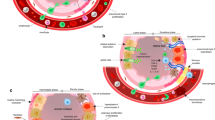
Abstract
-
The lungs are particularly sensitive to RT, and are often the primary dose-limiting structure during thoracic therapy.
-
The alveolar/capillary units and pneumocytes within the alveoli appear to be particularly sensitive to RT.
-
Hypoxia may be important in the underlying physiology of RT-associated lung injury.
-
The cytokine transforming growth factor-beta (TGF-β), plays an important role in the development of RT-induced fibrosis.
-
The histopathological changes observed in the lung after RT are broadly characterized as diffuse alveolar damage.
-
The interaction between pre-treatment PFTs and the risk of symptomatic lung injury is complex. Similarly, the link between changes in PFTs and the development of symptoms is uncertain.
-
The incidence of symptomatic lung injury increases with increase in most dosimetric parameters. The mean lung dose (MLD) and V20 have been the most-often considered parameters. MLD might be a preferable metric since it considers the entire 3D dose distribution.
-
Radiation to the lower lobes appears to be more often associated with clinical symptoms than is radiation to the upper lobes. This might be related to incidental cardiac irradiation. In pre-clinical models, there appears to be a complex interaction between lung and heart irradiation.
-
TGF-β has been suggested in several studies to predict for RT-induced lung injury, but the data are still somewhat inconsistent.
-
Oral prednisone (Salinas and Winterbauer 1995), typically 40–60 mg daily for 1–2 weeks with a slow taper, is usually effective in treating pneumonitis. There are no widely accepted treatments for fibrosis.
-
A number of chemotherapeutic agents have been suggested to be associated with a range of pulmonary toxicities.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Single Photon Emission Compute Tomography
- Lung Injury
- Malignant Pleural Mesothelioma
- Total Body Irradiation
- Stereotactic Body Radiation Therapy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Radiation therapy (RT) is an important treatment modality for multiple thoracic malignancies, including breast, lung, and esophageal cancer, as well as malignant lymphomas involving the mediastinum. The thoracic cavity contains the heart, great vessels, esophagus, and lungs, surrounded by the musculoskeletal constituents of the chest wall. The lungs are particularly sensitive to RT, and are consequentially a dose-limiting structure when planning therapy.
The lungs are complex organs, consisting of the large central airways such as the trachea and mainstem bronchi, the smaller conducting airways, a complex vascular network, and the alveolar sacs where gas exchange occurs. Irradiation of the trachea and proximal bronchi can lead to mucosal irritation, clinically manifesting as a dry cough. However, late complications of the conducting airways (e.g., fistula or stenosis) are unusual with conventional doses of RT. The proximal vascular network is generally considered to be relatively insensitive to the effects of RT. The primary toxicity from lung irradiation is related to the alveolar/capillary complex.
The processes that ultimately culminate in clinically apparent alveolar injury are complex but thought to begin with reactive oxygen and nitrogen species (ROS/RNS). In addition to being directly toxic to cells, ROS/RNS instigate a host of molecular cascades which alter the cytokine milieu leading to chronic inflammation, tissue damage, and remodeling. Edema and extravasation of proteinaceous material into the alveoli can be appreciated within a few weeks after RT as increased density on radiographs (Skoczylas et al. 2000). If the volume of inflammed lung is large, pulmonary symptoms can develop 1–6 months after RT. Within 3 months after completing RT (and maybe sooner), there is a reduction in pulmonary function tests secondary to thickening of the intraalveolar septum (edema and fibrosis) and loss of alveoli. The RT-induced microenvironment of chronic inflammation, hypoxia, and fibrosis appears to be self-sustaining leading to progressive decline in pulmonary function. Our understanding of the cellular and biochemical changes that occur in the lung after RT continues to evolve (Brush et al. 2007; Fleckenstein et al. 2007; Tsoutsou and Koukourakis 2006) (see Sect. 3).
Concern for RT-induced lung injury must be taken in the context of the cancer that is being targeted. For example, a small volume of the anterior ipsilateral lung is typically included within tangent fields used to treat patients with breast cancer. Most patients with breast cancer have ample lung reserve, especially relative to the small volume of lung within the RT fields. Therefore, the incidence of clinical symptoms is low. Typical pneumonitis rates are in the range of 1–5 %, with severe injury being even less common.
Conversely, patients irradiated for lung cancer usually have a history of tobacco use, and usually have poor baseline lung function. Primary lung tumors typically invade into, and replace, normal lung. Further, the primary lung tumor or enlarged hilar/mediastinal lymph nodes can compress central airways/vessels that can decrease overall cardiopulmonary function. In these instances, the damaging effects of RT on the lung are often, at least partially, off-set by RT-induced reductions in tumor mass that may increase lung function. For such patients, the resultant change in lung function after thoracic RT is typically a balance between the radiotoxic effects on the normal lung and improvement in lung function resulting from tumor shrinkage.
A variety of dose/volume parameters have been linked to pulmonary injury and provide rationale clinical guidelines (see Sect. 5). However, predictive models for RT-induced lung injury are suboptimal. Further, only limited progress has been made in developing compounds that prevent and/or mitigate the detrimental effects of RT. In this chapter, we will review many facets of RT-induced lung injury and provide dose/volume recommendations for the clinician. Biocontinuum of adverse early and late effects are shown in Fig. 1.
2 Anatomy and Histology
2.1 Gross Anatomy
The lungs are paired organs, each contained in its own pleural sac within the thoracic cavity. Both lungs are subdivided into anatomically distinct lobes. The right lung normally has three lobes separated by two fissures. The horizontal fissure separates the upper and middle lobes while the oblique fissure separates the lower lobe from the middle and upper lobes. The left lung normally has two lobes (upper and lower) divided by an oblique fissure. The lobes can be further subdivided into segments. The lingula (Latin-little tongue) is part of the upper lobe and is composed of a superior and inferior segments (Fig. 2).
The architecture of the lung can be considered as subunits arranged in both parallel and series. Centrally, the structure is “in series” as the large vessels and airways in the mediastinum and hilum support all of the distal vessels and airways to which they are connected. Fibrosis/stenosis of a central vessel or bronchus will render the downstream alveoli nonfunctional. Conversely, in the periphery, the smaller alveolar/capillary units function relatively independently (i.e., “in parallel”). Resection or injury to a region of lung will not compromise function of adjacent regions. The alveolar/capillary units appear far more sensitive to the effects of RT than the central conducting airways and vessels. Therefore, most RT-induced injury is referable to these “peripheral” structures and the lung is classically considered a “parallel” organ regarding RT-induced effects.
2.2 Histology and the Functional Subunit
The functional unit of the lung is the pulmonary lobule which consists of a terminal bronchiole and the lung parenchyma distal to it (i.e., alveolar/capillary unit) (Fig. 3a, b). The terminal bronchiole is the final conducting branch of the tracheobronchial tree not involved in gaseous exchange. Terminal bronchioles branch into transitional airways (respiratory bronchioles and alveolar ducts) which contribute to gaseous exchange. The transitional airways terminate in alveolar sacs which open into the alveoli.
The principal function of the lungs is to facilitate gas exchange between alveoli and adjoining capillaries. Gas exchange is most efficient when the thickness of intervening tissues is minimal. Adjacent alveoli share a common wall, termed the interalveolar septum, which contains capillaries and a fine connective tissue framework. Within the interalveolar septum, the adjacent basal lamina of the alveolar epithelium and endothelium are often fused providing a thin barrier between the erythrocytes in the capillaries and the alveolar air space (~0.5 μm). The capillaries wrap around the alveoli creating a prodigious surface area for diffusion (50–100 m2).
Several cell types are present in the pulmonary lobule. Type 1 pneumocytes are flat epithelial cells with minimal cytoplasm and few organelles that form a complete, thin (0.2 μm), lining of the alveoli. Type II pneumocytes are as numerous as type I pneumocytes but only cover 2–5% of the alveolar surface. They are round cells with larger nuclei, and contain prominent lamellar bodies (cytosomes) which contain dipalmitoyl phosphatidylcholine (surfactant), which reduces surface tension within the alveoli. Type II pneumocytes can differentiate into type I pneumocytes in response to damage to the alveolar lining. Alveolar macrophages (dust cells) derive from circulating monocytes and phagocytose microorganisms and other particulate matter that is deposited in the alveoli.
3 Biology, Physiology, and Pathophysiology
3.1 Biology: Molecular Mechanisms of RT-Induced Lung Injury
Radiation injury of the lung is categorized as two distinct phases: acute inflammatory (pneumonitis) and late fibroproliferative. The progression of radiation injury is well established in both mice and humans. In humans, the pneumonitis phase peaks between 2–3 months after radiation exposure. This phase is characterized by a dense inflammatory cell infiltrate, exudation of proteinaceous material, and edema. The chronic fibroproliferative phase occurs months to years after exposure and is characterized by diffuse interstitial fibrosis, focal scarring, and impaired ventilation.
The lung is considered to be a late responding tissue, as the time between exposure and symptomatic injury can last months to years, presumably as a result of innate tissue kinetics. During the asymptomatic latency period, radiographic changes can be observed (Skoczylas et al. 2000; Brush et al. 2007; Fleckenstein et al. 2007). Although the period of time between exposure and symptomatic injury may appear to be quiet, there is a firestorm of events occurring at the molecular and cellular level that is just beginning to be better understood.
At the time of the initial irradiation, exposed cells rapidly spread the “danger signal” to neighboring cells as far away as 1 mm (Tsoutsou and Koukourakis 2006; Pusey 1905). This results in an orchestrated response of tissue-specific cells, the vascular endothelium, and inflammatory mediators in order to resolve the injury (Skoczylas et al. 2000; Fleckenstein et al. 2007; Tsoutsou and Koukourakis 2006; Bernier et al. 2004).
The intercellular conversation is initiated at the moment of irradiation when injury to the cell membrane, DNA, cytoplasmic bodies, and other cell components occurs, resulting in an immediate release of cytokine mRNA. The identification of a “perpetual cytokine cascade” (Fleckenstein et al. 2007) caused a major paradigm shift from the classical “target cell theory” (α/β) to one that posits a dynamic molecular crosstalk among numerous cells in normal tissue (the lung has more than 40 cell types (Evans and Leucutia 1925)) that propagates the injurious process (Fig. 4).
The cytokine and chemokine reaction observed by Rubin and colleagues clearly demonstrated the absence of a molecular and cellular latent period prior to observed histologic changes. These findings suggest that radiation initiates a downstream sequence of spatial and temporal events that eventually express themselves as clinical manifestations months later.
Coincident with the release of inflammatory cytokines is the development of oxidative stress. Free radical species can participate in a host of cellular signaling events. Indeed, many of the signaling pathways implicated in radiation injury, such as the TGF-β pathway, are sensitive to changes in cellular redox status (Tyler and Blackman 1922; Groover et al. 1922). The transient nature of ionizing radiation suggests that a mechanism of amplification of ROS/RNS occurs after radiation. Proposed mechanisms include activation of oxidant-generating enzymes, such as NADPH oxidases, mitochondrial leakage, and the respiratory burst from activated inflammatory mediators recruited to the site of injury. The role of NADPH oxidases is supported by the fact that TGF-β is activated by radiation and that in turn, TGF-β can activate the NADPH oxidase isoform, Nox4. Newer data coming to light recently have shown a critical role for Nox4 in TGF-β mediated apoptosis (Hines 1922) and downstream Smad signaling (Davis 1924).
In the lung, chronic oxidative stress has been observed using markers for DNA oxidation (Skoczylas et al. 2000) and lipid peroxidation (Groover et al. 1922; Fleckenstein et al. 2007). Oxidative stress can contribute to a number of changes within injured tissue including endothelial cell activation, increased vascular permeability and edema, lipid peroxidation, inflammation, and fibrosis (Skoczylas et al. 2000; Bernier et al. 2004; Johnston et al. 1996; Rubin et al. 1995). The end result is a cyclic progression of oxidative stress and activation of growth factors, transcription factors, and cytokines, all of which can contribute to lung injury.
It is hypothesized that the imbalance between oxidant-generating enzymes and antioxidant capacity is a primary cause of hypoperfusion following radiation (Skoczylas et al. 2000; Vujaskovic et al. 2001). Fleckenstein et al. demonstrated that tissue perfusion begins to decrease 3 days after radiation exposure, resolves for approximately 2 weeks, and then progressively decreases throughout the time of disease progression (Skoczylas et al. 2000). In parallel, the lung tissue becomes increasingly hypoxic (Skoczylas et al. 2000). The late decrease in tissue oxygenation is likely a result of fibrous obliteration of capillaries and decreased gas exchange due to increased alveolar wall thickness and edema/congestion within the alveolar space. The early drop in perfusion cannot be explained by these events and is more likely a result of vasoconstriction, although this is not confirmed. The activation of NADPH oxidase after radiation increases superoxide production, which may suppress vasodilation by oxidizing key enzymes within the NO signaling pathway or through rapid reaction with NO itself to form peroxynitrite. Both of these reactions can interfere with NO signaling leading to vasoconstriction, a transient loss in tissue perfusion, and tissue hypoxia. Tissue hypoxia, a fundamental feature underlying the development of lung injury, develops several weeks after lung irradiation and progresses with time, presumably as a result of injury to the pulmonary vascular and decreased perfusion (Skoczylas et al. 2000; Bernier et al. 2004). Changes in pulmonary perfusion have been observed within months following high doses of radiation in humans (Bentzen 2006; Mikkelsen and Wardman 2003) and within weeks in rodent models (Skoczylas et al. 2000; Rubin et al. 1995; Jackson et al. 2006). In studies by Fleckenstein et al. (Skoczylas et al. 2000), the transient decrease in perfusion was associated with increased edema and elevated expression of hypoxia-inducible factor-1 alpha (HIF-1α) and TGF-β. In that study, the vascular perfusion changes were noted before the development of histologically apparent and functional disease.
The same study showed that tissue hypoxia and perfusion changes coincide with the accumulation and activation of macrophages within the residual alveolar space and interstitium, as well as enhanced cytokine and growth factor production. This could, in part, contribute to the presence of cytokines in the irradiated tissue weeks to months after exposure (Skoczylas et al. 2000). Jackson et al. (Johnston et al. 1996) found that in vitro TGF-β production by macrophages could be induced by hypoxia (0.5% O2), prior to the onset of VEGF production. In that study, incubation with a superoxide dismutase mimetic led to decreased TGF-β and VEGF production (Johnston et al. 1996). Thus, it appears that ROS signaling plays a key role in macrophage-associated expression of the above cytokines under hypoxic conditions.
The pro-inflammatory/pro-fibrogenic cytokine transforming growth factor-beta (TGF-β) has been most extensively studied in regard to its role in the development of radiation-induced lung injury (Fleckenstein et al. 2007; Novakova-Jiresova et al. 2007; Kang et al. 2003; Feletou et al. 1995). TGF-β is secreted as an inactive polypeptide by a number of cells including macrophages, fibroblasts, and epithelial cells of the lung. The inactive precursor is sequestered in the extracellular matrix with its latency-associated peptide (LAP), which has been suggested to be the “sensory” portion of the protein (Marks et al. 1997; Theuws et al. 2000; Peterson et al. 1992; Dor et al. 2001; Li et al. 2001). The LAP is sensitive to free radicals, changes in cellular pH, and integrins, all of which can induce activation of TGF-β which readily binds the TGF-β type II receptor to stimulate the Smad second messenger signaling pathway (Bartholdi et al. 1997). Activation of TGF-β and subsequent translocation of Smad2/3 protein into the nucleus of the responding cell leads to transcription of genes involved in fibroproliferation and tissue repair (Li et al. 2007; van Hinsbergh et al. 2001). An increase in TGF-β protein can be observed within 24 hours following radiation exposure (Skoczylas et al. 2000; Dor et al. 2001; Anscher et al. 1998, 2006). Epperly et al. (Rodemann et al. 1996) found a biphasic TGF-β surge associated with the early and delayed radiation response in mice after 20 Gy whole lung irradiation. The establishment of TGF-β as a mediator of radiation-induced lung injury has led to a number of studies evaluating circulating serum TGF-β levels as a potential biomarker for increased risk of lung injury as well as pre-clinical testing of inhibitors of TGF-β and/or its signaling pathway. These studies have used either neutralizing antibodies or adenoviral vectors to demonstrate significantly reduced histological and functional damage in rodent models of lung injury following radiation exposure. Reduced lung damage was also associated with decreased cytokine activity, fewer macrophages, and less oxidative stress (Kang et al. 2003; Barcellos-Hoff 1996; Rahimi and Leof 2007).
In summary, our knowledge of the mechanisms of radiation injury has vastly improved over the past 30 years. It is now established that chronic oxidative stress and continuous production of cytokines, particularly TGF-β, play a significant role in sustaining inflammation and fibroproliferation in irradiated lung during the progression of disease. Symptomatic and histologically relevant lung injury is preceded by cytokine production and communication, inflammatory cell infiltration, changes in blood flow and perfusion, and increased vascular permeability (Bernier et al. 2004; Roberts 1999; Barcellos-Hoff 1993; Barcellos-Hoff et al. 1994; Stone et al. 2003, Vujaskovic et al. 2000). Although great progress has been made in identifying the mechanisms underlying radiation-induced lung injury, more comprehensive studies are needed to link the events occurring at the molecular level to cellular and tissue pathology.
Despite these laboratory findings, the results in the clinic are less encouraging. To date, the observed associations between changes in a variety of biological markers and clinical pneumonitis have been inconsistent (Table 1).
3.2 Pathophysiology (Cellular Dynamics and the Radiation Response)
3.2.1 Animal Models
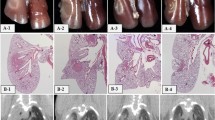
The ultrastructural changes in lung were documented by Penney et al. in a mouse model. These are documented for a wide range of single doses from 5 to 13 Gy over a period of months (1 h–60 weeks) (Table 2) (Penney 1087). Examples of corresponding histologic images are shown in Fig. 5a, b.
a Radiation pneumonitis: pneumonitis in a C57L/J mouse 14 weeks after thoracic irradiation with a single dose of 10 Gy. Alveolar inflammation and perivascular lymphocytic cuffing can be seen. b Radiation fibrosis in a C57BL/6J mouse 24 weeks after thoracic irradiation with a single dose of 12.5 Gy. Both are Masson’s trichrome stain of paraffin embedded tissues
The Biocontinuum ‘roller coaster’ curve for radiation pneumonitis hypothesized by Rubin and Casarett was confirmed in an elegant series of studies in mice using breathing rates by Travis et al. (1980). The increased breathing rates at 16–36 weeks are associated with radiation pneumonitis and then becomes elevated again at 52 weeks associated with fibrosis. Utilizing a split dose exposure, the early pneumonitis phase was not expressed, however, the late occurrence of pulmonary fibrosis appeared between 6–12 months. This was confirmed in similar studies by Siemann et al. (1982) who utilized different fractionation schedules in mice and confirmed the appearance of a late fibrotic stage without an early pneumonitic phase. There observations support the concept of differing underlying mechanisms for pneumonitis and fibrosis.
The importance of host response is well-recognized clinically. A number of investigators have compared the variation in pulmonary response comparing a radioresistant strain (C3H) with a radiosensitive strain (C57BL) to radiation (Jackson et al. 2010; Williams et al. 2010). The basis for the variation could be explained by differences in the elicited cytokine cascade to similar doses. Utilizing knock out mice for 1 CAM expression compared to wild type mice, Hallahan demonstrated a lower incidence of lung injury due to blocking the 1 CAM-1 pathway (Hallahan et al. 2002). Similarly in TGF β knock out mice, the induction of radiation-induced fibrosis in both skin and lung is inhibited (Martin et al. 2000).
3.2.2 Clinical Observations
The lungs are complex organs containing conducting airways, a complex vascular network, and the alveolar/capillary units where gas exchange occurs. The trachea and proximal airways are lined with pseudostratified ciliated columnar epithelial cells with admixed mucus producing goblet cells. Since these cells have a relatively rapid turnover rate, the mucosa can become acutely denuded during RT. Mild dry cough and/or sore throat during RT is likely related to RT-induced changes in the epithelial lining and decreased clearance of intratracheal debris. Due to the rapid cellular turnover, the depleted mucosa is typically replenished promptly after completing RT and the associated symptoms are transient.
The supporting vascular and other connective tissues are comprised primarily of quiescent “mature” cells with a relatively slow mitotic rate. Although no clinical/pathologic findings are typically observed acutely in these tissues during thoracic RT, late normal tissue injury is possible. For example, bronchial stenosis has been observed following high-dose external beam RT (Miller et al. 2005) as well as brachytherapy (Speiser and Spratling 1993). Similarly, we have seen a case of diffuse pulmonary hypoperfusion after high-dose external beam RT, apparently due to fibrosis of central vascular structures. Months to years following RT, a fibrotic reaction develops within irradiated lung parenchyma. Whether this is a direct effect of RT on the cells comprising the supporting connective tissues, or due to an RT response in other cells, is unclear. While this fibrotic reaction is generally considered to be progressive and irreversible, there is limited experimental data suggesting that this process can be modified and perhaps even reversed (Delanian et al. 1994, 1999).
The more peripheral vascular structures, such as the alveolar capillaries, appear to be more sensitive to RT than the central vasculature. With even modest doses of RT, reductions in regional perfusion, apparently due to sclerosis of these small vessels, has been observed. The extent of reduction in regional lung perfusion has been associated with the degree of change in global lung function as assessed by pulmonary function tests (Fan et al. 2001; Theuws et al. 1998).
A reduction in regional ventilation is also observed after RT, apparently corresponding to a fibrotic/inflammatory reaction within the distal airways. However, the decline in ventilation after RT appears to be less than the corresponding decline in perfusion. Since optimal respiratory function requires both adequate ventilation and perfusion, RT-induced reductions in either will result in ventilation/perfusion mismatch with corresponding decline in overall pulmonary function. That ventilatory and perfusion changes occur in parallel suggests that normal physiologic compensatory mechanisms within both the ventilatory and circulatory systems are contributing to the changes noted after RT.
The histopathological changes observed in the lung after RT are broadly characterized as diffuse alveolar damage. Acutely, increased vascular permeability leads to edema of the interalveolar septa and extravasation of proteinaceous material into the alveoli. Type I pneumocytes are depleted and type II pneumocytes proliferate to restore the integrity of the alveolar epithelium. However, type II pneumocytes are also known to be damaged by RT, leading to the release of surfactant into the alveolar lumen. Increased levels of alveolar surfactant can be seen within hours of RT and can persist for 2–6 weeks (Fleckenstein et al. 2007).
3.2.2.1 Airway Diameter and Airway Resistance
Resistance to airflow within the tracheobronchial tree increases the work of breathing. Assuming laminar flow, the pressure required to produce a given flow rate in a tube is inversely proportional to the fourth power of the radius. This is known as Poiseuille’s law and can be expressed as:
ΔP/V = resistance = 8 nl/πr 4 where ΔP refers to the pressure difference between the two ends of a tube, V is the volume of flow per time period, n is the coefficient of viscosity, l is the length of the tube, and r is the radius of the tube (Comroe 1965). Because the length of the tracheobronchial tree is constant, and the viscosity of air is nearly constant, resistance to airflow is almost entirely dependent on the radius of the airways. Even modest reductions may be sufficient to cause pulmonary symptoms. For example, when the radius of a tube is decreased by a modest 10%, the resistance to airflow is increased by 46%.
Symptomatic narrowing of the tracheobronchial tree is not a common clinical problem after conventional-dose EBRT. However, this has been reported following brachytherapy (Speiser and Spratling 1993) and with higher than conventional doses of external beam RT (≥70 Gy) (Miller et al. 2005; Kelsey et al. 2006). Whether asymptomatic narrowing of the bronchi occurs after conventional doses (60–70 Gy) is not known.
3.2.2.2 Gas Exchange
The rate at which a gas diffuses between the alveoli and capillaries is proportional to the partial pressure gradient of that gas and the available surface area, but inversely proportional to the thickness of the intervening tissues. Disease processes that affect any of these parameters have the potential to limit gaseous exchange. Decreased lung compliance from pulmonary fibrosis or increased airway resistance from bronchial stenosis can decrease the partial pressure of oxygen in the alveoli. Further, RT results in edema of the intraalveolar septa and extravasation of proteinaceous material into the alveoli, both of which negatively affects gas exchange. Later, deposition of scar tissue within the interalveolar septi will impede gas exchange while destruction of alveoli decreases the surface area available for gas exchange.
3.2.2.3 Ventilation and Perfusion
In healthy individuals, alveolar ventilation (V) and pulmonary capillary perfusion (Q) are closely matched for optimal efficiency. For example, if there is decreased alveolar ventilation to a portion of the lung, alveolar and interstitial P 02 will decrease. Local arterioles respond by constricting, diverting blood to better ventilated portions of the lung. On the other hand, if alveolar P 02 rises above normal, local arterioles dilate to maximize oxygen transport. In similar manner, local ventilation is regulated (albeit to a lesser degree than perfusion) in response to local levels of CO2 to optimize the V/Q ratio and maximize the efficiency of gas exchange in the lung.
RT negatively affects both ventilation and perfusion, with perfusion being affected to a greater degree than ventilation (Allavena et al. 1992; Bell et al. 1988). RT reduces the number of perfused alveoli, with a corresponding increase in the alveolar dead-space (i.e., ventilated but under- or nonperfused alveoli). This increased dead-space and reduced functional units leads to a reduction in gas exchange that can cause dyspnea.
4 Clinical Syndromes (Endpoints)
Scoring lung toxicity is difficult for many reasons. For example, a variety of endpoints can be appraised including symptoms, radiographic abnormalities, and pulmonary function tests. These endpoints can be somewhat arbitrarily segregated as reflecting focal versus global, and clinical versus subclinical, changes (Table 3). The SOMA LENT system provides a reasonable way to categorize and grade these various toxicities (Table 4). Each of these endpoints are discussed separately. In broad terms, symptoms occur in approximately 1–40% of patients who receive thoracic RT, depending on the volume of lung irradiated and the dose that is given. Radiologic changes occur in almost all patients if a sensitive imaging tool is used. Changes in PFT’s following RT are common and reflect the competing effects of RT-induced functional loss and recovery of function with tumor regression (for patients with gross intrathoracic lesions).
4.1 Symptoms
Radiation pneumonitis, typically manifested as shortness of breath, dry cough, and occasionally fever, occurring 1–6 months after treatment (Tsoutsou and Koukourakis 2006), appears to result from an acute inflammatory process within the alveolar spaces. While the radiologic changes associated with this inflammatory reaction are generally limited to the irradiated regions of the lung (Mah et al. 1987; Marks et al. 2000), radiologic changes have been occasionally observed to extend beyond the irradiated field (Gibson et al. 1988; Roberts et al. 1993). Furthermore, bronchoalveolar lavage (BAL) following unilateral irradiation reveals increased lymphocytes from both the irradiated and unirradiated lung (Gibson et al. 1988; Roberts et al. 1993; Martin et al. 1999). These observations suggest that radiation pneumonitis may be type of hypersensitivity pneumonitis (bilateral lymphocytic alveolitis) (Abratt and Morgan 2002). Further, the lymphocytes retrieved with BAL are activated T-lymphocytes (Martin et al. 1999), suggesting that immunologic processes are participating in the radiation reaction. The observation that pneumonitis typically responds well to oral prednisone (Salinas and Winterbauer 1995) also supports the inflammatory/immunologic nature of radiation pneumonitis.
In patients with symptomatic pneumonitis, physical exam is often normal, although rales or a friction rub can sometimes be appreciated. Radiological studies are often, but not always, abnormal. Thus, pneumonitis is a clinical diagnosis. Radiographic abnormalities without symptoms usually do not warrant intervention. Indeed, asymptomatic patients frequency has radiologic findings similar to those seen in symptomatic patients. Thus symptoms and radiologic findings do not necessarily parallel each other. Further, since abnormalities on CT and CXR are very common following RT, reported studies that consider asymptomatic radiologic findings as ‘an event’ likely over-estimate the rate of ‘clinical toxicity’.
Differentiating radiation pneumonitis from other etiologies (e.g., tumor progression, infection, pulmonary emboli, heart disease) can be challenging (Kocak et al. 2005). Ideally, one should be reasonably sure that symptoms suggestive of pneumonitis are not due to these other conditions before therapy is initiated. A short course of antibiotics is occasionally useful if there is concern for infection, with a rapid institution of steroids if symptoms do not respond.
The culminating event in RT-induced lung injury is replacement of normal lung parenchyma with fibrosis, which is often appreciated on radiographic studies but is usually asymptomatic (Marks et al. 2000). However, if the baseline pulmonary reserve of a patient is limited and/or the region of fibrosis is extensive, symptoms can develop. In this circumstance, dyspnea can be progressive and difficult to manage, often requiring long-term steroids, oxygen, and/or rehabilitation. The severity of radiographic abnormalities is not always well-correlated with the presence or severity of pulmonary symptoms (Marks et al. 2000). The time course for the development of RT-associated lung symptoms is shown in Fig. 6a, b.
Time course for the development of RT-associated lung symptoms. a Data from Duke with patients individually noted as having “pneumonitis”, “fibrosis”, as well as cases where the diagnosis was somewhat uncertain due to concurrent illnesses (e.g. infection). b Data from several different centers as shown. (Adapted from Marks et al. 2000)
In patients treated with high doses of radiation (e.g., ≥70 Gy), unusual pulmonary complications, such as bronchial stenosis, bronchopleural fistula, and fatal hemoptysis have been reported (Miller et al. 2005; Socinski et al. 2004).
4.2 Imaging
Multiple radiological studies have been used to assess regional lung injury. The most widely studied include chest X-ray and CT (which evaluate structure and tissue density), and single photon emission computed tomography (SPECT) (which can evaluate the functional endpoints of ventilation and perfusion). The sensitivity of these radiological modalities varies, and thus, the incidence of lung injury depends on the modality utilized. Furthermore, radiological changes are common in patients with lung cancer (who receive relatively large doses to large volumes of lung), and less common in patients with breast cancer or lymphoma (where the doses/volumes are lower). In general, 3D imaging modalities are more sensitive than 2D modalities (CT is more sensitive than CXR), and SPECT-based ventilation/perfusion imaging is more sensitive than either CXR or CT. Comparisons between studies are challenging since the patient populations and RT doses used are variable. Nevertheless, these generalizations appear to be true in studies that have considered multiple imaging modalities (Jackson et al. 2006).
4.2.1 Chest X-ray
The most common finding on plain radiographs after thoracic RT is increased density (radiopacity). This may be secondary to increased interstitial edema and/or alveolar consolidation acutely and pulmonary fibrosis at later time points. Other common findings are nonanatomic margination (corresponding to the RT field edge), volume loss with midline shift, and pleural thickening. Radiographic changes develop shortly after completion of RT, peak at approximately 6 months (Skoczylas et al. 2000; Monson et al. 1998; Lind et al 2000), and typically stabilize by 12 months. However, continued follow-up may show either further progression or even regression in a small number of patients (Skoczylas et al. 2000).
4.2.2 Computed Tomography
The abnormalities noted with CT and chest X-ray are similar. However, CT appears to be more sensitive, in part because paramediastinal abnormalities may be obscured by the cardiac or aortic silhouette on chest X-ray. The typical acute findings are often described as early ground-glass opacities and patchy infiltrates (Fig. 7a). Late findings typically include fibrotic changes with consolidation and volume loss (Fig. 7b). It can sometimes be difficult to differentiate mass-like fibrosis from persistent/recurrent tumor (Koenig et al. 2002). Registration of the planning CT scan (and hence the 3D dose distribution) to the post-RT CT, demonstrates that there are CT-defined increases in tissue density, which are dose-dependent, and largely seen in lung regions receiving ≥40 Gy (Theuws et al. 1998; Kocak and Marks 2005) (Fig. 7e).
CT image of a patient 3 months post-RT to the mediastinum for lung cancer. Characteristic increased interstitial markings are seen in the medial lung bilaterally, essentially demarcating the volume of lung that received ≥40 Gy (a). CT image about 2 years post-RT illustrating late fibrotic changes with volume loss. Representative overlying isodose curves shown (b). Transverse SPECT lung perfusion images obtained pre-RT (c) and post-RT (d) in a single patient irradiated for lung cancer. Representative isodose lines are shown. Note the reduction in regional perfusion post-RT. By considering the CT density or perfusion in each pixel independently, and pooling pixels into dose bins, one can create a dose response curve; example panel (e)
Other abnormalities include traction bronchiectasis, diminished pulmonary vascularity, as well as narrowing of the proximal bronchi. Traction bronchiectasis develops from tethering of the bronchial wall by RT-induced fibrosis. Decrease in pulmonary vasculature has been demonstrated outside of the RT field. This maybe secondary to irradiation of central tumors with injury to proximal vessels and/or bronchi (Bell et al. 1988). However, in our studies using SPECT to assess changes in regional perfusion post-RT, we have not typically seen reduced perfusion in unirradiated lung, suggesting that perfusion through larger central vessels is preserved after conventional doses of RT. Symptomatic bronchial stenosis is not commonly observed after conventional doses of RT. With dose escalation, bronchial stenosis has been observed in both symptomatic (Miller et al. 2005) and asymptomatic patients (Kelsey et al. 2006).
4.2.3 Single Photon Emission Computed Tomography
Nuclear medicine imaging of regional perfusion and/or ventilation can also be used to assess regional lung function. Imaging can be planer or 3D (via SPECT). The latter is more sensitive than the former (Bell et al. 1988), analogous to the utility of CT over CXR. Example pre- and post-RT SPECT perfusion images are shown in Fig. 7c, d. Registration of the planning CT scan to pre-and post-RT SPECT scans allows one to quantitatively relate regional RT doses to changes in regional perfusion (Fig. 7e). RT-induced changes in ventilation and perfusion appear to be more common than corresponding changes on CT (Theuws et al. 1998).
Dose-dependent reductions in perfusion are seen as early as 6 weeks after RT (Theuws et al. 2000; Woel et al. 2002), and peaking by approximately 6 months (Woel et al. 2002). Whether recovery occurs with time is unclear. Following modest doses of RT for breast cancer and lymphoma, investigators at the Netherlands Cancer Institute (NKI) report up to 50% recovery by 18 months (Theuws et al. 2000; Boersma et al. 1996). At Duke, we have not seen such recovery, but our study population was predominantly patients with lung cancer receiving higher doses of RT (Woel et al. 2002).
Since the alveolar network is structured in parallel, it is reasonable to hypothesize that changes in global lung function will be related to the extent and severity of regional injury. This is similar to what has been done by surgeons who have tried to relate the percent decline in pulmonary function with the percent of alveoli that are resected (Beckles et al. 2003). We and investigators at the NKI have attempted to relate the sum of the changes in regional perfusion (also termed the integrated response) to changes in pulmonary symptoms and PFTs, with mixed success. While there is a strong correlation between the sum of regional injuries and changes in global function, the correlation coefficients are weak, suggesting that there may be numerous other factors affecting global pulmonary function (Fan et al. 2001; Theuws et al. 1998). The correlation coefficients are higher for the patients with breast and lymphoma than for the patients with lung cancer.
4.2.4 Other Modalities
Magnetic resonance imaging (MRI) has not been widely used to evaluate RT-induced lung toxicity. A preliminary study demonstrated T1 and T2 changes within normal lung parenchyma as early as 17 days after RT. These changes increased over several months before beginning to resolve (Yankelevitz et al. 1994). Another study demonstrated distinct perfusion changes associated with radiation pneumonitis (greater enhancement with both first-pass and redistribution phases) and fibrosis (decreased enhancement with first-pass but greater enhancement with the distribution phase) (Ogasawara et al. 2002). Further MRI investigations may be warranted.
Like MRI, there are few studies evaluating RT-induced lung injury using positron emission tomography (PET). Increased uptake in the lungs of patients with radiation pneumonitis has been reported (Hassaballa et al. 2005; Lin et al. 2000). Another study demonstrated an apparent linear dose response (Guerrero et al. 2007). As expected, there was marked inter-patient variation suggesting an underlying biological susceptibility to injury.
4.3 Pulmonary Function Tests
PFTs objectively assess multiple pulmonary parameters including lung volumes, the amount and rate of air flow (spirometry), and the ability of the lung to transfer gas at the alveolar level. Diffusing capacity of the lung for carbon monoxide (DLCO) is likely the best parameter to assess lung function after RT, since the ultimate role of the lungs is to facilitate gas exchange. Loss of alveolar surface area and thickening of the intraalveolar septi are the primary causes for reductions in DLCO. Thus, both acute (edema, extravasation of material into the alveoli) and chronic (fibrosis, loss of alveoli) injury can affect DLCO. The forced expiratory volume in 1 s (FEV1) is another useful parameter and often interpreted in the context of the FEV1/FVC ratio (FVC = forced vital capacity). A decreased FEV1 with a normal FEV1/FVC ratio is most consistent with a restrictive process, such as fibrosis. A decreased FEV1 and FEV1/FVC ratio is more consistent with an obstructive process.
Assessing the impact of RT on PFTs in patients with lung cancer is challenging. Many patients with lung cancer have baseline pulmonary dysfunction from smoking, such as chronic obstructive pulmonary disease, and some patients continue to smoke after RT with continued accelerated diminutive effects on function. Also, pulmonary function may transiently improve as large central tumors regress. Finally, there is limited data on long-term PFT changes since most patients with lung cancer succumb to their disease within 1–2 years.
With these caveats, most studies show a decline in pulmonary function after RT. Reductions in FEV1 are appreciated approximately 3–6 months post-RT (Table 5), sometimes followed by partial/complete recovery at ≈12 months. This transient decline may be coincident with the acute inflammatory reaction associated with pneumonitis. DLCO typically is reduced to a greater degree than FEV1 (Table 5), and many studies appears to show less recovery at ≈12 months than FEV1. Most studies show a decline in both FEV1 and FVC (Borst et al. 2005; Miller et al. 2003), such that the FEV1/FVC ratio remains in the normal range (Myers et al. 2005), consistent with a restrictive process. In the few patients with long-term changes in PFTs systematically studied beyond one year, there appears to be long-term continued diminution in PFTs 2–8 years post-RT (Miller et al. 2003).
Multiple studies, primarily in children, have confirmed that pulmonary function tests are abnormal after whole lung irradiation (Benoist et al. 1982; Ellis et al. 1992; Littman et al. 1976; Miller et al. 1986; Weiner et al. 2006). In general, these studies demonstrate decreased lung volumes (vital capacity, total lung capacity), impaired air flow (forced expiratory volume in 1 second), and perhaps diminished diffusing capacity (DLCO). Multiple factors likely play a role, including impaired chest wall growth, a reduction in the number of functioning alveolar units, and fibrotic changes within the lung. Whether function improves over time (Ellis et al. 1992) or continues to deteriorate (Weiner et al. 2006) is unclear.
5 Radiation Tolerance and Predicting Radiation-Induced Lung Injury
5.1 Whole Lung Irradiation
Irradiation of both lungs occurs in multiple clinical contexts. This includes total body irradiation (TBI) as part of the preparative regimen for stem cell transplants, hemibody irradiation in the setting of diffuse symptomatic metastatic disease, and whole lung irradiation for pulmonary metastases from various malignancies (not commonly done recently). Clinical experience in these settings has permitted the tolerance of whole lung irradiation to be estimated.
5.1.1 Single Fraction (Total Body RT and Hemibody RT)
With single fraction whole lung irradiation, the risk of interstitial pneumonitis is primarily dependent on total dose and dose rate. For low-dose rate TBI (5–10 cGy/min), assuming lung density corrections, an early study concluded that the threshold dose for pneumonitis is approximately 9 Gy, the TD5 (dose resulting in a 5% complication rate) is approximately 10 Gy, with a TD50 (dose resulting in a 50% complication rate) of 11–12 Gy (Keane et al. 1981). When higher dose rates are utilized, principally for hemibody irradiation, the dose response curve is shifted to the left. In this circumstance, the threshold dose was estimated to be approximately 7 Gy, with a TD5 of 8 Gy and a TD50 of approximately 9.3 Gy (Keane et al. 1981). In a more recent, comprehensive review, the TD5 (with cyclophosphamide) was estimated to be much lower, approximately 5 Gy for low-dose rate TBI (Sampath et al. 2005). Both studies demonstrated that the dose response curve for whole lung irradiation is steep such that a difference of only 1–2 Gy increases the risk of pneumonitis significantly (Fig. 8) The development of pneumonitis after whole lung irradiation is an ominous sign, proving fatal in up to 80% of patients (Fryer et al. 1978). The difference in mortality for similar doses between TBI and HBI dose response appears to have been due to the low-dose rate protraction for TBI. Decreasing the dose rate from 0.5 to 0.1 by per minute decreases the incidence of pneumonitis associated with whole lung irradiation during TBI. This was a byproduct of greater distance from the radiation source, required to produce a large field to encompass the whole body.
The risk of pneumonitis following whole lung RT as part of TBI. The addition of chemotherapy appears to move the dose response curve to the left, and fractionating the RT moves the dose response curve to the right (Adopted from QUANTEC, Marks et al. 2010, IJROBP)
5.1.2 Multiple Fractions
Two randomized studies treated patients with osteogenic sarcoma with prophylactic, fractionated whole lung irradiation. A Mayo clinic trial treated patients with 15 Gy over 2 weeks with concurrent actinomycin D (Rab et al. 1976). A European study treated patients with 17.5 Gy in 2 weeks without chemotherapy (Breur et al. 1978). Lung density corrections were not utilized in either study, and thus the true total physical doses delivered were likely in the neighborhood of 17 and 20 Gy in these two studies, respectively. No cases of pneumonitis were reported in either trial. A retrospective study of whole lung irradiation for a variety of tumor histologies, mostly pediatric, reported no cases of pneumonitis after doses ranging from 15–25 Gy (Newton and Spittle 1969). Furthermore, in several retrospective studies in which TBI was utilized in the preparative regimen prior to stem cell transplant, the risk of interstitial pneumonitis appeared to be lower with fractionation (Cosset et al. 1989; Pino et al. 1982; Shank et al. 1983). These studies suggest that fractionation increases lung tolerance. However, in two randomized studies comparing single fraction TBI with fractionated TBI, there was no difference in the risk of interstitial pneumonitis (virus + idiopathic) (Table 6). There were less cases of idiopathic pneumonitis with fractionation (Deeg et al. 1986; Girinsky et al. 2000).
Pulmonary toxicity after allogeneic stem cell transplantation, particularly after myeloablative conditioning, is relatively common. Pulmonary toxicity includes both infectious (bacterial, viral, or fungal) and non-infectious (interstitial pneumonia, adult respiratory distress syndrome, diffuse alveolar hemorrhage, bronchiolitis obliterans, etc.) etiologies. However, in immunocompromised transplant patients, it is often difficult to definitively differentiate one etiology from another (e.g., radiation pneumonitis versus bacterial pneumonia) and often requires invasive tests such as bronchoscopy.
Most, if not all, TBI protocols are now fractionated. Total doses are typically 10–14 Gy given in once or twice daily fractions, usually with the lungs attenuated to ~7–10 Gy. Patients also receive high-dose chemotherapy which appears to lower the threshold for pneumonitis. In the absence of chemotherapy, single doses of up to 6–8 Gy and fractionated doses of up to 25–28 Gy appear tolerable. Conversely, for patients undergoing TBI as part of a transplant regimen that includes chemotherapy, far lower doses are tolerated.
5.2 Partial Lung Irradiation: Conventional Fractionation
Most patients receiving thoracic RT receive fractionated partial lung irradiation. Determining tolerance doses and accurately predicting the risk of RT-associated pulmonary toxicity after partial lung irradiation is challenging. Multiple clinical (patient- and tumor-specific), biologic, and dosimetric parameters have been evaluated. Dosimetric parameters appear to be the most useful and will thus receive the most attention.
5.2.1 Clinical Parameters
In both retrospective and prospective studies, several clinical factors have been associated with an increased risk of developing symptomatic pneumonitis, including underlying lung disease (Monson et al. 1998; Rancati et al. 2003), decreased baseline pulmonary function (Monson et al. 1998; Robnett et al. 2000), low performance status (Monson et al. 1998; Robnett et al. 2000), female gender (Robnett et al. 2000), history of smoking (Monson et al. 1998), and not actively smoking. However, these findings have been inconsistent across studies, such that none of these parameters have been found to be a robust and reliable risk factor for the development of RT-induced lung injury.
Preclinical and clinical data suggest that irradiation of lower lung regions may be more clinically important than irradiation of upper lung regions. This may be secondary to nonuniform distribution of gas exchange throughout the lung and/or incidental heart dose. In mice, Travis et al. (1997) demonstrated that the lower lobes are more radiosensitive (increased respiratory rate, lethality) than the upper lobes. In another rodent study, the respiratory rate following thoracic RT was related to both the volume of lung and heart irradiated (van Luijk et al. 2005). Similarly, RT-induced changes in breathing rate were greater with left- versus right-sided RT in rats (Wiegman et al. 2003), suggesting that incidental dose to the heart may contribute to symptomatic RT-induced lung injury. Many (Graham et al. 1999; Hope et al. 2006; Seppenwoolde et al. 2004; Yorke et al. 2005), but not all (Robnett et al. 2000; Tsujino et al. 2003; Wang et al. 2006) clinical studies have shown increased rates of radiation pneumonitis with treatment of lower lobe tumors. As stated above, part of these effects may be related to incidental cardiac irradiation, as a greater volume of heart is the field with lower lobe targets.
It seems logical to assume that pre-RT pulmonary function would be an important factor in predicting RT-induced lung injury. In patients undergoing surgery, there is an association between the percent decline in PFTs and the percent of lung volume resected. Thus, pre-surgical pulmonary function is predictive of the patient’s ability to tolerate surgery. For patients receiving RT, it has been more challenging to quantitatively relate pre-RT PFTs to the risk of subsequent RT-induced lung injury. One of the challenges for considering pulmonary function in the pre-RT assessment is that it may be compromised by the tumor itself, and that treatment may lead to improvements in pulmonary function with tumor shrinkage.
5.2.2 Biological Parameters
Many studies have also addressed the role of potential biologic predictors of RT-induced lung injury. These are markers found in the blood prior to, during, or after completing RT and are thought to reflect a predisposition for, or the ongoing evolution of, RT-induced lung injury. A host of cytokines have been implicated, including transforming growth factor-beta (TGF-β). This is a multifunctional regulator of cell growth and differentiation that stimulates connective tissue formation and decreases collagen degradation, which can result in fibrosis (Anscher et al. 1998). In patient subsets, TGF-β has been suggested to predict RT-induced lung injury (Anscher et al. 1994; Fu et al. 2001). However, further investigation will be necessary before biological predictors of lung injury can be used clinically because the present data regarding such cytokines are conflicting (Table 1).
Rare disorders (e.g., ataxia-telangiectasia), suggest that genetic differences, including single nucleotide polymorphisms (SNPs), may mediate RT sensitivity. A recent study demonstrated a reduced risk of radiation pneumonitis in lung cancer patients who harbored a polymorphism at position 869 within the TGF-β gene (Yuan et al. 2009). Further studies are ongoing.
5.2.3 Dosimetric Parameters
The risk of developing acute pneumonitis has been associated with a variety of dosimetric parameters (Kim et al 2005; Yorke et al 2002, Willner et al 2003, Oetzel et al 1995, Fay et al 2005) including the mean lung dose (MLD) and the percent of lung receiving doses in excess of a specified threshold dose, as summarized in the recent QUANTEC review (Marks et al. 2010) (Fig. 9a, b). These parameters are readily extractable from a dose–volume histogram (DVH) and/or treatment planning software. However, lung volumes vary with the breathing cycle and tumor response during RT may lead to increased lung exposure (with normal lung replacing space previously occupied by tumor). These factors are not easily accounted but should be kept in mind.
Incidence of radiation pneumonitis versus mean lung dose (a) and the percent lung exposed to doses exceeding different thresholds [Vx] (b). Vx = percent of lung receiving ≥x Gy. (Adopted from QUANTEC, Marks et al. 2010, IJROBP)
Strict dose/volume guidelines are difficult to define and implement clinically. Traditional DVHs assume that all regions of the lung contribute equally to pulmonary function, which is often an erroneous assumption since many patients have heterogenous regional lung function (e.g., from tumor or COPD). Furthermore, metrics such as the V20 or V30 (volume of lung receiving at least 20 or 30 Gy, respectively), consider only a single point on the cumulative DVH. It is apparent that two DVHs with markedly different shape may overlap at the same point (V20, for instance). MLD might be a preferable metric since it considers the entire 3D dose distribution. In practice, it may not be particularly useful to try to determine which parameter is truly “optimal” since there tends to be strong correlations between the different dosimetric parameters, at least with relatively uniform treatment techniques (Fan et al. 2001; Graham et al. 1999; Kong et al. 2006).
In most series, the majority of patients are treated with MLDs in the lower aspect of the range shown in Fig. 9a. Thus, even though the incidence of pneumonitis is less when the MLD is lower, the absolute number of patients with pneumonitis derived from the low-MLD group is relatively high. In this regard, MLD is not particularly sensitive (i.e., it does not predict a high fraction of the pneumonitis cases). An alternative manner to illustrate the approximate relationship between dose/volume/risk is shown in Fig. 10.
A suggested manner to illustrate the relationship between DVH-based parameters and the risk for lung toxicity. The numbers in the rectangles are the estimated risks of injury with the corresponding dose (left y-axis) and volume (x-axis). The right y-axis indicates the tolerance dose ranges for the TD5 and TD50 for whole organ (lungs) irradiation, as well as the TD5 and TD50 for the partial organ volume (POV) irradiation (with permission from Clinical Oncology 8th Edition)
5.3 Partial Lung Irradiation: Intensity-Modulated Radiation Therapy
Intensity-modulated radiation therapy (IMRT) facilitates conformal treatment of irregularly shaped and concave targets. This is especially useful for targets adjacent to critical structures. However, IMRT planning usually delivers lower doses of radiation to larger volumes of surrounding normal tissue. The impact of large-volume/low-dose lung irradiation in the context of curative treatment of lung cancer is not known. The M.D. Anderson Cancer Center recently published the largest clinical experience using IMRT in 68 patients with lung cancer (Yom et al. 2007). In this report, the incidence of grade ≥3 radiation pneumonitis was significantly lower in those patients treated using IMRT versus their comparison group treated with conventional 3D-conformal RT (8 vs. 32% at 12 months, p = 0.002). In the IMRT group, patients with V5 values (volume of lung receiving more than 5 Gy) greater than 70% appeared to be at higher risk of pneumonitis. Other dosimetric parameters (MLD, V20) were not assessed for their association with pneumonitis. Investigators at Memorial Sloane Kettering Hospital noted a similarly low rate of clinical lung injury in patients with non-small cell cancer treated with IMRT (Sura et al. 2008). Additional experience is necessary to confirm the safety and efficacy of IMRT for lung cancer. Further, the dosimetric predictors of pneumonitis might be different with a limited number of conformal beams versus IMRT.
5.4 Partial Lung Irradiation: Hypofractionation
There is increasing interest in the use of stereotactic body radiation therapy (SBRT) for patients with medically inoperable stage I NSCLC and patients with oligometastases involving the lung. In contrast to the standard method of treating lung cancer (1.8–2 Gy daily fractions given over a period of 6–7 weeks) this unique approach uses only a few very large fractions given over 5–10 days. Currently used fractionation schemes of 18 Gy × 3, 12 Gy × 4, and 10 Gy × 5 appear to provide a reasonable therapeutic ratio for small lesions. These large daily doses are feasible because only small volumes are treated with conformal techniques, which minimizes dose to surrounding critical structures.
Within 6–12 months after SBRT, most patients develop increased tissue density on CT in the high-dose region (Hof et al. 2003; Kyas et al. 2007), which can be difficult to differentiate from persistent tumor. Symptoms consistent with radiation pneumonitis have occurred in 2–25% of patients (Stephans et al. 2009; Hoyer et al. 2006; Nagata et al. 2005; Timmerman et al. 2003). Serious, and sometimes lethal, pulmonary toxicity has associated with treatment of perihilar/central tumors (Song et al. 2009; Timmerman et al. 2006), especially with the 20 Gy × 3 regimen. Whether more protracted regimens (e.g., 10 Gy × 5) are safer for central tumors is not presently clear and dose-escalation studies are ongoing. PFT changes after SBRT appear to be minimal (Stephans et al. 2009). As experience with this radiation technique is being rapidly gained, our understanding of the effects of hypofractionated, high-dose RT to very small volumes of lung will become more broadly known in the near future.
Investigators at the University of Michigan have systematically studied the role of dose escalation for non-small cell lung cancer and their findings with conventional fractionation may have relevance to SBRT. The dose of RT was determined based on the predicted risk of grade 2 or higher radiation pneumonitis using Lyman’s NTCP model (Lyman and Wolbarst 1987). In this manner, extremely high doses of RT (up to 102.9 Gy) were successfully delivered (Hayman et al. 2001) with ~15% risk of radiation pneumonitis and no cases of grade 4–5 toxicity (Kong et al. 2006).
5.5 Recommended Dose/Volume Constraints
The lung is one of the most challenging organs to define dose/volume constraints. Many patients with lung cancer require large treatment fields due to tumor extent. The radiation oncologist does not always have the option to reduce the volume of lung irradiated. Further, there is marked inter-patient variation in pre-RT lung disease (e.g., COPD), and tumor-related pulmonary compromise. It may not be appropriate to force strict dose/volume constraints based on pre-RT pulmonary status, since poor pre-RT function maybe due to the tumor itself, and hence may actually improve with RT. Therefore, patients need to be counseled about the possible risks of lung injury and the uncertainty in defining individual risk. With these caveats in mind, there are nevertheless some broad guidelines that can be clinically useful (Table 7).
We recommend limiting the volume of lung receiving over 20 Gy (V20) to 30–35% (Graham et al. 1999) and limiting the MLD to 20–23 Gy (Hernando et al. 2001; Kwa et al. 1998). Recent data suggest that the volume of lung exposed to relatively low doses of RT (e.g., 5–15 Gy) may be more predictive for pneumonitis than V20–30 (Schallenkamp et al. 2007; Uno et al. 2006; Wang et al. 2006). This is particularly relevant when IMRT techniques are utilized or when patients with mesothelioma are treated after extrapleural pneumonectomy. However, further investigation is necessary to understand the biological and clinical ramifications of low-dose RT to large volumes of lung. Symptomatic bronchial stenosis has been observed with doses >70 Gy, thus care should be taken when irradiating central structures to high doses. Dose guidelines for SBRT are in a state of rapid flux as the number of clinical reports appears to be rapidly increasing.
6 Chemotherapy
6.1 Combined Treatment
A number of chemotherapeutic agents have been shown to be associated with a range of pulmonary toxicities, including dyspnea, pulmonary edema, bronchospasm, acute and interstitial pneumonitis, and even pulmonary fibrosis. Toxicities are often dose-dependent and related to other factors such as concurrent administration of other chemotherapeutic drugs and/or RT. Although many toxicities are reversible with discontinuation of medication, some are permanent and may occur as part of late manifestations of treatment. As a result, a high index of suspicion is needed for patients who present with respiratory symptoms, particularly in those for whom the treatment is known to been associated with an increased risk of lung damage. Lung toxicity has been reported with a number of chemotherapeutic agents (Table 8) (Carver et al. 2007). It is important to note, however, that chemotherapy-induced lung toxicity is largely considered a diagnosis of exclusion after fully considering and exploring other differential diagnoses (i.e., typical and atypical infections, COPD exacerbation, pulmonary embolism, etc.).
The first recognition that chemotherapy could produce late effect fibrosis in lung without pneumonitis was a tragic event. In children with brain tumors, the administration of the nitrosoureas (BUDR) was continued and prolonged, judging toxicity hematologically. Eventually, 35% of brain tumor survivors died of lung fibrosis. Twelve percent died within three years of treatment, however, 24% died of progressive lung fibrosis being symptom free for 7–12 years.
Bleomycin, as an example, has been one of the most studied agents for chemotherapy-induced lung injury, and is a feared complication of treatment for germ cell tumors with an estimated occurence of ~10% of patients (Jules-Elysee and White 1990). Although the exact mechanism is unknown, the pulmonary specific drug toxicity is felt to be in part due to the fact that the inactivating enzyme, bleomycin hydrolase, is less expressed in normal lung tissue compared to other normal tissues (Sikic 1986). Exposure to high FiO2 has also been associated with exacerbation of bleomycin toxicity in animal models and humans (Gilson and Sahn 1985; Allen et al. 1981; Toledo et al. 1982; Tryka et al. 1982). Clinical manifestations include an acute hypersensitivity pneumonitis or a fibrosing alveolitis that may occur acutely, subacutely, or >6 months following exposure. Symptoms are nonspecific, as is the case in many presentations of chemotherapy-induced lung toxicity, and may include fever, cough, chest pain, dyspnea, tachypnea, hypoxemia, etc. Although treatment in the acute setting has traditionally consisted of prompt discontinuation of bleomycin and initiation of corticosteroids, reports are anecdotal and nonrandomized (Maher and Daly 1993; White and Stover 1984).
The interactions between chemotherapy and RT within the normal lung are still being deduced, although it has been observed that many chemotherapeutic drugs, including those without significant pulmonary toxicity, appear to potentiate the toxic effects of RT. For example, in a small series of 24 lung cancer patients treated with 40 Gy of RT with concurrent doxorubicin (10 mg/m2), 13 (54%) developed radiation pneumonitis. Combinations of RT and chemotherapeutic agents with known pulmonary toxicity have generally been associated with higher rates of radiation pneumonitis, including mitomycin-C (Rancati et al. 2003) and bleomycin (Einhorn et al. 1976). The taxane docetaxel, with RT in the locally advanced setting and with gemcitabine in the metastatic setting (Kouroussis et al. 2004), has also been associated with high rates of pneumonitis. For example, in a Japanese phase II study (Onishi et al. 2003), lung cancer patients were treated with 60–66 Gy with concurrent weekly docetaxel (20 mg/m2). The rate of grade 3–4 pneumonitis was 47%. Fortunately, the chemotherapeutic agents most commonly utilized with RT for lung cancer, such as cisplatin, carboplatin, paclitaxel, and etoposide, have not been consistently shown to increase the risk of pneumonitis (Robnett et al. 2000; Graham et al. 1999; Hope et al. 2006; Hernando et al. 2001). The impact of chemotherapy in the setting of TBI is shown in Fig. 8.
7 Special Topic
7.1 Abscopal Effects: Autoimmune Events
Contralateral pneumonitis following incidental ipsilateral lung irradiation for breast cancer utilizing tangential parallel fields was first reported in 1964. This was intensely studied by Morgan and Breit who lavaged the contralateral lung in a series of breast cancer patients post-irradiation. Although asymptomatic, the lavaged contralateral lung yielded an increase in lymphocytes and macrophages which was termed an autoimmune effect. Finklestein, Rubin and Williams observed a dramatic increase in the contralateral lung abnormalities following ipsilateral lung irradiation. Other investigators have confirmed abscopal out-of-field effects when confining radiation to the apex or base of lung attributed to cytokine cascades, inducing an inflammatory response in shielded lung segments. DNA damage in micronuclei are also evident in addition to lymph acidic infiltrates. The induction of an acute respiratory distress syndrome (ARDS) in out of field lung, induced by in field radiation pneumonitis can be fatal. Bennett and Million reported that 8% (6/72) of lung cancer patients at necropsy had ARDSas did 3% of patients iiradiated for breast cancer, which had been attributed to bilateral radiation pneumonitis.
7.2 Malignant Pleural Mesothelioma
Local disease progression is the primary obstacle to cure for patients with pleural mesothelioma, even after aggressive surgery. Tumor can spread anywhere within the pleural space, including the fissures, diaphragmatic and pericardial surfaces, and the pleural recesses. RT techniques have historically been suboptimal to fully encompass sites at risk while respecting normal tissue tolerance. Recently, there has been growing interest in IMRT for these patients as it tends to be well suited for the delivery of the necessary concave dose distribution. In an initial report from MD Anderson, a low rate of lethal radiation pneumonitis was noted (1.6%, 1/62) (Stevens et al. 2005). A subsequent report from Harvard could not replicate the safety of this approach. Of 13 patients treated with IMRT, 6 experienced lethal radiation pneumonitis (46%, 6/13) (Allen et al. 2006). The MLD was 15 Gy for patients who developed pneumonitis (all lethal) versus 13 Gy for those who did not (p = 0.07). Similarly, the V20 was 18% for patients who developed pneumonitis versus 11% for those who did not (p = 0.08). In a reanalysis of the data from MD Anderson, fatal pulmonary toxicities (whether previously ascribed to RT or not) were noted to have occurred in 10% of patients. These events were more common in patients with higher V20 values (Rice et al. 2006). While IMRT may be particularly suited for the concave targets encountered with the postoperative treatment of mesothelioma, dose to the contralateral lung must be minimized. In this setting, we recommend limiting the V20 to 4–10% and the MLD to <8 Gy (Allen et al. 2006; Rice et al. 2006) (Table 7).
7.3 Functional Imaging
There is growing interest in utilizing functional imaging, such as SPECT, in the RT treatment planning process. In lung perfusion SPECT, injected radiolabeled microspheres are trapped in the small capillaries of the lung. The local concentration, in principle, is proportional to regional pulmonary blood flow. Lung function is nonuniform in most patients with lung cancer secondary to COPD. SPECT provides a three-dimensional map of functioning alveolar–capillary units.
When selecting beam orientations for a patient with an intrathoracic tumor, one can orient the beam such that it preferentially passes through hypoperfused regions of lung (Marks et al. 1995). Similarly, the quantitative functional information from SPECT can be used to define dose/volume constraints for IMRT planning (McGuire et al. 2006; Shioyama et al. 2007). These approaches assume that hypofunctional regions of lung are permanently nonfunctioning. However, if hypoperfusion is caused by an obstructing tumor, then it may be temporary and may improve with tumor shrinkage, and this should be taken into account in the RT planning process. In our experience, areas of hypoperfusion that are adjacent to large central lung lesions often demonstrate reperfusion on post-RT scans. Conversely, areas of hypoperfusion that are “apart” from the gross tumor do not demonstrate reperfusion post-RT. Thus, care must be taken when incorporating such functional information into the planning process.
7.4 Respiratory Motion
The most effective way to reduce the complications of pulmonary irradiation is to decrease the volume of lung that is exposed to the beam. It has been amply demonstrated that tumors within the lung may move with respiration. This is most commonly accounted for by adding a safety margin (the CTV → PTV expansion accounts for daily setup error and organ motion). Though this is an easy solution to the problem, it is clearly detrimental as it leads to increased normal tissue exposure.
Three strategies to account for respiratory motion are breath hold (often at deep inspiration, termed DIBH), respiratory gating (Mageras and Yorke 2004; Jiang 2006), and tracking. In DIBH, treatment planning and delivery is performed with the patient holding their breath in deep inspiration. DIBH requires a compliant patient with sufficient pulmonary function to hold their breath for a moderate length of time. DIBH has the added advantage of delivering treatment to an expanded lung, thus irradiating a smaller fraction of the lung for a given target size. Respiratory gating, on the other hand, allows the patient to breathe freely and the beam is turned on during a specified phase of the respiratory cycle and turned off when that phase has passed (Giraud et al. 2006). This technology requires a signal, usually an external marker acting as a surrogate for respiratory motion, to gate the linear accelerator. It is essential to have CT images corresponding to the desired phase of the respiratory cycle for treatment planning, often accomplished using 4D CT technology. Tracking refers to systems where the treatment beam adjusts, in real time, to movements of the intra-thoracic contents. This is most commonly performed using a CyberKnife (Accuray, Sunnyvale, CA) (Nuyttens et al. 2006), but has also been suggested to be possible with synchronized movement of multi-leaf collimators (Keall et al. 2005). Respiratory gating and tracking are potentially less demanding on a patient compared with the breath-hold techniques.
8 Prevention
The therapeutic ratio is derived from differences between the tumor control probability (TCP) curve and the normal tissue complication probability (NTCP) curve. Shifting the NTCP curve away from the TCP curve will theoretically improve the therapeutic ratio, such that the same RT dose will be associated with a lower risk of injury or an escalated dose of RT can be administered with the same risk of injury.
Several pharmacologic agents have been employed to mitigate the acute and chronic consequences of incidental RT on normal lung tissue (shift the NTCP curve). Amifostine and pentoxifylline have shown the most promise and are discussed further here.
8.1 Amifostine
Amifostine (WR-2721; Ethyol, Medimmune Inc, Gaithersburg, MD), is a thio-organic pro-drug which is converted in vivo to the active free thiol (WR-1065) by vascular endothelial cell alkaline phosphatase. Amifostine is believed to scavenge harmful free radicals produced by the interaction of ionizing radiation and water molecules. Multiple randomized studies and a meta-analysis (Sasse et al. 2006) have tested the utility of amifostine in patients receiving RT for lung cancer with conflicting results (Table 9). The largest study was performed by the RTOG (Movsas et al. 2005). The incidence of grade ≥3 esophagitis, the primary endpoint of the study, was 30% with amifostine versus 34% without (p = 0.9). The incidence of grade ≥3 pulmonary toxicity was lower in patients receiving amifostine (9 vs. 16%), but this was not statistically significant. This study has been criticized since the drug was given once daily (4 days/week) and the RT was given twice daily (5 days/week), and thus 60% of the RT fractions were delivered without the protector. Conversely, there are several single-institutional randomized trials suggesting protection with amifostine, in which the drug was used during a larger fraction of radiation treatments (Table 9). Presently, given the acute toxicity associated with amifostine (nausea/vomiting, hypotension, infection, rash), and the mixed clinical results, amifostine is not currently utilized widely for patients receiving thoracic RT.
8.2 Pentoxifylline
Pentoxifylline (Trental, sanofi aventis, Bridgewater, NJ) is an ethyl xanthine derivative with immunomodulating and anti-inflammatory activity. It is primarily prescribed for patients with intermittent claudication secondary to its ability to improve erythrocyte flexibility. Preclinical studies of pentoxifylline are mixed. Koh et al. (1995) showed that pentoxifylline did not mitigate acute decline in lung perfusion after single fraction RT in rats. However, it did seem to facilitate late recovery in perfusion compared to rats receiving RT alone. In contrast, Stelzer et al. (1996) showed that rats receiving pentoxifylline maintained higher perfusion ratios than irradiated controls during weeks 3–5. However, the protection was transient and subsequent recovery from lung injury was inhibited at later times.
In a small study of 40 patients irradiated for lung or breast cancer, the addition of pentoxifylline appeared to reduce the incidence of lung injury (Ozturk et al. 2004). In particular, there were decreased perfusion defects, decreased chest X-ray abnormalities, and smaller decline in DLCO in patients receiving pentoxifylline. Additional study of this approach may be warranted.
9 Management
Evans and Leucutia stated in 1925 that “the treatment of the changes produced by radiation [in the lung] can only be symptomatic (Evans and Leucutia 1925)”. This remains true today. Studies evaluating the optimal treatment of RT-induced lung injury are scarce and restricted to retrospective reports. Most recommendations are based on expert opinion.
9.1 Acute Pneumonitis
The traditional treatment for acute pneumonitis is oral steroids, typically prednisone. Symptomatic improvement after steroid therapy was first reported by Cosgriff and Kligerman (1951), who noted that although the acute symptoms rapidly abated, fibrosis eventually developed. There are no robust studies assessing the optimal corticosteroid or dosing schedule, though it has been observed that symptoms may recur with a rapid taper or when corticosteroids, prescribed for other reasons, are discontinued after completion of RT (Castellino et al. 1974). We recommend oral prednisone (Salinas and Winterbauer 1995), typically 40–60 mg daily for 1–2 weeks, followed by a slow taper (reducing ~10 mg every 1–2 weeks). It must be remembered that there is a differential diagnosis for shortness of breath occurring after RT. This includes tumor progression, drug toxicity, cardiac disease, pulmonary embolus, anemia, and infection. Since steroids can exacerbate an infection, an initial short course of antibiotics may be considered. The majority of patients with pneumonitis recover. However, symptoms can be serious enough to require oxygen or hospitalization, and can be potentially fatal.
9.2 Chronic Fibrosis
It is generally assumed that established radiation fibrosis is irreversible. However, as our understanding of the physiology of radiation-induced fibrosis has increased, several pharmacologic agents have been evaluated and some have shown some promise in reversing subcutaneous fibrosis, which may be relevant to fibrosis in the lung. Such agents have included corticosteroids, pentoxifylline, alpha-tocopherol, hyperbaric oxygen, superoxide dismutase, angiotensin-converting enzymes, and combined pentoxifylline and alpha-tocopherol (Delanian and Lefaix 2007).
After promising results were obtained in a phase II study (Delanian et al. 1999), performed a randomized study in which 24 women with breast cancer and superficial RT-induced fibrosis were randomized to pentoxifylline (800 mg/day) and alpha-tocopherol (Vitamin E, 1000 U/day), pentoxifylline plus placebo, alpha-tocopherol plus placebo, or double placebo (Delanian et al. 2003). Treatment was continued for 6 months. There was a significant reduction in both fibrotic surface regression (60 vs. 43%, p = 0.04) and volume regression as based by ultrasound (73 vs. 51%, p = 0.054), comparing the combined treatment arm with the control arm. Neither pentoxifylline alone nor alpha-tocopherol alone was effective. Interestingly, there was a marked decrease in fibrosis in the double placebo arm. Another agent that has shown preliminary promise is liposomal superoxide dismutase. In a preclinical study (Lefaix et al. 1996), this agent has been shown to reverse RT-induced fibrosis with regeneration of normal tissue in the previously fibrotic region. A single arm clinical study has also been reported (Delanian et al. 1994). Patients (n = 34) with palpable radiation-induced fibrosis were treated with 3 weeks of liposomal Cu/Zn superoxide dismutase. All patients showed some clinical regression within weeks of initiation of treatment. Though promising, further study is necessary to confirm these findings. Currently there are no widely accepted treatments for lung fibrosis and management continues to be entirely supportive.
10 Future Directions
Progress regarding the predictors of RT-induced lung injury requires further understanding of the following.
Endpoint interaction
The study of RT-induced lung injury is confounded by the use of ambiguous endpoints. Many scoring systems combine radiologic, functional, and symptomatic criteria to define a “global score”. Because each endpoint may have different dose–volume dependence, this approach may be counterproductive. Therefore, we recommend that further study of lung injury explicitly consider symptomatic, functional, and radiographic endpoints separately.
Impact of clinical factors
The impact of clinical factors (e.g., pre-RT functional status, tobacco use) and systemic agents (e.g., chemotherapy) on the risk of RP needs further study.
Organ interactions
Some pre-clinical data suggest that there may be interactions between the lung and heart in the development of RT-associated dyspnea. In rats, the respiratory rate after thoracic RT was related to the volume of lung and heart irradiated (Barcellos-Hoff and Dix 1996; Ewan et al. 2002; Yi et al. 1996).
Impact of an in situ lung cancer on the risk of radiation-induced lung injury
The data for whole lung radiation are derived essentially from patients without primary lung cancers (e.g., elective lung RT for sarcoma), versus fractionated partial lung radiation, often derived from patients with gross lung cancers. The confounding effect of tumor in the lung makes the study of RT-induced lung injury extremely challenging. Indeed, in several studies, the ability to predict for RT-induced lung injury is improved in patients without large central or occluding tumors. Thus, it might be relevant to develop separate predictive models in patients with intact intraparenchymal lung tumors versus those without such a lesion (i.e., postresection RT for lung cancer, or RT for other thoracic tumors).
Radiation response modifiers
Amifostine is a thio-organic prodrug believed to scavenge harmful free radicals mediating RT-induced injury. Several randomized studies in patients receiving RT for lung cancer note a reduction in RP in the amifostine arm (Ehrhart et al. 1997; Rube et al. 2000; Epperly et al. 1999), although the largest study (from RTOG) was negative (Haiping et al. 2006). However, this study has been criticized because the drug was administered once daily (4 days/week), whereas the RT was delivered twice daily (5 days/week), and thus 60% of the RT fractions were delivered without the protector. Such mixed results, combined with the acute toxicities of amifostine (nausea/vomiting, hypotension, infection, and rash), have dissuaded many from using it in routine practice. One small randomized study demonstrated a protective effect of pentoxifylline, but pentoxifylline is not currently used in routine clinical practice (Nishioka et al. 2004).
Biomarkers
Additional work is needed to assess the predictive ability offered by biomarkers (see Bentzen et al. in this issue), such as TGF-β (measured before and/or during RT) (Table 1) (Denham and Hauer-Jensen 2002).
11 History and Literature Landmarks
Almost immediately after Wilhelm Conrad Röentgen discovered “a new kind of ray” in 1895, X-rays were employed to shrink malignant tumors (Pusey 1905) (Table 10). Primitive X-ray machines were limited by their low energy, and were consequently only able to treat superficial tumors effectively (Bernier et al. 2004). Initially, most tumors were managed with only a few relatively large doses of RT. Although reasonable tumor control was achieved, it was noted that fibrosis often developed in surrounding soft tissues (Evans and Leucutia 1925; Tyler and Blackman 1922). Pulmonary and pleural reactions were, as expected, seldom observed (Evans and Leucutia 1925). Technological advances in the early twentieth century allowed deeper-seated tumors to be treated. The effects of RT on normal tissues deep to the skin surface were subsequently noted. In 1921, Groover et al. (1922) reported the following to the Southern Medical Association:
In a considerable number of cancers of the breast which have been treated…an irritative, unproductive cough has developed….with a certain amount of dyspnea. Shortly afterward certain pulmonary changes have been demonstrable in the roentgenogram in cases which have previously shown no evidence of intra-thoracic pathology…In at least one case the infiltration assume(d) a more fibrotic appearance. The natural assumption would be that these pulmonary phenomena are due to metastasis, but certain clinical observations have led us to consider seriously the possibility of their being caused by the treatment instead…It is at once apparent that it is of vital importance to determine the significance of these pulmonary phenomena…and if due to treatment…they become of very great therapeutic significance.
Shortly thereafter, Tyler and Blackman (1922), Hines (1922), and others reported similar findings. Kenneth Davis, a fellow in Roentgenology at the Mayo Clinic, designed the first extensive animal studies that specifically evaluated the effects of RT on the lungs (Davis 1924). His experiments demonstrated thickening of the alveolar septi and obliteration of alveolar sacs, thickening of the arterial tunica media, and extravasation of “coagulum” and desquamated epithelial cells into the alveoli. The study of RT-induced lung injury has expanded dramatically since these initial reports nearly a century ago.
References
Abratt RP, Willcox PA (1995) The effect of irradiation on lung function and perfusion in patients with lung cancer. Int J Radiat Oncol Biol Phys 31:915–919
Abratt RP, Morgan GW (2002) Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer 35:103–109
Allavena C, Conroy T, Aletti P et al (1992) Late cardiopulmonary toxicity after treatment for Hodgkin’s disease. Br J Cancer 65:908–912
Allen SC, Riddell GS, Butchart EG (1981) Bleomycin therapy and anaesthesia. The possible hazards of oxygen administration to patients after treatment with bleomycin. Anaesthesia 36:60–63
Allen AM, Czerminska M, Janne PA et al (2006) Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 65:640–645
Anscher MS, Murase T, Prescott DM et al (1994) Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys 30:671–676
Anscher MS, Kong FM, Jirtle RL (1998) The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 19:109–120
Anscher MS, Thrasher B, Rabbani Z et al (2006) Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys 65:876–881
Antonadou D, Coliarakis N, Synodinou M et al (2001) Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys 51:915–922
Antonadou D, Throuvalas N, Petridis A et al (2003) Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 57:402–408
Barcellos-Hoff MH (1993) Radiation-induced transforming growth factor beta and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res 53:3880–3886
Barcellos-Hoff MH (1996) Latency and activation in the control of TGF-beta. J Mammary Gland Biol Neoplasia 1:353–363
Barcellos-Hoff MH, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10:1077–1083
Barcellos-Hoff MH, Derynck R, Tsang ML et al (1994) Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93:892–899
Barlesi F, Villani P, Doddoli C et al (2004) Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol 18:85–91
Bartholdi D, Rubin BP, Schwab ME (1997) VEGF mRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur J Neurosci 9:2549–2560
Beckles MA, Spiro SG, Colice GL et al (2003) The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 123:105S–114S
Bell J, McGivern D, Bullimore J et al (1988) Diagnostic imaging of post-irradiation changes in the chest. Clin Radiol 39:109–119
Benoist MR, Lemerle J, Jean R et al (1982) Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax 37:175–180
Bentzen SM (2006) Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6:702–713
Bernier J, Hall EJ, Giaccia A (2004) Radiation oncology: a century of achievements. Nat Rev Cancer 4:737–747
Boersma LJ, Damen EM, de Boer RW et al (1996) Recovery of overall and local lung function loss 18 months after irradiation for malignant lymphoma. J Clin Oncol 14:1431–1441
Borst GR, De Jaeger K, Belderbos JS et al (2005) Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys 62:639–644
Breur K, Cohen P, Schweisguth O et al (1978) Irradiation of the lungs as an adjuvant therapy in the treatment of osteosarcoma of the limbs. An E.O.R.T.C. randomized study. Eur J Cancer 14:461–471
Brush J, Lipnick SL, Phillips T et al (2007) Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol 17:121–130
Buzdar AU, Legha SS, Luna MA et al (1980) Pulmonary toxicity of mitomycin. Cancer 45:236–244
Cannon GW (1997) Methotrexate pulmonary toxicity. Rheum Dis Clin North Am 23:917–937
Carver JR, Shapiro CL, Ng A et al (2007) American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25:3991–4008
Castellino RA, Glatstein E, Turbow MM et al (1974) Latent radiation injury of lungs or heart activated by steroid withdrawal. Ann Intern Med 80:593–599
Chang AY, Kuebler JP, Pandya KJ et al (1986) Pulmonary toxicity induced by mitomycin C is highly responsive to glucocorticoids. Cancer 57:2285–2290
Chen Y, Williams J, Ding I et al (2002) Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 12:26–33
Choi NC, Kanarek DJ (1994) Toxicity of thoracic radiotherapy on pulmonary function in lung cancer. Lung Cancer 10(Suppl 1):S219–S230
Choi NC, Kanarek DJ, Grillo HC (1990) Effect of postoperative radiotherapy on changes in pulmonary function in patients with stage II and IIIA lung carcinoma. Int J Radiat Oncol Biol Phys 18:95–99
Ciotti R, Belotti G, Facchi E et al (1999) Sudden cardio-pulmonary toxicity following a single infusion of gemcitabine. Ann Oncol 10:997
Comroe J (1965) Physiology of respiration, 1st edn. Year Book Medical Publishers, Chicago
Cosgriff S, Kligerman M (1951) Use of ACTH and cortisone in the treatment of post-irradiation pulmonary reaction. Radiology 57:536–540
Cosset JM, Baume D, Pico JL et al (1989) Single dose versus hyperfractionated total body irradiation before allogeneic bone marrow transplantation: a non-randomized comparative study of 54 patients at the Institut Gustave-Roussy. Radiother Oncol 15:151–160
Czarnecki A, Voss S (2006) Pulmonary toxicity in patients treated with gemcitabine and a combination of gemcitabine and a taxane: investigation of a signal using postmarketing data. Br J Cancer 94:1759–1760
Dajczman E, Srolovitz H, Kreisman H et al (1995) Fatal pulmonary toxicity following oral etoposide therapy. Lung Cancer 12:81–86
Davis K (1924) Intrathoracic changes following X-ray treatment: a clinical and experimental study. Radiology 3:301–321
De Jaeger K, Seppenwoolde Y, Kampinga HH et al (2004) Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 58:1378–1387
Deeg HJ, Sullivan KM, Buckner CD et al (1986) Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow Transpl 1:151–157
Delanian S, Lefaix JL (2007) Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol 17:99–107
Delanian S, Baillet F, Huart J et al (1994) Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol 32:12–20
Delanian S, Balla-Mekias S, Lefaix JL (1999) Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol 17:3283–3290
Delanian S, Porcher R, Balla-Mekias S et al (2003) Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol 21:2545–2550
Denham JW, Hauer-Jensen M (2002) The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol 63:129–145
Dor Y, Porat R, Keshet E (2001) Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol 280:C1367–C1374
Dunsford ML, Mead GM, Bateman AC et al (1999) Severe pulmonary toxicity in patients treated with a combination of docetaxel and gemcitabine for metastatic transitional cell carcinoma. Ann Oncol 10:943–947
Ehrhart EJ, Segarini P, Tsang ML et al (1997) Latent transforming growth factor beta1 activation in situ: quantitative and functional evidence after low-dose gamma-irradiation. Faseb J 11:991–1002
Einhorn L, Krause M, Hornback N et al (1976) Enhanced pulmonary toxicity with bleomycin and radiotherapy in oat cell lung cancer. Cancer 37:2414–2416
Ellis ER, Marcus RB Jr, Cicale MJ et al (1992) Pulmonary function tests after whole-lung irradiation and doxorubicin in patients with osteogenic sarcoma. J Clin Oncol 10:459–463
Epperly MW, Bray JA, Krager S et al (1999) Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys 43:169–181
Esteban E, Villanueva N, Muniz I et al (2008) Pulmonary toxicity in patients treated with gemcitabine plus vinorelbine or docetaxel for advanced non-small cell lung cancer: outcome data on a randomized phase II study. Invest New Drugs 26(1):67–74
Evans W, Leucutia T (1925) Intrathoracic changes induced by heavy radiation. Am J Roentgenol Radium Ther 13:203–220
Evans ES, Kocak Z, Zhou SM et al (2006) Does transforming growth factor-beta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine 35:186–192
Ewan KB, Henshall-Powell RL, Ravani SA et al (2002) Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res 62:5627–5631
Fan M, Marks LB, Hollis D et al (2001) Can we predict radiation-induced changes in pulmonary function based on the sum of predicted regional dysfunction? J Clin Oncol 19:543–550
Fay M, Tan A, Fisher R et al (2005) Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 61:1355–1363
Feingold ML, Koss LG (1969) Effects of long-term administration of busulfan. Report of a patient with generalized nuclear abnormalities, carcinoma of vulva, and pulmonary fibrosis. Arch Intern Med 124:66–71
Feletou M, Girard V, Canet E (1995) Different involvement of nitric oxide in endothelium-dependent relaxation of porcine pulmonary artery and vein: influence of hypoxia. J Cardiovasc Pharmacol 25:665–673
Fleckenstein K, Gauter-Fleckenstein B, Jackson IL et al (2007a) Using biological markers to predict risk of radiation injury. Semin Radiat Oncol 17:89–98
Fleckenstein K, Zgonjanin L, Chen L et al (2007b) Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys 68:196–204
Fryer CJ, Fitzpatrick PJ, Rider WD et al (1978) Radiation pneumonitis: experience following a large single dose of radiation. Int J Radiat Oncol Biol Phys 4:931–936
Fu XL, Huang H, Bentel G et al (2001) Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys 50:899–908
Garbes ID, Henderson ES, Gomez GA et al (1986) Procarbazine-induced interstitial pneumonitis with a normal chest X-ray: a case report. Med Pediatr Oncol 14:238–241
Garg S, Garg MS, Basmaji N (2002) Multiple pulmonary nodules: an unusual presentation of fludarabine pulmonary toxicity: case report and review of literature. Am J Hematol 70:241–245
Gibson PG, Bryant DH, Morgan GW et al (1988) Radiation-induced lung injury: a hypersensitivity pneumonitis? Ann Intern Med 109:288–291
Giles FJ, Smith MP, Goldstone AH (1990) Chlorambucil lung toxicity. Acta Haematol 83:156–158
Gilson AJ, Sahn SA (1985) Reactivation of bleomycin lung toxicity following oxygen administration. A second response to corticosteroids. Chest 88:304–306
Giraud P, Yorke E, Ford EC et al (2006) Reduction of organ motion in lung tumors with respiratory gating. Lung Cancer 51:41–51
Girinsky T, Benhamou E, Bourhis JH et al (2000) Prospective randomized comparison of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies. J Clin Oncol 18:981–986
Graham MV, Purdy JA, Emami B et al (1999) Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323–329
Groover T, Christie A, Merritt E (1922) Observations on the use of the copper filter in the roentgen treatment of deep-seated malignancies. South Med J 15:440–444
Guerrero T, Johnson V, Hart J et al (2007) Radiation pneumonitis: local dose versus [(18)F]-fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys 68(4):1030–1035
Gupta N, Ahmed I, Steinberg H et al (2002) Gemcitabine-induced pulmonary toxicity: case report and review of the literature. Am J Clin Oncol 25:96–100
Gurjal A, An T, Valdivieso M et al (1999) Etoposide-induced pulmonary toxicity. Lung Cancer 26:109–112
Haiping Z, Takayama K, Uchino J et al (2006) Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther 13:864–872
Hallahan DE, Geng L, Shyr Y (2002) Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst 94:733–741
Hankins DG, Sanders S, MacDonald FM et al (1978) Pulmonary toxicity recurring after a six week course of busulfan therapy and after subsequent therapy with uracil mustard. Chest 73:415–416
Hart JP, Broadwater G, Rabbani Z et al (2005) Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int J Radiat Oncol Biol Phys 63:1448–1454
Hassaballa HA, Cohen ES, Khan AJ et al (2005) Positron emission tomography demonstrates radiation-induced changes to nonirradiated lungs in lung cancer patients treated with radiation and chemotherapy. Chest 128:1448–1452
Hauer-Jensen M, Fink LM, Wang J (2004) Radiation injury and the protein C pathway. Crit Care Med 32:S325–S330
Haupt HM, Hutchins GM, Moore GW (1981) Ara-C lung: noncardiogenic pulmonary edema complicating cytosine arabinoside therapy of leukemia. Am J Med 70:256–261
Hayman JA, Martel MK, Ten Haken RK et al (2001) Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol 19:127–136
Hernando ML, Marks LB, Bentel GC et al (2001) Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 51:650–659
Hines L (1922) Fibrosis of the lung following roentgen-ray treatments for tumor. J Am Med Assoc 79:720–722
Hoelzer KL, Harrison BR, Luedke SW et al (1986) Vinblastine-associated pulmonary toxicity in patients receiving combination therapy with mitomycin and cisplatin. Drug Intell Clin Pharm 20:287–289
Hof H, Herfarth KK, Munter M et al (2003) Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 56:335–341
Hope AJ, Lindsay PE, El Naqa I et al (2006) Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys 65:112–124
Hoyer M, Roed H, Hansen AT et al (2006) Prospective study on stereotactic radiotherapy of limited-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 66:S128–S135
Hurst PG, Habib MP, Garewal H et al (1987) Pulmonary toxicity associated with fludarabine monophosphate. Invest New Drugs 5:207–210
Jackson IL, Batinic-Haberle I, Sonveaux P et al (2006) ROS production and angiogenic regulation by macrophages in response to heat therapy. Int J Hyperthermia 22:263–273
Jackson IL, Vujaskovic Z, Down JD (2010) Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res 173:10–20
Jiang SB (2006) Technical aspects of image-guided respiration-gated radiation therapy. Med Dosim 31:141–151
Joerger M, Gunz A, Speich R et al (2002) Gemcitabine-related pulmonary toxicity. Swiss Med Wkly 132:17–20
Johnston CJ, Piedboeuf B, Rubin P et al (1996) Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res 145:762–767
Jules-Elysee K, White DA (1990) Bleomycin-induced pulmonary toxicity. Clin Chest Med 11:1–20
Kane GC, McMichael AJ, Patrick H et al (1992) Pulmonary toxicity and acute respiratory failure associated with fludarabine monophosphate. Respir Med 86:261–263
Kang SK, Rabbani ZN, Folz RJ et al (2003) Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys 57:1056–1066
Kataoka M, Kawamura M, Nishiyama Y et al (1992) A case with delayed-onset radiation pneumonitis suspected to be induced by oral etoposide. Nippon Igaku Hoshasen Gakkai Zasshi 52:641–645
Keall PJ, Joshi S, Vedam SS et al (2005) Four-dimensional radiotherapy planning for DMLC-based respiratory motion tracking. Med Phys 32:942–951
Keane TJ, Van Dyk J, Rider WD (1981) Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int J Radiat Oncol Biol Phys 7:1365–1370
Kelsey CR, Kahn D, Hollis DR et al (2006) Radiation-induced narrowing of the tracheobronchial tree: an in-depth analysis. Lung Cancer 52:111–116
Kim TH, Cho KH, Pyo HR et al (2005) Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 235:208–215
Kocak Z, Marks LB (2005) Radiation-induced lung injury. In: Perez CA, Bradey LW, Halperin EC et al (eds) Principles and practice of radiation oncology, Vol 1, 4th edn. Lippincott Williams & Wilkins Healthcare, New York
Kocak Z, Evans ES, Zhou SM et al (2005) Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys 62:635–638
Koenig TR, Munden RF, Erasmus JJ et al (2002) Radiation injury of the lung after three-dimensional conformal radiation therapy. AJR Am J Roentgenol 178:1383–1388
Koh WJ, Stelzer KJ, Peterson LM et al (1995) Effect of pentoxifylline on radiation-induced lung and skin toxicity in rats. Int J Radiat Oncol Biol Phys 31:71–77
Komaki R, Lee JS, Milas L et al (2004) Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys 58:1369–1377
Kong FM, Hayman JA, Griffith KA et al (2006) Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 65:1075–1086
Konits PH, Aisner J, Sutherland JC et al (1982) Possible pulmonary toxicity secondary to vinblastine. Cancer 50:2771–2774
Kouroussis C, Mavroudis D, Kakolyris S et al (2004) High incidence of pulmonary toxicity of weekly docetaxel and gemcitabine in patients with non-small cell lung cancer: results of a dose-finding study. Lung Cancer 44:363–368
Kwa SL, Lebesque JV, Theuws JC et al (1998) Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 42:1–9
Kyas I, Hof H, Debus J et al (2007) Prediction of radiation-induced changes in the lung after stereotactic body radiation therapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 67:768–774
Lateef O, Shakoor N, Balk RA (2005) Methotrexate pulmonary toxicity. Expert Opin Drug Saf 4:723–730
Lauta VM, Valerio G, Greco A et al (1987) Early-onset diagnosis of lung toxicity caused by cyclophosphamide, melphalan and procarbazine therapy. Tumori 73:351–358
Lefaix JL, Delanian S, Leplat JJ et al (1996) Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys 35:305–312
Leong SS, Tan EH, Fong KW et al (2003) Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage III non-small-cell lung cancer. J Clin Oncol 21:1767–1774
Li YQ, Ballinger JR, Nordal RA et al (2001) Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61:3348–3354
Li F, Sonveaux P, Rabbani ZN et al (2007) Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26:63–74
Lin P, Delaney G, Chu J et al (2000) Fluorine-18 FDG dual-head gamma camera coincidence imaging of radiation pneumonitis. Clin Nucl Med 25:866–869
Lind PA, Bylund H, Wennberg B et al (2000) Abnormalities on chest radiographs following radiation therapy for breast cancer. Eur Radiol 10:484–489
Linette DC, McGee KH, McFarland JA (1992) Mitomycin-induced pulmonary toxicity: case report and review of the literature. Ann Pharmacother 26:481–484
Littman P, Meadows AT, Polgar G et al (1976) Pulmonary function in survivors of Wilm’s tumor. Patterns of impairment. Cancer 37:2773–2776
Luedke D, McLaughlin TT, Daughaday C et al (1985) Mitomycin C and vindesine associated pulmonary toxicity with variable clinical expression. Cancer 55:542–545
Lyman JT, Wolbarst AB (1987) Optimization of radiation therapy, III: a method of assessing complication probabilities from dose-volume histograms. Int J Radiat Oncol Biol Phys 13:103–109
Mageras GS, Yorke E (2004) Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol 14:65–75
Mah K, Van Dyk J, Keane T et al (1987) Acute radiation-induced pulmonary damage: a clinical study on the response to fractionated radiation therapy. Int J Radiat Oncol Biol Phys 13:179–188
Maher J, Daly PA (1993) Severe bleomycin lung toxicity: reversal with high dose corticosteroids. Thorax 48:92–94
Mahmood T, Mudad R (2002) Pulmonary toxicity secondary to procarbazine. Am J Clin Oncol 25:187–188
Malik SW, Myers JL, DeRemee RA et al (1996) Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med 154:1851–1856
Marks LB, Spencer DP, Sherouse GW et al (1995) The role of three dimensional functional lung imaging in radiation treatment planning: the functional dose-volume histogram. Int J Radiat Oncol Biol Phys 33:65–75
Marks LB, Munley MT, Spencer DP et al (1997) Quantification of radiation-induced regional lung injury with perfusion imaging. Int J Radiat Oncol Biol Phys 38:399–409
Marks LB, Fan M, Clough R et al (2000) Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol 76:469–475
Marks L et al (2010) Radiation dose-volume effects in the lung. Int. J. Radiat Oncol Biol. Phys 76(Suppl 3):S70–S76
Martin C, Romero S, Sanchez-Paya J et al (1999) Bilateral lymphocytic alveolitis: a common reaction after unilateral thoracic irradiation. Eur Respir J 13:727–732
Martin M, Lefaix J, Delanian S (2000) TGF- β1 and radiation fibrosis: a master switch and a specific therapeutic target? IJROBP 47(2):277–290
McGuire SM, Zhou S, Marks LB et al (2006) A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys 66:1543–1552
McKenna KE, Burrows D (2000) Pulmonary toxicity in a patient with psoriasis receiving methotrexate therapy. Clin Exp Dermatol 25:24–27
Mikkelsen RB, Wardman P (2003) Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 22:5734–5754
Miller RW, Fusner JE, Fink RJ et al (1986) Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 14:202–207
Miller KL, Zhou SM, Barrier RC Jr et al (2003) Long-term changes in pulmonary function tests after definitive radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 56:611–615
Miller KL, Shafman TD, Anscher MS et al (2005) Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys 61:64–69
Min KW, Gyorkey F (1968) Interstitial pulmonary fibrosis, atypical epithelial changes and bronchiolar cell carcinoma following busulfan therapy. Cancer 22:1027–1032
Monson JM, Stark P, Reilly JJ et al (1998) Clinical radiation pneumonitis and radiographic changes after thoracic radiation therapy for lung carcinoma. Cancer 82:842–850
Movsas B, Scott C, Langer C et al (2005) Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98–01. J Clin Oncol 23:2145–2154
Myers JN, O’Neil KM, Walsh TE et al (2005) The pulmonary status of patients with limited-stage small cell lung cancer 15 years after treatment with chemotherapy and chest irradiation. Chest 128:3261–3268
Nagata Y, Takayama K, Matsuo Y et al (2005) Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 63:1427–1431
Newton KA (1960) Total thoracic irradiation combined with intravenous injection of autogenous marrow. Clin Radiol 11:14–21
Newton KA, Spittle MF (1969) An analysis of 40 cases treated by total thoracic irradiation. Clin Radiol 20:19–22
Nishioka A, Ogawa Y, Mima T et al (2004) Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys 58:1235–1241
Novakova-Jiresova A, Van Gameren MM, Coppes RP et al (2004) Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol 71:183–189
Novakova-Jiresova A, van Luijk P, van Goor H et al (2007) Changes in expression of injury after irradiation of increasing volumes in rat lung. Int J Radiat Oncol Biol Phys 67:1510–1518
Nuyttens JJ, Prevost JB, Praag J et al (2006) Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: marker placement and early results. Acta Oncol 45:961–965
Oetzel D, Schraube P, Hensley F et al (1995) Estimation of pneumonitis risk in three-dimensional treatment planning using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys 33:455–460
Ogasawara N, Suga K, Karino Y et al (2002) Perfusion characteristics of radiation-injured lung on Gd-DTPA-enhanced dynamic magnetic resonance imaging. Invest Radiol 37:448–457
Oliner H, Schwartz R, Rubio F et al (1961) Interstitial pulmonary fibrosis following busulfan therapy. Am J Med 31:134–139
Onishi H, Kuriyama K, Yamaguchi M et al (2003) Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer 40:79–84
Ozturk B, Egehan I, Atavci S et al (2004) Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys 58:213–219
Pavlakis N, Bell DR, Millward MJ et al (1997) Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer 80:286–291
Penney DP (1087) Ultrasound organization of the distal lung and potential target cells of ionizing radiation. In: Paper presented at: international conference on new biology of lung and lung injury and their implications for oncology; 1087; Porvoo, Finland
Peterson LM, Evans ML, Thomas KL et al (1992) Vascular response to fractionated irradiation in the rat lung. Radiat Res 131:224–226
Pino Y, Torres JL, Bross DS, Lam WC et al (1982) Risk factors in interstitial pneumonitis following allogenic bone marrow transplantation. Int J Radiat Oncol Biol Phys 8:1301–1307
Provenzano G (2003) Chronic pulmonary toxicity of methotrexate and rheumatoid arthritis. Rheumatology (Oxford) 42:802–803; author reply 803–804
Pusey W (1905) The therapeutic use of X-rays. J Am Med Assoc 44:1496–1504
Rab GT, Ivins JC, Childs DS Jr et al (1976) Elective whole lung irradiation in the treatment of osteogenic sarcoma. Cancer 38:939–942
Raderer M, Kornek G, Hejna M et al (1996) Acute pulmonary toxicity associated with high-dose vinorelbine and mitomycin C. Ann Oncol 7:973–975
Rahimi RA, Leof EB (2007) TGF-beta signaling: a tale of two responses. J Cell Biochem 102(3):593–608
Rancati T, Ceresoli GL, Gagliardi G et al (2003) Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol 67:275–283
Rice D, Liao Z, Vaporciyan AA et al (2006) V20 predicts fatal pulmonary toxicity after extrapleural pneumonectomy and intensity modulated radiation therapy. Proc ASTRO 66:S63
Roberts AB (1999) TGF-beta signaling from receptors to the nucleus. Microbes Infect 1:1265–1273
Roberts CM, Foulcher E, Zaunders JJ et al (1993) Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med 118:696–700
Robnett TJ, Machtay M, Vines EF et al (2000) Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 48:89–94
Rodemann HP, Binder A, Burger A et al (1996) The underlying cellular mechanism of fibrosis. Kidney Int Suppl 54:S32–S36
Rube CE, Uthe D, Schmid KW et al (2000) Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys 47:1033–1042
Rubin P, Casarett GW (1968) Alimentary tract: esophagus and stomach. In: Rubin P, Casarett GW (eds) Clinical radiation pathology, vol 1, 1 edn. W. B. Saunders Company, Philadelphia p 517
Rubin P, Johnston CJ, Williams JP et al (1995) A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 33:99–109
Rudders RA, Hensley GT (1973) Bleomycin pulmonary toxicity. Chest 63:627–628
Salinas FV, Winterbauer RH (1995) Radiation pneumonitis: a mimic of infectious pneumonitis. Semin Respir Infect 10:143–153
Sampath S, Schultheiss TE, Wong J (2005) Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys 63:876–884
Sasse AD, Clark LG, Sasse EC et al (2006) Amifostine reduces side effects and improves complete response rate during radiotherapy: results of a meta-analysis. Int J Radiat Oncol Biol Phys 64:784–791
Schallenkamp JM, Miller RC, Brinkmann DH et al (2007) Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys 67:410–416
Seppenwoolde Y, De Jaeger K, Boersma LJ et al (2004) Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 60:748–758
Shank B, Chu FC, Dinsmore R et al (1983) Hyperfractionated total body irradiation for bone marrow transplantation. Results in seventy leukemia patients with allogeneic transplants. Int J Radiat Oncol Biol Phys 9:1607–1611
Shioyama Y, Jang SY, Liu HH et al (2007) Preserving functional lung using perfusion imaging and intensity-modulated radiation therapy for advanced-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 68:1349–1358
Siemann DW, Hill RP, Penney DP (1982) Early and late pulmonary toxicity in mice evaluated 180 and 420 days following localized lung irradiation. Radiat Res 89:396–407
Sikic BI (1986) Biochemical and cellular determinants of bleomycin cytotoxicity. Cancer Surv 5:81–91
Skoczylas JZ, Bentzen SM, Overgaard M et al (2000) Time course of radiological lung density changes after postmastectomy radiotherapy. Acta Oncol 39:181–187
Sleijfer S (2001) Bleomycin-induced pneumonitis. Chest 120:617–624
Socinski MA, Morris DE, Halle JS et al (2004) Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol 22:4341–4350
Song SY, Choi W, Shin SS et al (2009) Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 66:89–93
Speiser BL, Spratling L (1993) Radiation bronchitis and stenosis secondary to high dose rate endobronchial irradiation. Int J Radiat Oncol Biol Phys 25:589–597
Stelzer KJ, Koh WJ, Peterson LM et al (1996) Effect of high-dose pentoxifylline on acute radiation-induced lung toxicity in a rat lung perfusion model. Int J Radiat Oncol Biol Phys 34:111–115
Stemmer SM, Cagnoni PJ, Shpall EJ et al (1996) High-dose paclitaxel, cyclophosphamide, and cisplatin with autologous hematopoietic progenitor-cell support: a phase I trial. J Clin Oncol 14:1463–1472
Stephans KL, Djemil T, Reddy CA et al (2009a) A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol 4:976–982
Stephans KL, Djemil T, Reddy CA et al (2009b) Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 4:838–844
Stevens C, Forster K, Zhu X et al (2005) Excellent local control and survival after extrapleural pneumonectomy and IMRT for mesothelioma. Proc ASTRO 63:S103
Stone HB, Coleman CN, Anscher MS et al (2003) Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 4:529–536
Sura S, Gupta V, Yorke E et al (2008) Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol 87:17–23
Tempero MA, Brand R (1998) Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer 82:1800–1801
Theuws JC, Kwa SL, Wagenaar AC et al (1998a) Prediction of overall pulmonary function loss in relation to the 3-D dose distribution for patients with breast cancer and malignant lymphoma. Radiother Oncol 49:233–243
Theuws JC, Kwa SL, Wagenaar AC et al (1998b) Dose-effect relations for early local pulmonary injury after irradiation for malignant lymphoma and breast cancer. Radiother Oncol 48:33–43
Theuws JC, Seppenwoolde Y, Kwa SL et al (2000) Changes in local pulmonary injury up to 48 months after irradiation for lymphoma and breast cancer. Int J Radiat Oncol Biol Phys 47:1201–1208
Timmerman R, Papiez L, McGarry R et al (2003) Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 124:1946–1955
Timmerman R, McGarry R, Yiannoutsos C et al (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24:4833–4839
Toledo CH, Ross WE, Hood CI et al (1982) Potentiation of bleomycin toxicity by oxygen. Cancer Treat Rep 66:359–362
Travis EL, Down JD, Holmes SJ et al (1980) Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res 84:133–143
Travis EL, Liao ZX, Tucker SL (1997) Spatial heterogeneity of the volume effect for radiation pneumonitis in mouse lung. Int J Radiat Oncol Biol Phys 38:1045–1054
Tryka AF, Skornik WA, Godleski JJ et al (1982) Potentiation of bleomycin-induced lung injury by exposure to 70% oxygen. Morphologic assessment. Am Rev Respir Dis 126:1074–1079
Tsoutsou PG, Koukourakis MI (2006) Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 66:1281–1293
Tsujino K, Hirota S, Endo M et al (2003) Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 55:110–115
Tyler A, Blackman J (1922) Effect of heavy radiation on the pleurae and lungs. J Radiol 3:469–475
Uno T, Isobe K, Kawakami H et al (2006) Dose-volume factors predicting radiation pneumonitis in patients receiving salvage radiotherapy for postlobectomy locoregional recurrent non-small-cell lung cancer. Int J Clin Oncol 11:55–59
van Hinsbergh VW, Collen A, Koolwijk P (2001) Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 936:426–437
van Luijk P, Novakova-Jiresova A, Faber H et al (2005) Radiation damage to the heart enhances early radiation-induced lung function loss. Cancer Res 65:6509–6511
Veltkamp SA, Meerum Terwogt JM, van den Heuvel MM et al (2007) Severe pulmonary toxicity in patients with leiomyosarcoma after treatment with gemcitabine and docetaxel. Invest New Drugs 25:279–281
Vujaskovic Z, Marks LB, Anscher MS (2000) The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 10:296–307
Vujaskovic Z, Anscher MS, Feng QF et al (2001) Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 50:851–855
Wang S, Liao Z, Wei X et al (2006a) Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 66:1399–1407
Wang SL, Liao Z, Vaporciyan AA et al (2006b) Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 64:692–699
Weiner DJ, Maity A, Carlson CA et al (2006) Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 46:222–227
Weinstein AS, Diener-West M, Nelson DF et al (1986) Pulmonary toxicity of carmustine in patients treated for malignant glioma. Cancer Treat Rep 70:943–946
Weiss RB, Shah S, Shane SR (1979) Pulmonary toxicity from carmustine (BCNU): a case report. Med Pediatr Oncol 6:255–259
White DA, Stover DE (1984) Severe bleomycin-induced pneumonitis. Clinical features and response to corticosteroids. Chest 86:723–728
Wiegman EM, Meertens H, Konings AW et al (2003) Loco-regional differences in pulmonary function and density after partial rat lung irradiation. Radiother Oncol 69:11–19
Williams JP et al (2010) Animal models for medical countermeasures to radiation exposure. Radiat Res 173:557–578
Willner J, Jost A, Baier K et al (2003) A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy. Strahlenther Onkol 179:548–556
Woel RT, Munley MT, Hollis D et al (2002) The time course of radiation therapy-induced reductions in regional perfusion: a prospective study with >5 years of follow-up. Int J Radiat Oncol Biol Phys 52:58–67
Yankelevitz DF, Henschke CI, Batata M et al (1994) Lung cancer: evaluation with MR imaging during and after irradiation. J Thorac Imaging 9:41–46
Yi ES, Bedoya A, Lee H et al (1996) Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation 20:339–352
Yom SS et al (2007) Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 68:94–102
Yorke ED, Jackson A, Rosenzweig KE et al (2002) Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 54:329–339
Yorke ED, Jackson A, Rosenzweig KE et al (2005) Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys 63:672–682
Yuan X, Liao Z, Liu Z et al (2009) Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol 27:3370–3378
Zhao L, Wang L, Ji W et al (2007) Association between plasma angiotensin-converting enzyme level and radiation pneumonitis. Cytokine 37:71–75
Acknowledgments
Supported in part by National Institutes of Health Grant CA69579 (L.B.M.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kelsey, C.R., Vujaskovic, Z., Jackson, I.L., Riedel, R.F., Marks, L.B. (2014). Lung. In: Rubin, P., Constine, L., Marks, L. (eds) ALERT • Adverse Late Effects of Cancer Treatment. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75863-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-540-75863-1_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-75862-4
Online ISBN: 978-3-540-75863-1
eBook Packages: MedicineMedicine (R0)