinatl et al. 2003). This suggests that the virus has evolved strategies to overcome the antiviral IFN response in infected host cells. Type I IFNs are rarely detected in acute SARS patients, and SARS-CoV is sensitive to the antiviral effect of pegylated IFN-α in a cynomolgus monkey model (Haagmans et al. 2004). Data from in vitro experiments show that suppression of the type I IFN response in SARS-CoV infected cells is due to the inactivation of interferon regulatory factor 3 (IRF-3), which is a latent cytoplasmic transcriptional factor that regulates IFN transcription (Spiegel et al. 2005). Other accessory proteins of SARS-CoV also function as potent IFN antagonists via various mechanisms. N protein inhibits interferon synthesis, whereas Orf 3b and Orf 6 proteins inhibit both IFN synthesis and signaling. Orf 6 protein also inhibits nuclear translocation of STAT1 (Kopecky-Bromberg et al. 2006). Recently, Orf 3b has been shown to be a shuttling protein and inhibits induction of type I IFN induced by retinoic acid-inducible gene 1 and the mitochondrial signaling protein (Freundt et al. 2009). Moreover, M protein inhibits type I IFN production by impeding the formation of the TRAF3–TANK–TBK1/IKKε complex (Siu et al. 2009). In addition, NSP1 protein of SARS-CoV could suppress host IFN-β gene expression by promoting host mRNA degradation and inhibiting translation (Narayanan et al. 2008). Papain-like protease (PLpro), a polyprotein from SARS-CoV replicase proteins, inhibits the phosphorylation and nuclear translocation of IRF-3, thereby disrupting the activation of type I IFN responses through either the Toll-like receptor 3 or the retinoic acid-inducible gene I/melanoma differentiation-associated gene 5 pathway. Such inhibition of the IFN response is independent of the protease activity of PLpro (Devaraj et al. 2007). Besides, SARS-CoV infected cells activate both PKR and PERK, resulting in a sustained eIF2alpha phosphorylation. However, viral replication and viral-induced apoptosis are unaffected (Krahling et al. 2009).
5 T Cell Responses
Lymphopenia is a common hematological feature in acute SARS infection, as with other acute viral infections such as measles, Ebola, and respiratory syncytial virus (Schneider-Schaulies et al. 2001; Formenty et al. 1999; Openshaw et al. 2001). Lymphopenia is observed in most SARS patients (>98%) in different studies, and most of them have reduced T-helper (CD4) and T-cytotoxic/suppressor (CD8) cell counts during the early phase of their illness; these reach their lowest values on day 5 and 7 from disease onset (fever), and restore gradually during the recovery phase (Wong et al. 2003; Lam et al. 2004; He et al. 2005). Low CD4 and CD8 lymphocyte counts at presentation are associated with adverse outcomes. B-lymphocyte count and the ratio of T-helper to T-cytotoxic/suppressor lymphocytes (CD4:CD8) remain normal and stable. Lymphopenia as a result of enhanced cell death during the acute SARS infection is attributed to the activation of cytoplasmic caspase 3 in both CD4 and CD8 lymphocytes (Chen et al. 2006). Transfection of SARS E protein in Jurkat T cells could also induce T-cell apoptosis by inactivating antiapoptotic protein Bcl-xL (Yang et al. 2005a). However, very little is known of whether apoptosis of bystander uninfected T cells is present in acute SARS infection.
There is little data available regarding the kinetics and correlation of SARS-specific T-cell responses in viral clearance and disease severity during the acute phase of primary SARS infection. Most of the information on the antigen-specific T-cell response in humans is from retrospective studies using PBMC samples from convalescent SARS patients (Chen et al. 2005; Zhou et al. 2006; Peng et al. 2006a, 2006b; Yang et al. 2006, 2007; Poccia et al. 2006; Li et al. 2008). However, antigen-specific responses from convalescent samples provide information on the memory response of T lymphocytes after primary SARS infection; this is important in the generation of the design of prophylactic SARS vaccines. Recent advances have provided more sensitive techniques to detect and enumerate low frequency antigen-specific T cells by ELISPOT assay, and to identify their surface, maturation, and functional phenotypes by multiparameter flow cytometry at a single cell level.
Using a matrix of overlapping peptides spanning the entire SARS-CoV proteome and an IFN-γ Elispot assay, it was shown that 50% of 128 convalescent SARS patients from endemic areas of the SARS outbreak retain memory T cells against SARS-CoV protein in their peripheral blood one year after SARS infection (Li et al. 2008). The memory response is polyclonal, and SARS-specific T-cell responses are directed against different SARS-CoV proteins and against multiple epitopes (Fig. 16.1). Despite the large genome size of SARS-CoV, only 3% of the proteome is immunogenic. Eight of fourteen SARS Orfs are able to stimulate T-cell responses: replicase, S, Orf 3, Orf 4, E, M, Orf 13 and N. Most responses are focused on structural proteins (S, E, M, and N). S protein induces the most dominant T-cell responses, the majority of which are CD4 responses, suggesting that these may provide help for the B cells to produce neutralizing antibodies. In fact, there is a strong association between the CD4 memory response and neutralizing antibody against S protein in recovered SARS patients. However, overall the SARS-specific CD8 response predominates over CD4 in terms of frequency and magnitude of responses. In terms of quality of T cells by polyfunctional status, most SARS-specific CD4 cells produce mainly one cytokine, and they tend to be of a central memory phenotype (CD27+ and CD45RO+). Most SARS-specific CD8 responses are IFN-γ positive, but a significant proportion of the responding cells could also produce TNF-α and degranulate (based upon the mobilization of CD107a to the cell membrane). Observations in the mild–moderate and severe groups show that the frequency of SARS-specific memory CD4 producing IFN-γ, IL2, and TNF-α is significantly higher in the severe group than in the mild–moderate group. The influence of preexisting cross-reactive T cells from other human coronaviruses on the outcome of SARS infection is unlikely, as most published human SARS epitopes do not share amino acid sequence homology with other known human coronaviruses such as NL63, OC43, 229E, and HKU1.
Distribution of peptide recognition and magnitude of SARS-specific T-cell responses across the entire expressed SARS-CoV genome in convalescent SARS patients, as determined by immediate IFN-γ release ELISPOT assay. Freshly isolated PBMC from convalescent SARS samples were stimulated overnight with overlapping peptides from different proteins across the SARS genome, and the IFN-γ response was measured by ELISPOT assay. The individual 1,843 overlapping peptides of different proteins are represented on the x-axis. (a) The y-axis represents the percentage recognition of the study subjects to the individual peptides. (b) The y-axis represents the average magnitude of response by the study subjects to individual peptides
Data from other independent studies also show that SARS-specific T lymphocytes against S, M, E, and N proteins are detected in convalescent SARS samples from one to four years postinfection using overlapping peptides against individual structural proteins, rather than a genome-wide approach (Peng et al. 2006a, 2006b; Yang et al. 2006, 2007). Both CD4 and CD8 responses are detected for all proteins, and they are IFN-γ positive. Moreover, the S protein response is predominantly CD4-dependent (Yang et al. 2006; Libraty et al. 2007), and N-specific T cells could be detected two years postinfection in the absence of antigen stimulation (Peng et al. 2006a and b). In the absence of antigen and reexposure, memory T cells specific to SARS-CoV further declined and could be detected in a small proportion of convalescent patients (Fan et al. 2009). Using a panel of TCR-specific antibodies, effector/memory Vγ9Vδ2 cells were found in convalescent SARS patients (Poccia et al. 2006). These cells are associated with higher anti-SARS IgG levels. Stimulated Vγ9Vδ2 cells display IFN-γ-dependent anti-SARS-CoV activity and are able to kill SARS-CoV infected target cells. In addition, using a prediction algorithm to identify putative cytotoxic T-lymphocyte (CTL) epitopes, S-specific CTL in convalescent SARS patients show a differentiated effector phenotype, which is characterized by CD45RA+CCR7−CD62L−CCR5+CD44+ (Chen et al. 2005). In a separate study, the CTL epitopes identified could be used to induce CTL responses in A2 transgenic mice after DNA immunization (Zhou et al. 2006). Association of specific HLA alleles with susceptibility to SARS infection and disease severity has been proposed in several studies (Lin et al. 2003; Ng et al. 2004a). However, due to diverse polymorphism of HLA alleles and the small number of samples tested, the results are inconclusive.
6 Antibody and B-Cell Responses
Antibodies to SARS-CoV are present in patients after primary SARS infection. Using immunofluorescence assays and ELISA against N protein, serum IgG could be detected as early as four days after illness onset. Serum IgG, IgM, and IgA responses to SARS-CoV are present around the same time, with most patients sero-converted by day 14 after illness onset (Hsueh et al. 2004). In a 36-month follow-up study of 56 convalescent SARS patients, SARS-specific IgG and neutralizing antibodies peaked at month 4 and diminished thereafter (Liu et al. 2006; Cao et al. 2007). IgG and neutralizing antibodies are only detectable in 70% and 20% of convalescent patients respectively by month 36. There is no significant difference in the kinetics of specific antibodies with regard to disease severity, use of steroids, and type and number of coexisting conditions. In a large study of 623 SARS patients, antibodies were able to neutralize pseudotyped viruses bearing S proteins from four different SARS-CoV strains, suggesting that these antibodies are cross-reactive (Nie et al. 2004). Among structural proteins, only the S protein elicits neutralizing antibody (Buchholz et al. 2004). The major immunodominant epitope in S protein lies between amino acids 441 and 700 (Lu et al. 2004).
Memory B cells could be isolated from convalescent SARS patients and immortalized with Epstein–Barr virus to generate human monoclonal antibodies with sufficient affinities to protect from SARS infection (Traggiai et al. 2004). These antibodies could protect mice from viral replication in the lungs after nasal virus challenge. Randomized placebo-controlled trials evaluating passive postexposure prophylaxis in at-risk groups have not been undertaken; however, a retrospective analysis of outcomes in a limited number of SARS patients who continue to deteriorate but receive plasma therapy suggests that passive immunization may shorten hospital stays without obvious adverse effects in patients (Soo et al. 2004; Cheng et al. 2005). Currently, only hyperimmune globulin produced from plasma from convalescent patients and equine plasma produced by immunization with inactivated SARS-CoV are available for prophylactic trials in humans (Lu et al. 2005; Zhang et al. 2005). A human monoclonal IgG1 produced from a single-chain variable region fragment against the S1 domain from two nonimmune human antibody libraries has also been produced (Sui et al. 2004). One of the single-chain variable region fragments, 80R, blocks spike–ACE2 receptor interactions through binding to the S1 domain. Passive immunization of ferrets, hamsters, and mice with human mAbs is effective in suppressing viral replication in the lungs (ter Meulen et al. 2004; Traggiai et al. 2004; Roberts et al. 2006). Recently, potent cross-reactive monoclonal antibodies against highly conserved sites within the S protein, which can neutralize zoonotic or epidemic SARS-CoV, have been reported (He et al. 2006; Zhu et al. 2007). These broadly cross-reactive neutralizing antibodies may be useful for treatment options.
The role of antibodies in antibody-dependent enhancement (ADE) in SARS vaccine has been controversial and extensively debated (Perlman and Dandekar 2005; Chen and Subbarao 2007). However, most of the evidence is taken from limited data from in vitro and animal experiments. The interpretation of these findings with regard to implications for human disease is not clear. Concerns over the safety of SARS vaccines came from a previous animal study in which ADE was observed in domestic cats after vaccination against feline infectious peritonitis coronavirus (FIPV) (Vennema et al. 1990). Moreover, ferrets that are immunized with MVA vaccine expressing S protein suffer hepatitis whilst failing to develop protection (Weingartl et al. 2004). In addition, some variants of SARS-CoV are resistant to antibody neutralization, but the infection is enhanced by antibodies against a different variant (Yang et al. 2005b). Under in vitro conditions, entry into human B-cell lines by SARS-CoV virus could be carried out in an FcγRII-dependent and ACE2-independent fashion, indicating that ADE of virus entry is an alternative cell entry mechanism of SARS-CoV (Kam et al. 2007). However, there is no direct evidence that patients with SARS have had previous exposure to a related virus. Likewise, there is no evidence of enhanced disease in the lungs of animals that are infected with SARS-CoV following passive transfer of antibodies against SARS-CoV induced by infection or immunization. Also, macrophages are not productively infected by SARS-CoV (Subbarao et al. 2004; Bisht et al. 2004; Stadler et al. 2005; Yang et al. 2004b; Greenough et al. 2005; Kam et al. 2007). Hence, the potential for ADE following SARS vaccination is low. However, due to the lack of animal models that mimic the clinical disease in humans, care must be taken in the interpretation of the safety data of SARS vaccines.
7 Vaccines
SARS infection begins at the mucosal surface of the respiratory system, and the virus infects the lung epithelial cells via the surface S protein. An effective SARS vaccine is required to elicit neutralizing antibodies that abolish the binding of virus to the surface of host target cells and/or to eliminate the virus at the early stages of infection. Hope for active immunization with SARS vaccine stems from the findings that natural human infection with SARS-CoV leads to long-lived neutralizing antibody responses and immune sera capable of cross-neutralizing pseudotype viruses bearing S proteins from diverse but highly related human SARS-CoV isolates (Nie et al. 2004). In addition, long-lived memory T lymphocytes against different SARS-CoV structural proteins are present in the peripheral blood of convalescent SARS patients (Li et al. 2008; Peng et al. 2006; Fan et al. 2009).
Numerous immunization strategies with various vaccine expression vectors, routes of immunization, and adjuvants have been used to develop SARS vaccines (reviewed in Chen and Subbarao 2007; Roberts et al. 2008). They include inactivated whole virus vaccine, DNA immunization, recombinant viral vector (modified vaccinia Ankara (MVA), adenovirus, baculovirus, rhabdovirus, parainfluenza virus, Venezuelan equine encephalitis virus, measles virus), recombinant bacterial vector (Salmonella enteritica, Lactobacillus casei), recombinant protein, adjuvants (alum, ODN CpG, Protollin, MALP-2), and different combinations of prime-boost strategies. In most viral vectors, expression of the target gene in eukaryotic cells is codon optimized, and the immunogenicity is improved by using immunostimulatory sequences encoding IL-2 and internal ribosomal entry side (IRES).
Among all the structural proteins that are potential vaccine targets, S protein is the only protein that could induce neutralizing antibody and protect animals from challenge infection regardless of the method of antigen delivery (Callendret et al. 2007; Yang et al. 2004b; Kobinger et al. 2007; See et al. 2006; Bukreyev et al. 2004; Hu et al. 2007; Du et al. 2008). S protein protects hamsters from challenge when expressed in an attenuated parainfluenza type 3 vector (Buchholz et al. 2004). Mucosal immunization of African green monkeys with this parainfluenza type 3–S protein chimeric virus elicits neutralizing antibody and protection from viral replication in both the upper and lower respiratory tract after challenge with live SARS-CoV (Bukreyev et al. 2004). S protein-encoding DNA vaccines and modified vaccinia Ankara (MVA) expressing the S protein stimulate neutralizing antibody production and provide protection from live virus challenge in mice (Yang et al. 2004b). The protective mechanism is mediated by antibody and not by T cells.
Taken together, both neutralizing antibody (B-cell) responses and T-cell responses to the S protein are dominant in patients recovered from SARS, suggesting that an S protein-based vaccine may be sufficient to induce protective immunity by prophylactic vaccination. Moreover, there is a good correlation between neutralizing antibody and T-cell responses (Li et al. 2008). In humans, only two independent Phase 1 clinical trials of SARS vaccines have been published. Inactivated SARS-CoV vaccine administered via two intramuscular immunizations was reported to be well tolerated and immunogenic in 36 healthy adult volunteers in China (Lin et al. 2007). All subjects had sero-converted by day 42 postvaccination. The geometric mean titer of neutralizing antibody peaked two weeks after the second vaccination, but decreased four weeks later. In a separate DNA vaccine trial where S antigen was used as the target, 80% of the subjects had sero-converted after receiving 2–3 immunizations (Martin et al. 2008). The neutralizing antibody response peaked between weeks 8 and 12, and 50% of the subjects remained positive at week 32 postvaccination. In addition, the DNA vaccine induced 100% spike-specific T-lymphocyte response in all subjects from week 2 postvaccination. The spike-specific T-cell response was mainly CD4-dependent. The peak T-cell response occurred between weeks 8 and 12, and was maintained throughout the 32-week trial period.
8 Summary and Perspectives
Following the initial outbreaks of SARS which occurred in hospital-based clusters, the virus was rapidly isolated, sequenced, and characterized as a result of an international public health effort. This knowledge was quickly transformed into diagnostic reagents and tools to control community outbreaks of SARS infection. Despite the wealth of active scientific research and information, the mechanisms of viral clearance, immune correlates of protection, and the immunopathogenesis of SARS infection remain unclear. The risk of SARS-CoV to humans is still high due to the large number of animal reservoirs of SARS-CoV-like coronaviruses and the genome instability of RNA coronaviruses. Currently available animal models are inadequate, and development of models that mimic the clinical symptoms of human SARS infection is urgently required for mechanistic study and vaccine evaluation. Serial analysis of acute SARS samples to analyze innate, antibody, and T-cell responses would be useful to develop treatment strategies and prophylactic SARS vaccines.
References
Anonymous (2004) The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am J Clin Pathol 121(4):507–511
Bisht H, Roberts A et al (2004) Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA 101(17):6641–6646
Bosch BJ, van der Zee R et al (2003) The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol 77(16):8801–8811
Bosch BJ, Martina BE et al (2004) Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci USA 101(22):8455–8460
Buchholz UJ, Bukreyev A et al (2004) Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA 101(26):9804–9809
Bukreyev A, Lamirande EW et al (2004) Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet 363(9427):2122–2127
Callendret B, Lorin V et al (2007) Heterologous viral RNA export elements improve expression of severe acute respiratory syndrome (SARS) coronavirus spike protein and protective efficacy of DNA vaccines against SARS. Virology 363(2):288–302
Cameron MJ, Ran L et al (2007) Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol 81(16):8692–8706
Cao WC, Liu W et al (2007) Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 357(11):1162–1163
Chan CM, Ma CW et al (2007) The SARS-Coronavirus membrane protein induces apoptosis through modulating the Akt survival pathway. Arch Biochem Biophys 459(2):197–207
Chen J, Subbarao K (2007) The immunobiology of SARS. Annu Rev Immunol 25:443–472
Chen JH, Chang YW et al (2004) Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc Natl Acad Sci USA 101(49):17039–17044
Chen H, Hou J et al (2005) Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J Immunol 175(1):591–598
Chen RF, Chang JC et al (2006) Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS). Microbes Infect 8(1):122–127
Cheng Y, Wong R et al (2005) Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 24(1):44–46
Cheng VC, Lau SK et al (2007) Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 20(4):660–694
Cheung CY, Poon LL et al (2005) Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol 79(12):7819–7826
Chien JY, Hsueh PR et al (2006) Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 11(6):715–722
Chu CM, Poon LL et al (2004) Initial viral load and the outcomes of SARS. CMAJ 171(11):1349–1352
Chua KB, Bellini WJ et al (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288(5470):1432–1435
Cinatl J, Morgenstern B et al (2003) Treatment of SARS with human interferons. Lancet 362(9380):293–294
Devaraj SG, Wang N et al (2007) Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J Biol Chem 282(44):32208–32221
Drosten C, Gunther S et al (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348(20):1967–1976
Du L, He Y et al (2008) Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today 44(1):63–73
Fan YY, Huang ZT et al (2009) Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch Virol 154(7):1093–1099
Formenty P, Hatz C et al (1999) Human infection due to Ebola virus, subtype Cote d'Ivoire: clinical and biologic presentation. J Infect Dis 179(Suppl 1):S48–S53
Freundt EC, Yu L et al (2009) Molecular determinants for subcellular localization of the severe acute respiratory syndrome coronavirus open reading frame 3b protein. J Virol 83(13):6631–6640
Frieman M, Baric R (2008) Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation. Microbiol Mol Biol Rev 72(4):672–685
Glass WG, Subbarao K et al (2004) Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol 173(6):4030–4039
Greenough TC, Babcock GJ et al (2005) Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J Infect Dis 191(4):507–514
Gu J, Gong E et al (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–424
Haagmans BL, Kuiken T et al (2004) Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 10(3):290–293
Hamming I, Timens W et al (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637
He Z, Zhao C et al (2005) Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis 9(6):323–330
He Y, Li J et al (2006) Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein. J Immunol 176(10):6085–6092
Ho MS, Chen WJ et al (2005) Neutralizing antibody response and SARS severity. Emerg Infect Dis 11(11):1730–1737
Hofmann H, Pohlmann S (2004) Cellular entry of the SARS coronavirus. Trends Microbiol 12(10):466–472
Hofmann H, Pyrc K et al (2005) Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA 102(22):7988–7993
Hogan RJ (2006) Are nonhuman primates good models for SARS? PLoS Med 3(9):e411; author reply e415
Hsueh PR, Huang LM et al (2004) Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect 10(12):1062–1066
Hu MC, Jones T et al (2007) Intranasal Protollin-formulated recombinant SARS S-protein elicits respiratory and serum neutralizing antibodies and protection in mice. Vaccine 25(34):6334–6340
Huang KJ, Su IJ et al (2005) An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 75(2):185–194
Huang IC, Bosch BJ et al (2006) SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J Biol Chem 281(6):3198–3203
Imai Y, Kuba K et al (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436(7047):112–116
Imai Y, Kuba K et al (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133(2):235–249
Ip WK, Chan KH et al (2005) Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis 191(10):1697–1704
Jeffers SA, Tusell SM et al (2004) CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA 101(44):15748–15753
Jiang Y, Xu J et al (2005) Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 171(8):850–857
Kam YW, Kien F et al (2007) Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine 25(4):729–740
Kielian M, Rey FA (2006) Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol 4(1):67–76
Kobinger GP, Figueredo JM et al (2007) Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine 25(28):5220–5231
Kopecky-Bromberg SA, Martinez-Sobrido L et al (2006) 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J Virol 80(2):785–793
Krahling V, Stein DA et al (2009) Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J Virol 83(5):2298–2309
Ksiazek TG, Erdman D et al (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348(20):1953–1966
Kuba K, Imai Y et al (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11(8):875–879
Lam CW, Chan MH et al (2004) Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev 25(2):121–132
Lau SK, Woo PC et al (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA 102(39):14040–14045
Law HK, Cheung CY et al (2005a) Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 106(7):2366–2374
Law PT, Wong CH et al (2005b) The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J Gen Virol 86(Pt 7):1921–1930
Lee N, Hui D et al (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348(20):1986–1994
Li W, Moore MJ et al (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426(6965):450–454
Li W, Shi Z et al (2005) Bats are natural reservoirs of SARS-like coronaviruses. Science 310(5748):676–679
Li CK, Wu H et al (2008) T cell responses to whole SARS coronavirus in humans. J Immunol 181(8):5490–5500
Libraty DH, O'Neil KM et al (2007) Human CD4(+) memory T-lymphocyte responses to SARS coronavirus infection. Virology 368(2):317–321
Lin M, Tseng HK et al (2003) Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet 4:9
Lin JT, Zhang JS et al (2007) Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther 12(7):1107–1113
Liu S, Xiao G et al (2004) Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 363(9413):938–947
Liu W, Fontanet A et al (2006) Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis 193(6):792–795
Lu L, Manopo I et al (2004) Immunological characterization of the spike protein of the severe acute respiratory syndrome coronavirus. J Clin Microbiol 42(4):1570–1576
Lu JH, Guo ZM et al (2005) Preparation and development of equine hyperimmune globulin F(ab')2 against severe acute respiratory syndrome coronavirus. Acta Pharmacol Sin 26(12):1479–1484
Marra MA, Jones SJ et al (2003) The genome sequence of the SARS-associated coronavirus. Science 300(5624):1399–1404
Martin JE, Louder MK et al (2008) A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 26(50):6338–6343
Mossel EC, Wang J et al (2008) SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 372(1):127–135
Murray K, Selleck P et al (1995) A morbillivirus that caused fatal disease in horses and humans. Science 268(5207):94–97
Narayanan K, Huang C et al (2008) Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol 82(9):4471–4479
National Research Project for SARS, Beijing Group (2004) The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome
Ng MH, Lau KM et al (2004a) Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J Infect Dis 190(3):515–518
Ng PC, Lam CW et al (2004b) Inflammatory cytokine profile in children with severe acute respiratory syndrome. Pediatrics 113(Pt 1):e7–e14
Nicholls JM, Poon LL et al (2003) Lung pathology of fatal severe acute respiratory syndrome. Lancet 361(9371):1773–1778
Nicholls JM, Butany J et al (2006) Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med 3(2):e27
Nie Y, Wang G et al (2004) Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis 190(6):1119–1126
Openshaw PJ, Culley FJ et al (2001) Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20(Suppl 1):S27–S31
Peiris JS, Lai ST et al (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361(9366):1319–1325
Peng H, Yang LT et al (2006a) Human memory T cell responses to SARS-CoV E protein. Microbes Infect 8(9–10):2424–2431
Peng H, Yang LT et al (2006b) Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 351(2):466–475
Perlman S, Dandekar AA (2005) Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5(12):917–927
Poccia F, Agrati C et al (2006) Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J Infect Dis 193(9):1244–1249
Poutanen SM, Low DE et al (2003) Identification of severe acute respiratory syndrome in Canada. N Engl J Med 348(20):1995–2005
Roberts A, Thomas WD et al (2006) Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis 193(5):685–692
Roberts A, Lamirande EW et al (2008) Animal models and vaccines for SARS-CoV infection. Virus Res 133(1):20–32
Schneider-Schaulies S, Niewiesk S et al (2001) Measles virus induced immunosuppression: targets and effector mechanisms. Curr Mol Med 1(2):163–181
See RH, Zakhartchouk AN et al (2006) Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol 87(Pt 3):641–650
Sheahan T, Morrison TE et al (2008) MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog 4(12):e1000240
Shi Z, Hu Z (2008) A review of studies on animal reservoirs of the SARS coronavirus. Virus Res 133(1):74–87
Simmons G, Gosalia DN et al (2005) Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA 102(33):11876–11881
Siu KL, Kok KH et al (2009) Severe acute respiratory syndrome Coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3{middle dot}TANK{middle dot}TBK1/IKK{epsilon} complex. J Biol Chem 284(24):16202–16209
Soo YO, Cheng Y et al (2004) Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 10(7):676–678
Spiegel M, Pichlmair A et al (2005) Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J Virol 79(4):2079–2086
Stadler K, Masignani V et al (2003) SARS–beginning to understand a new virus. Nat Rev Microbiol 1(3):209–218
Stadler K, Roberts A et al (2005) SARS vaccine protective in mice. Emerg Infect Dis 11(8):1312–1314
Stockman LJ, Bellamy R et al (2006) SARS: systematic review of treatment effects. PLoS Med 3(9):e343
Subbarao K, McAuliffe J et al (2004) Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol 78(7):3572–3577
Sui J, Li W et al (2004) Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA 101(8):2536–2541
Tan YJ, Fielding BC et al (2004) Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol 78(24):14043–14047
Temperton NJ, Chan PK et al (2005) Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis 11(3):411–416
ter Meulen J, Bakker AB et al (2004) Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363(9427):2139–2141
To KF, Tong JH et al (2004) Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol 202(2):157–163
Traggiai E, Becker S et al (2004) An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med 10(8):871–875
Tripet B, Howard MW et al (2004) Structural characterization of the SARS-coronavirus spike S fusion protein core. J Biol Chem 279(20):20836–20849
Tsang KW, Ho PL et al (2003) A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348(20):1977–1985
Tseng CT, Perrone LA et al (2005) Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol 174(12):7977–7985
Vennema H, de Groot RJ et al (1990) Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol 64(3):1407–1409
Weingartl H, Czub M et al (2004) Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol 78(22):12672–12676
Wong RS, Wu A et al (2003) Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 326(7403):1358–1362
Wong CK, Lam CW et al (2004) Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136(1):95–103
Yang ZY, Huang Y et al (2004a) pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol 78(11):5642–5650
Yang ZY, Kong WP et al (2004b) A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 428(6982):561–564
Yang Y, Xiong Z et al (2005a) Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem J 392(Pt 1):135–143
Yang ZY, Werner HC et al (2005b) Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci USA 102(3):797–801
Yang LT, Peng H et al (2006) Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol 120(2):171–178
Yang L, Peng H et al (2007) Persistent memory CD4+ and CD8+ T-cell responses in recovered severe acute respiratory syndrome (SARS) patients to SARS coronavirus M antigen. J Gen Virol 88(Pt 10):2740–2748
Yen YT, Liao F et al (2006) Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J Virol 80(6):2684–2693
Yilla M, Harcourt BH et al (2005) SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res 107(1):93–101
Yoshikawa T, Hill T et al (2009) Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol 83(7):3039–3048
Zhang Y, Li J et al (2004) Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 72(8):4410–4415
Zhang Z, Xie YW et al (2005) Purification of severe acute respiratory syndrome hyperimmune globulins for intravenous injection from convalescent plasma. Transfusion 45(7):1160–1164
Zhao GP (2007) SARS molecular epidemiology: a Chinese fairy tale of controlling an emerging zoonotic disease in the genomics era. Philos Trans R Soc Lond B Biol Sci 362(1482):1063–1081
Zhao X, Nicholls JM et al (2008) Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem 283(6):3272–3280
Zhou M, Xu D et al (2006) Screening and identification of severe acute respiratory syndrome-associated coronavirus-specific CTL epitopes. J Immunol 177(4):2138–2145
Zhu Z, Chakraborti S et al (2007) Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci USA 104(29):12123–12128
Zinkernagel RM, Hengartner H (2004) On immunity against infections and vaccines: credo 2004. Scand J Immunol 60(1–2):9–13
Acknowledgements
This project was supported by Medical Research Council UK, Beijing Municipal Government, and the European Commission Euro-Asian SARS-DTV Network (SP22-CT-2004-511064). Expert editorial assistance and critical reading by Dr. Sarah Bangs is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Li, C.Kf., Xu, X. (2010). Host Immune Responses to SARS Coronavirus in Humans. In: Lal, S. (eds) Molecular Biology of the SARS-Coronavirus. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-03683-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-642-03683-5_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-03682-8
Online ISBN: 978-3-642-03683-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)