Abstract
The use of extracorporeal membrane oxygenation (ECMO) to support children with acute respiratory failure has steadily increased over the past several decades, with major advancements having been made in the care of these children. There are, however, many controversies regarding indications for initiating ECMO in this setting and the appropriate management strategies thereafter. Broad indications for ECMO include hypoxia, hypercarbia, and severe air leak syndrome, with hypoxia being the most common. There are many disease-specific considerations when evaluating children for ECMO, but there are currently very few, if any, absolute contraindications. Venovenous rather than veno-arterial ECMO cannulation is the preferred configuration for ECMO support of acute respiratory failure due to its superior side-effect profile. The approach to lung management on ECMO is variable and should be individualized to the patient, with the main goal of reducing the risk of VILI. ECMO is a relatively rare intervention, and there are likely a minimum number of cases per year at a given center to maintain competency. Patients who have prolonged ECMO runs (i.e., greater than 21 days) are less likely to survive, though no absolute duration of ECMO that would mandate withdrawal of ECMO support can be currently recommended.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Acute respiratory distress syndrome
- Artificial respiration
- Bronchoscopy
- Extracorporeal life support
- Extracorporeal membrane oxygenation
- Hypoxia
- Pediatrics
- Respiratory insufficiency
A 2-year-old girl has developed hypoxemic respiratory failure, severe pediatric acute respiratory distress syndrome, and sepsis secondary to influenza and Staphylococcus aureus pneumonia. On day 4 of mechanical ventilation, she is supported with high-frequency oscillatory ventilation with mean airway pressure set at 30 cmH 2 O. Her inspired oxygen requirement is 70%, and she has been unable to be weaned over the past 12 h. Her arterial blood gas shows pH 7.26, PaCO 2 65 mmHg, PaO 2 58 mmHg, and base deficit − 2. Epinephrine (0.06 mcg/kg/min) and dopamine (5 mcg/kg/min) infusions are required for hemodynamic support. Relevant clinical questions for the care of this child include:
-
What clinical criteria can be utilized to determine if extracorporeal membrane oxygenation is indicated to support this child’s hypoxemic respiratory failure?
-
Which extracorporeal modality and cannulation approach is most appropriate in this clinical scenario?
-
Which mechanical ventilation strategies and other respiratory therapies can help to optimize her chances of recovery?
Introduction
Extracorporeal membrane oxygenation (ECMO) is a form of extracorporeal life support utilized to rescue neonatal, pediatric, and adult patients with respiratory, cardiac, or combined cardiopulmonary failure that is refractory to conventional supportive and therapeutic measures. As a modified form of cardiopulmonary bypass, modern-day ECMO circuitry utilizes either a semi-occlusive roller pump or a centrifugal pump combined with a hollow fiber membrane oxygenator for gas exchange. As a supportive modality, ECMO can provide extended physiologic respiratory and cardiac support for days to weeks, thereby allowing the clinical team time to diagnose and treat the patient’s underlying disease process. To date, the Extracorporeal Life Support Organization registry contains more than 87,000 ECMO runs in its history, of which approximately 10% are children supported for a pulmonary indication [1].
Despite the growing use of ECMO across critical care settings, a discrepancy exists in the available data regarding the effectiveness and benefit of ECMO for various patient populations. For neonates with refractory respiratory failure, clinical trials have shown that ECMO decreases mortality and is cost-effective [2,3,4]. Likewise, ECMO has been demonstrated to be a valid treatment option for critically ill adults with refractory respiratory failure, [5] yet no clinical trials evaluating the efficacy of ECMO have been performed for children with respiratory failure. Current evidence for use of ECMO for children with respiratory failure relies mostly on registry reports and single-center experiences. A recent secondary analysis of the RESTORE trial [6] utilizing matching techniques to compare patients who did and did not receive ECMO showed no mortality benefit from ECMO as compared to ventilation management strategies [7]. This report, while thought provoking, was a secondary analysis of a study designed to evaluate a nurse-driven sedation protocol, and thus the results should be interpreted with caution. Examining ECMO in children with respiratory failure is further limited by the heterogeneity of this patient population; variations in patient age, disease processes, and patient comorbidities all contribute to this heterogeneity. Lastly, the clinical management of ECMO varies greatly across pediatric centers.
Without reliable data by which to guide clinical decision-making, pediatric intensivists managing respiratory failure often must resort to their “gut feeling” when initiating and managing ECMO. The stakes are high – acuity of illness and rapid deterioration often do not allow much time for these life-saving decisions. Prognostication of outcome is difficult, and the costs (e.g., patient morbidity, financial burden, resource utilization) are potentially immense. Given these constraints, we attempt to summarize the available literature regarding ECMO support for pediatric respiratory failure and provide an organized framework by which clinicians can make logical decisions for these challenging patients.
Indications for ECMO
Indications for initiating ECMO in the setting of pediatric respiratory failure can be divided into two general frameworks (Table 2.1). The first clinical scenario is one of progressive hypoxemia and associated hemodynamic instability that remains refractory despite escalation in ventilatory support and other ancillary therapies. Quite simply, the child will die without ECMO, and the decision to initiate ECMO is not a difficult one. The second scenario is one in which the toxicities of medical therapy may begin to outweigh their clinical benefit. In the setting of respiratory failure and severe lung disease, the concept of ventilator-induced lung injury (VILI) becomes pertinent. Mechanical ventilation (MV) has been shown to initiate or worsen lung injury through the mechanisms of volutrauma, barotrauma, oxygen toxicity, and atelectrauma [8]. Current recommendations to limit VILI in children with acute respiratory failure (ARF) include a low tidal volume strategy (5–8 mL/kg predicted body weight), limiting plateau pressures to <28 cmH2O, and titration of positive end-expiratory pressure (PEEP) in an effort to achieve alveolar recruitment and reduce fraction of inspired oxygen concentration (FiO2) to non-toxic levels [9]. Permissive hypercapnia (maintaining a pH > 7.25) and mild hypoxemia (PaO2 40–50 mmHg) are acceptable consequences of these maneuvers, provided adequate systemic oxygen delivery and hemodynamics are maintained [9]. In children with severe lung disease, these lung-protective strategies may have to be exceeded to provide adequate ventilation and oxygenation, with progressive VILI as an untoward consequence. Initiating ECMO provides respiratory support allowing for reduction in ventilator settings to non-toxic levels and possibly to avoid further VILI. In either scenario, the most important principle when deciding upon ECMO suitability is to identify those children with a high probability of mortality yet having potentially reversible lung disease.
Hypoxemic Respiratory Failure and Pediatric Acute Respiratory Distress Syndrome
Pediatric acute respiratory distress syndrome (pARDS) is a clinical syndrome characterized by decreased lung compliance and difficulties with oxygenation. Mortality from pARDS ranges from 18% to 35% [10, 11]. Clinical predictors of mortality from pARDS could help clinicians identify children who would benefit from ECMO support for refractory hypoxemic respiratory failure. Candidate predictors include alveolar dead space fraction (utilized in the studies below as [PaCO2 – end tidal CO2]/PaCO2), the PaO2/FiO2, and the oxygenation index (OI). Nuckton et al. prospectively measured dead space fraction in adult patients with ARDS early in the course of their illness and found increasing dead space fraction to be an independent risk factor for mortality [12]. From a pediatric perspective, in a retrospective review of 217 children requiring mechanical ventilation for acute hypoxemic respiratory failure, the dead space fraction at disease onset and day one both correlated with mortality, though not independently associated when controlled for severity of illness, 24-h maximal inotrope score, and oxygenation index [13]. On the other hand, in a cohort of 266 children with pARDS, Yehya et al. recently showed that the alveolar dead space fraction at the onset of pARDS was significantly higher in non-survivors (0.31 vs 0.13), was independently associated with mortality, and functioned better as a predictor of mortality than the initial PaO2/FiO2 ratio or oxygenation index [14]. This predictive value of dead space fraction however was not observed at 24 h. Functionally, a single numerical value at disease onset may not be practical from the standpoint of clinical decision-making, as intensivists may attempt other modalities and therapies (e.g., high-frequency oscillatory ventilation, prone position, inhaled nitric oxide, etc.) before proceeding with ECMO.
The PaO2/FiO2 (PF) ratio and the oxygenation index (OI = [(mean airway pressure × FiO2 × 100)/PaO2]) have both served as markers of lung disease severity in children with hypoxemic respiratory failure, but the OI has become preferred in contemporary pediatric critical care practice, as it incorporates the mean airway pressure (MAwP) required to maintain oxygenation goals [15]. Over the past decade, many retrospective and prospective studies have demonstrated an association between higher OI and mortality in children with hypoxemic respiratory failure [16,17,18,19]. Historically, an OI of greater than 20 has been used as an indication to transition from conventional mechanical ventilation to high-frequency oscillatory ventilation (HFOV) [20, 21]. In these studies, an OI greater than 20 is associated with mortality rates of more than 40%. The trend in OI value is likely more informative than any single data point, as pARDS is an evolving disease process. For example, utilizing data from preexisting cohorts with pARDS, the Pediatric Acute Lung Injury Consensus Conference evaluated the following variables as predictors of mortality: initial PF ratio, initial OI, worst PF ratio during the first 3 days of mechanical ventilation, and worst OI values during the first 3 days of mechanical ventilation. The worst (highest) OI value during the first 3 days of ventilation was the best discriminator for non-survival, with an area under the receiver operating characteristic curve of 0.75 [15]. Recent data suggests that incorporating an inflammatory cytokine profile alongside the oxygenation index is superior in predicting outcomes in pARDS compared to the oxygenation index alone [22]. These data are intriguing, as they suggest the possibility of a future biomarker array that could help identify patients with pARDS at risk for mortality and thus stratify those candidates who should be considered for earlier ECMO support. Until then, it can be concluded that for patients with pARDS that progress to ECMO, higher pre-ECMO oxygenation index is at higher risk of mortality [23, 24].
Currently, the Extracorporeal Life Support Organization recommends an OI sustained above 40 as the indication for initiation of ECMO in children with respiratory failure [25]. However, there are likely children with hypoxemic respiratory failure and OI values below this threshold that could benefit from ECMO’s potential ability to mitigate further VILI. For example, in an analysis of a cohort of children in our institution with hypoxemic respiratory failure who were ventilated with HFOV, an OI greater than 25 at 48 h following the onset of pARDS conferred a significantly higher odds of mortality (odds ratio: 5, 95% CI 1.3 to 16.7, p < 0.05) [19]. While the predictive value of the oxygenation index continues to grow in the arena of pediatric critical care, to date, no definitive OI threshold exists above which pediatric intensivists can be 100% certain regarding the optimal time point for the initiation of ECMO for pARDS. The Pediatric Acute Lung Injury Consensus Conference from 2015 states that “it is not possible to apply strict criteria for the selection of children who will benefit from ECMO in pARDS” [26].
In the absence of strict numerical criteria surrounding oxygenation, intensivists are left to rely on the gestalt clinical picture when deciding upon the initiation of ECMO to support children with hypoxemic respiratory failure. Key components within this framework include trends in ventilator support and oxygenation measures over time, hemodynamic stability, organ function, and acid-base status. First, with regard to the trajectory of respiratory support and oxygenation, serial assessments serve more valuable than an evaluation at a single time point [26]. It is our practice to obtain arterial blood gases and calculate the oxygenation index every 4 h. Concurrently, we also track trends in peak inspiratory pressures as a marker for the potential of evolving ventilator-induced lung injury. Second, children with evolving hypoxemic respiratory failure can develop hemodynamic instability. Etiologies for this instability can be multifactorial, including impaired oxygen delivery to the myocardium, impaired right ventricular preload from increased intrathoracic pressure, increased right ventricular afterload from hypercarbia and acidosis, and concurrent sepsis. Next, the cascade of inflammatory cytokines released during ARDS as well as with VILI has been demonstrated to result in direct organ dysfunction, fluid retention, and subsequent fluid overload [27]. All of the above factors may culminate in a progressive metabolic acidosis, the effect of which is noteworthy in critically ill children. In a retrospective cohort of children with hypoxemic respiratory failure supported with HFOV, the presence of a metabolic acidosis was independently associated with a higher risk of mortality [19]. Likewise, studies examining pre-ECMO variables in this patient population have shown that the presence of acidosis is associated with worse survival [23, 24]. For example, in a study by Dimico and colleagues containing data from 1325 children within the ELSO registry, odds of survival to hospital discharged increased by 15% for every 0.1 increase in pre-ECMO pH.
Hypercarbic Respiratory Failure
Though severe refractory hypoxemia is the most common indication for pediatric respiratory ECMO, there are patients who require ECMO due to severe ventilation impairment leading to hypercarbia. Acute hypercarbic respiratory failure is defined as a PaCO2 greater than 50 mmHg, typically associated with respiratory acidosis. Modern ventilation strategies are focused on limiting VILI and allowing for permissive hypercarbia [28, 29]. The possible detrimental effects of moderate hypercarbia (50–75 mmHg) have been debated, but they are inconsequential compared to the risks associated with ECMO [30]. Thus, ECMO is not advised for moderate hypercarbia (i.e., PaCO2 up to 75 mmHg). However, as PaCO2 rises and respiratory acidosis becomes more severe, dysfunction of other organ systems becomes more significant, particularly that of the cardiovascular system. Therefore, severe hypercarbic respiratory failure with acidosis and hemodynamic instability is an indication for initiation of ECMO [30]. ECMO can correct hypercarbia rapidly with a resultant improvement in acidosis and organ function. During this process, clinicians must be aware of possible detrimental consequences of rapid correction of PaCO2, which includes cerebral vasoconstriction and the development of alkalosis.
Status asthmaticus is the most common disease causing severe hypercarbia requiring ECMO, but patients with status asthmaticus only represented 3% of the pediatric respiratory cases reported to ELSO from 2009 to 2015 [31, 32]. Patients who require ECMO for status asthmaticus typically have severe acidosis (pH < 7.0) and severe hypercarbia (PaCO2 > 100 mmHg) despite maximal medical therapies [32]. Children with status asthmaticus on ECMO have very good survival, with 88% surviving to hospital discharge. Moreover, status asthmaticus improves relatively quickly, with an average duration of ECMO of 92 h [32].
Cystic fibrosis (CF) with pulmonary exacerbation is another patient population that can require ECMO for severe hypercarbic respiratory failure. There were 73 ECMO runs in the ELSO registry for cystic fibrosis from 1998 to 2013, with 52% survival [33]. There has been debate if patients with CF are candidates for ECMO due to the progressive nature of their disease. Each patient must be evaluated individually. First, the reversibility of their respiratory failure should be assessed. The primary determination in this regard is if the child has a pulmonary infection that is potentially amenable to antibiotic therapy. If lung disease is not thought to be reversible, then candidacy for lung transplantation should be considered. Cystic fibrosis should only be considered an absolute contraindication to ECMO if the patient is deemed not to have the potential for recovery from the acute process and is not a candidate for lung transplantation. Candidacy should be considered for any intubated cystic fibrosis patient to help inform decisions of whether or not to pursue ECMO. This candidacy should be determined by working with your local pulmonologist and, if necessary, a regional lung transplant center.
Airway Disorders
Patients with significant air leaks may not meet typical oxygenation or ventilation criteria for ECMO. Indications for ECMO support in patients with air leak syndromes include recurrent pneumothoraces causing life-threatening events or persistent air leaks not improving with chest tube thoracostomy. Patients with broncho-pleural fistulae from pulmonary infection or surgery that do not heal spontaneously on mechanical ventilation can also be successfully managed with ECMO, which allows for reduction of peak airway pressure [34, 35]. ECMO can also be used intraoperatively for major airway surgeries and postoperatively to allow for healing of surgical sites without the effects of high positive airway pressures [36, 37].
Our recommendations are a general framework to utilize when deciding upon ECMO support for children with refractory respiratory failure who have a potentially reversible lung disease and no absolute contraindication to ECMO (Table 2.1).
Case Presentation
OI was calculated to be 36 without improvement over 12 h despite medical therapy and use of high-frequency oscillatory ventilation. Her inspired oxygen concentration could not be weaned below 70%. At this point, her refractory hypoxemic respiratory failure secondary to influenza and Staphylococcus aureus pneumonia was considered refractory to conventional therapy with no trajectory of improvement. There was also concern that her potentially injurious ventilator support and high inspired oxygen concentration would continue to worsen her underlying lung disease. Based on these data, cannulation for ECMO was being considered.
Patient Selection
Diagnosis
Critically ill children may develop respiratory failure from a wide array of infectious or noninfectious and direct or indirect etiologies [38]. Successful use of ECMO support for children with refractory lung injury from a broad variety of noninfectious causes has been reported, including burn injuries [39], trauma [40, 41], hydrocarbon aspiration [42], and rheumatologic diseases [43, 44]. In an analysis of factors associated with pediatric ECMO survival, Zabrocki et al. reviewed 3213 children supported with ECMO for a primary pulmonary indication from the years 1993 through 2007 [24]. In this review, diseases associated with the best survival rates include asthma (83%), aspiration pneumonia (71%), and RSV bronchiolitis (70%). As infection is the most common etiology of pARDS [45], the remainder of the focus in this section will be on specific infectious pathogens. Overall survival for children with bacterial and viral pneumonia who require ECMO is approximately 56–59% [24]. Infectious agents that portend worse outcomes include Bordetella pertussis, herpes simplex virus (HSV), fungal pneumonia, and methicillin-resistant Staphylococcus aureus (MRSA).
Over the past two decades, the overall survival for children with pertussis who are supported with ECMO is 39%, considerably lower as compared to children requiring ECMO for other viral and bacterial etiologies [24]. In a retrospective analysis, children with pertussis who survived their ECMO course were found to have higher pre-ECMO PEEP (11 cmH2O vs. 7 cmH2O, p = 0.006) and significantly higher pre-ECMO pH values (7.31 vs. 7.14, p 0.002) when compared to non-survivors [46]. Lung pathology obtained from young infants who died from fulminant pertussis shows necrotizing bronchiolitis with necrosis of the alveolar epithelium [47]; thus, an open-lung strategy achieved with high PEEP (i.e., 10–11 mmHg) may help to offset the pathophysiologic ramifications of this process. Refractory pulmonary hypertension and severe leukocytosis are two additional pathophysiologic mechanisms which contribute to the severity of fulminant pertussis infections. Significantly leukocytosis is observed in infants with the most severe infections, with white blood cell (WBC) counts in excess of 100,000/mm3 associated with increased mortality risk [48]. Postmortem examinations of these patients show leukocytes obstructing the pulmonary blood vessels [47]. This severe leukocytosis leads to vascular hyperviscosity and pulmonary arteriole thromboses and, when combined with the reactive pulmonary vasculature of the young infant, pulmonary hypertension refractory to conventional therapy. Given the role of leukocytosis in the pathology of this disease, an interesting single-center case series explored the utility of leukodepletion in infants with fulminant pertussis [49]. Implementation of a clinical protocol for leukodepletion for children with WBC count of greater than 50,000/mm3 resulted in improved survival, with a reduction in mortality from 44% to 10%. Leukofiltration for patients receiving ECMO support was accomplished by placing a WBC filter sited in the bridge of the ECMO circuit. While this report was a single-center study with a small volume of patients, a case-mix adjustment accounting for age, WBC count, and ECMO referral time revealed a significantly better observed mortality following the implementation of leukodepletion than predicted. Thus, while the prognosis for infants with fulminant pertussis requiring ECMO remains guarded, implementation of leukodepletion can be considered to help offset the pathologic consequences of severe leukocytosis and subsequent pulmonary hypertension.
Case reports exist which describe the use of ECMO to support both neonates [50] and adults [51] for HSV infections. ECMO was successful in the cases of isolated respiratory infection leading to ARDS. Conversely, the outcomes for use of ECMO with disseminated HSV infection remain quite poor. Disseminated neonatal HSV is a rapidly progressive infection, which leads to multiple organ failure and has a high mortality rate despite aggressive care. A 2010 review of 40 neonates with HSV infection supported with ECMO showed an overall survival to hospital discharge of 25% [52]. Survival remained constant across all decades, suggesting that the fulminant nature of this particular infection should cause one to approach initiating ECMO with caution.
Patients with active fungal infections prior to the initiation of ECMO are at risk of persistent seeding and contamination of the ECMO circuit. Candida was the predominate fungal species acquired both pre- and during ECMO in a study of the ELSO registry. While the presence of a fungal infection significantly increased the odds of mortality in all age groups, 82–89% of patients with fungal infections prior to the initiation of ECMO became culture negative at some point into their ECMO course [53]. Similarly, the presence of a fungal infection did not lead to an increase in circuit complications or circuit failure. Thus, the authors of this review concluded that while a fungal infection remains an important comorbidity to consider when initiating ECMO support, it, in and of itself, should not be considered an absolute contraindication to ECLS. However, caution must be exercised for pediatric patients with respiratory failure secondary to fungal infections. In the aforementioned review by Zabrocki et al., fungal pneumonia was the single diagnosis independently associated with the highest risk of mortality [24]. It is likely that the fungal infection alone does not portend to a grim prognosis but rather is a surrogate marker for patients with other diagnoses associated with poor outcomes on ECMO, such as patients with oncological diagnoses.
Community-acquired MRSA is an invasive bacterium that can cause skin and soft-tissue infections in previously healthy children. The invasive nature of this bacterium may lead to severe necrotizing pneumonia and ARDS in some children. Concurrent with the rise of invasive MRSA infections in the community over the past 25 years, the use of ECMO to support children with hypoxemic respiratory failure secondary to MRSA pneumonia has also risen [54]. Mortality from this pathogen remains high, with an approximate mortality rate of 50% for children who require ECMO; children older than 5 years seem to be particularly at risk. MRSA pneumonia and sepsis may result in thrombocytopenia-associated multiple organ failure (TAMOF), and there is some evidence which shows benefit from plasmapheresis performed during ECMO to reverse the sequelae of this disorder [55]. Combining plasmapheresis to the ECMO circuit adds a layer of technical complexity, and there is also increased heparin clearance during the procedure, thus necessitating careful monitoring of anticoagulation. Despite these challenges, recent data have shown that therapeutic plasma exchange can improve organ function in children with sepsis-induced multiorgan dysfunction who require ECMO support [56].
Duration of Mechanical Ventilation
As late as the 1990s, children with respiratory failure were not considered candidates for extracorporeal support after 7–10 days of mechanical ventilation due to a significant reduction in survival when ECMO was initiated beyond this timeframe [57, 58]. In this era, lung-protective ventilator strategies had not yet become standard practice, and, perhaps, significant VILI that had accrued in these children prior to ECMO initiation was irreversible. More contemporary data suggests that the optimal timing for ECMO initiation still lies within the first week of mechanical ventilation [59,60,61], but outcomes for children ventilated beyond 7 days prior to ECMO have improved such that these children should still be considered appropriate candidates for extracorporeal support. Domico et al. reviewed the relationship of pre-ECMO mechanical ventilation duration and the outcomes of 1352 pediatric patients who required ECMO for respiratory failure from 1999 to 2008 [23]. In this analysis, a significant reduction in survival was not observed until the pre-ECMO duration of ventilation exceeded 14 days. Similarly, in Zabrocki’s review of pediatric respiratory failure, analysis of pre-ECMO mechanical ventilation revealed that survival remained 56% or higher for children who had ECMO initiated within the first 14 days of mechanical ventilation but declined to 38% beyond this timeframe [24]. Fourteen days is now often considered a “cutoff” for duration of ventilation prior to ECMO; however, the outcomes for patients with certain diagnoses, such as viral pneumonia, can be good even when ECMO is initiated after 2 weeks of ventilation [21, 22]. Careful patient selection is therefore paramount to successful use of when ECMO if extracorporeal support is initiated after an extended course of mechanical ventilation. Factors that should be considered are the number of comorbidities, the number of non-pulmonary organ failures, and the primary cause of pARDS.
Patient Comorbidities
Historically, the ideal ECMO candidate was one with a known reversible illness, had single-organ failure and minimal comorbidities, was neurologically intact and not developmentally delayed, and had minimal bleeding risk. Practically speaking, very few children cared for in modern-day pediatric intensive care units fit this description. Over the past two decades, the use of ECMO for respiratory support in children with comorbidities has increased markedly. In 1993, 19% of children placed on ECMO had underlying comorbidities, compared to 47% in 2007 [24]. Not surprisingly, children with comorbidities have lower survival rates when compared to previously healthy children [24]. Comorbidities which have been shown to significantly increase mortality on ECMO include acute kidney injury, liver failure, cancer, primary immunodeficiency, and pre-ECMO cardiac arrest [24].
Acute kidney injury is a common occurrence in the intensive care setting, and its impact on outcomes of children requiring ECMO is notable. Recently, the Kidney Intervention During Extracorporeal Membrane Oxygenation Study Group showed that approximately 60% of children requiring ECMO support have acute kidney injury, and this comorbidity is associated with a longer duration of ECMO and an increased risk of mortality [62]. Closely related to acute kidney injury is fluid overload, which also impacts ECMO patient outcomes. In a recent analysis of 756 neonatal and pediatric ECMO patients, peak fluid overload, fluid overload at ECMO discontinuation, and the change in fluid overload during ECMO were significantly higher in patients who suffered mortality either while on ECMO or later during their hospitalization [63].
The status of a patient’s immune system is a significant consideration when deciding upon a child’s suitability for extracorporeal support. Historically, an immunocompromised condition was a relative contraindication for ECMO for several reasons:
-
Leukopenia from chemotherapy or the disease process itself confers a risk for subsequent super-infection.
-
Concurrent thrombocytopenia and coagulopathy place patients at a higher risk for hemorrhagic complications.
-
Multiorgan failure is often present prior to the initiation of ECMO.
-
Doubt or uncertainty may exist regarding the patient’s long-term prognosis with respect to their underlying disease.
A recent review examined the outcomes of 107 children with underlying malignancies (73 hematologic, 34 solid tumors) who received ECLS for various disease processes (the majority of which were acute respiratory failure) over a 13-year period [64]. Overall survival to hospital discharge was 35% (36% for respiratory failure, 29% for cardiac failure), which is worse than for other children receiving ECLS. Despite their immunosuppressed state, only 19% of these children acquired a new infection while on ECMO. Children with malignancies did have a higher rate of hemorrhagic complications, including cannula site bleeding, disseminated intravascular coagulation, and CNS hemorrhages when compared to children without cancer. Currently, most pediatric ECMO centers offer ECMO support for this patient population. Of 118 centers surveyed, 78% did not view the presence of a malignancy as a contraindication, and 17% considered pediatric cancer as only a relative contraindication to ECMO [64]. The support for the use of ECMO in this patient population, despite only a 35% overall survival, likely stems from the notion that these patients, the majority of whom have leukemia, have a good prognosis with regard to their underlying malignancy if their acute respiratory failure can be overcome. In this cohort, the median OI prior to initiation of ECMO was 52, reflecting that these patients likely have a higher degree of lung injury prior to ECMO initiation, relative to other patient populations. It remains to be seen if earlier initiation of ECMO within this patient population could potentially result in improved outcomes. Regardless, malignancy should not be considered an absolute contraindication from ECMO. Close communication with the child’s oncology team regarding the long-term prognosis from their underlying malignancy is an additional essential piece of information to be considered in this decision-making process.
Children undergoing hematopoietic stem cell transplantation (HSCT) are another immunosuppressed patient population where the decision to implement extracorporeal support may be controversial. These children can develop life-threatening complications in the immediate posttransplant period (e.g., diffuse alveolar hemorrhage, idiopathic pulmonary syndrome, various infections) and in the later stages following transplantation (e.g., graft versus host disease, bronchiolitis obliterans), all of which can lead to respiratory failure refractory to conventional care. Historical mortality rates for children requiring mechanical ventilation following HSCT are well over 50% [65,66,67]. More recent data suggests that an earlier transition from conventional ventilation to HFOV may improve survival, but mortality remains high [68]. With regard to the use of ECMO in this patient population, there are sparse case reports documenting the successful use of ECMO to support HSCT patients through posttransplant complications, including diffuse alveolar hemorrhage, [69] idiopathic pulmonary syndrome, [70] and sepsis secondary to neutropenic enterocolitis [71]. However, in an ELSO registry review of 19 children undergoing ECLS following HSCT, 79% died during the ECMO course: seven developed multiorgan failure, three had refractory hemorrhage, and five had support was withdrawn for others reasons [72]. Furthermore, only one of the four remaining children alive after ECMO survived to hospital discharge. A more recent ELSO registry review of children placed on ECMO support following hematopoietic stem cell transplant also showed poor outcomes, with only 10% of patients surviving to hospital discharge [73]. Based on this experience, it can be concluded that, at the present time, children undergoing HSCT are unlikely to survive refractory respiratory failure requiring ECMO. Broadly speaking, this population of children has had dismal outcomes when requiring intensive care admission, particularly if mechanical ventilation is necessary, and thus ECMO should be avoided.
In summary, while the historical approach may have been to reserve ECMO for previously healthy “salvageable” patients, contemporary ECMO utilization has shown that there are in fact very few conditions completely incompatible with ECMO. In the setting of pediatric respiratory failure, extracorporeal support serves as either a bridge to patient recovery or a bridge to consideration for lung transplantation in the event of non-recovery. Unless a disease process is deemed irreversible or the child is not a candidate for lung transplantation, ECMO remains a realistic option to support most critically ill children with refractory respiratory failure.
ECMO Modality and Cannulation Strategy
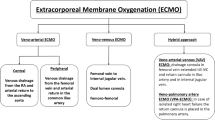
Once the decision has been made to initiate extracorporeal support for the child with worsening respiratory failure, the next determination is choice of ECMO modality. Venovenous (V-V) ECMO involves the removal of deoxygenated blood from the patient’s right atrium, oxygenation and ventilation as the blood traverses through the ECMO circuit, and then return of oxygenated blood into the child’s central venous circulation through a different port, often within the same cannula. V-V ECMO provides gas exchange but no direct cardiac support. In contrast, with veno-arterial (V-A) ECMO, oxygenated blood returning to the patient enters the arterial circulation, commonly through a different cannula in the carotid or femoral artery. Completely bypassing the patient’s native heart and lungs, V-A ECMO can provide complete cardiopulmonary support [74].
The inherent risks between these two ECMO modalities should be considered. With V-A ECMO, cannulation of the femoral artery incurs the risk of lower extremity ischemia [74], while cannulation of the carotid artery carries a substantial risk of stroke (23%) [75]. In an analysis of pediatric patients with respiratory failure, the use of V-A ECMO was independently associated with an increased risk of neurologic injury when compared to V-V ECMO [76]. Other risks associated with V-A ECMO include embolism of air or thrombi into the patient’s arterial circulation or increased systemic afterload leading to distension of the left ventricle, with subsequent risk of pulmonary hemorrhage. The primary disadvantage of V-V relative to V-A ECMO is its inability to provide cardiac support for patients with hemodynamic instability. However, there are important cardiac benefits that indirectly result from initiation of V-V ECMO for respiratory failure. First, with the ability to wean mechanical ventilation, intrathoracic pressure decreases, thereby improving preload to the right ventricle. Second, the ability of V-V ECMO to correct hypercarbia and acidosis can lead to a reduction in pulmonary vascular resistance and a corresponding decrease in right ventricular afterload. Lastly, oxygenated blood returning from the ECMO circuit passes through the pulmonary vasculature, eventually making its way into the left ventricle. A portion of this blood will enter the coronary circulation as it exits the aortic valve, providing a previously oxygen-deprived myocardium with a rich source of oxygen and a resultant improvement in ventricular function and hemodynamics.
In a single-center review of children requiring ECMO for acute respiratory failure from 1991 through 2002, Pettignano et al. illustrated the hemodynamic benefits of V-V ECMO [77]. In this cohort, 35% of patients required at least one vasopressor, and 41% required at least one inotropic infusion at the time of ECMO cannulation. After initiation of ECMO support, there was a significant reduction in vasoactive medication requirements, and all patients were free from vasoactive infusions by day 6 of ECMO. Similarly, an analysis of 4332 ECMO runs for children with sepsis showed significantly better survival for children receiving V-V support when compared to those on V-A ECMO [78]. This study was unable to account for pre-ECMO severity of illness and also did not factor in the type of hemodynamic derangement that characterized each patient’s type of sepsis (i.e., warm shock vs. cold shock). However, the fact that such a large number of children with sepsis were successfully supported with V-V ECMO gives credence to the ability of this modality to support critically ill children who have unstable hemodynamics.
The use of V-V ECMO in pediatric critical care is continuing to increase [79], and based on data from a large retrospective review, V-V ECMO appears to confer a survival advantage relative to V-A ECMO [24]. To date, there is no definitive formula or inotrope score by which to guide intensivists in choice of ECMO modality for children with acute respiratory failure and associated hemodynamic instability. Our own institutional practice relies greatly on echocardiogram imaging of myocardial performance to aid in this decision. A child with mild to moderately depressed right ventricular function, which is most often due to a combination of high pulmonary vascular resistance induced by lung disease and respiratory acidosis, is a candidate for V-V ECMO, even if requiring a moderate amount of vasoactive medications for hemodynamic support. V-V ECMO is recommended for these patients, even in the setting of hemodynamic instability, as correction of respiratory acidosis and an increase in right ventricular preload that occur after ECMO initiation often improve ventricular function. In contrast, a child with an echocardiogram showing significantly depressed left ventricular or biventricular function due to sepsis-induced myocardial depression should be cannulated for V-A ECMO.
ECMO cannula selection and configuration is an essential decision made in the process of initiating V-V ECMO support. One option for V-V cannulation is a multisite configuration with single-lumen ECMO catheters, the first being placed within the right internal jugular vein and extending into the right atrium and the second inserted into a femoral vein and extending upward into the inferior vena cava. The alternative cannulation option utilizes a dual-lumen venovenous (VVDL) catheter inserted into the right internal jugular vein, with both drainage and reinfusion lumens of the cannula residing within the right atrium. One disadvantage of the two-cannula technique includes the need to access multiple venous sites, thus being a more invasive and time-consuming procedure, along with increasing the sites for potential bleeding and infectious complications. Second, children weighing less than 15 kg typically do not have large enough femoral veins to accommodate the necessary-sized ECMO catheters. Given these constraints of the two-site cannulation technique, the application of the VVDL cannulation strategy to provide ECMO support to children with respiratory failure has increased significantly over the past decade [24, 80]. Zamaro et al. analyzed the performance and complication rates of these two cannulation strategies from 1323 pediatric V-V ECMO runs [80]. Compared to multisite cannulation, VVDL cannulation achieved greater weight-adjusted ECMO flow but had a slightly higher rate of mechanical (26.2% vs. 22.5%, p 0.004) and cardiac (24.4% vs. 21.7%, p 0.03) complications. Importantly, there was no significant difference in survival between the two cannulation techniques.
One drawback to early versions of VVDL ECMO catheters was their potential to bend and kink, thus raising the possibility of obstruction to blood flow and interruption of the ECMO circuit. The newer generation of VVDL catheters is manufactured with wire reinforcement to offset this potential complication. The only available pediatric literature comparing these two types of catheter designs was a single-center retrospective study of 25 neonates and infants, which found no difference in the incidence ECMO flow interruption between the wire-reinforced and non-wire cannulas within the first 72 h [81]. The two wire-reinforced VVDL ECMO catheters currently available for pediatric use are the OriGen® DL cannula (OriGen® Biomedical, Austin, TX) and Avalon® Elite Bicaval cannula (Avalon® Laboratories, LLC, Rancho Dominguez, CA). The OriGen® is placed in the right atrium and has a proximal drainage hole and distal reinfusion hole. The configuration of the bicaval Avalon® cannula places the drainage holes within the SVC and IVC, while the reinfusion port is located within the right atrium and is directed toward the tricuspid valve, offering the theoretical advantage of reducing the amount of recirculation. The use of bicaval ECMO cannulas has been shown to be effective and safe in the adult ECMO population [82, 83], but the smaller-sized venous and cardiac structures inherent to pediatric patients may raise concern regarding an increased rate of mechanical complications and increased need for catheter repositioning. Several pediatric ECMO centers have published their institutional experiences with the bicaval wire-reinforced cannulas and have noted minimal complications in both pediatric and neonatal patients [81, 84,85,86]. In an analysis of cannula complications by Zamaro and colleagues, there was no difference in rate of complications when wire-reinforced and non-wire-reinforced catheters were compared [80]. Proper imaging techniques during cannulation, including the combined use of echocardiography and fluoroscopy, have been shown to reduce complications and the need for catheter repositioning [86]. Lastly, successful and safe percutaneous ECMO cannulation of pediatric patients performed by intensivists has recently been described [87]. A single-center retrospective review of percutaneous ECMO cannulations performed by intensivists included 18 pediatric patients cannulated for V-V ECMO. In this cohort, the overall rate of successful cannulation was 98% [87]. In our current practice, we consider wire-reinforced VVDL ECMO catheters safe for pediatric patients and are the preferred modality by which to provide extracorporeal respiratory support.
Case Presentation
In addition to her persistently elevated OI, her relatively short duration of illness thus far and her potentially reversible viral influenza and Staphylococcus aureus pneumonia were determined to be characteristics of a good candidate for ECMO. The patient was receiving relative modest doses of vasoactive support – epinephrine 0.06 mcg/kg/min and dopamine 5 mcg/kg/min. These infusions were likely necessary due to the combination of hypoxemia, respiratory acidosis, and high MAwP, conditions that were considered amenable with venovenous ECMO. The decision was therefore made to cannulate the patient with a wire-enforced VVDL cannula in her right internal jugular vein for V-V ECMO support. Following successful cannulation, vasoactive medications were able to be easily weaned to off over the first 12 h on ECMO.
Pulmonary Management
Ventilator Management
There have been major strides in the study of mechanical ventilation in ARDS over the past decades [29]. The result of these studies has been the low tidal ventilation strategy, which consists of low tidal volumes (VT) (6–8 mL/kg), PEEP titrated to keep FiO2 < 0.6, and permissive hypercarbia. However, for patients who fail this strategy and are cannulated for ECMO, the optimal ventilator management strategy has not been well studied. The ideal mechanical ventilation strategy would limit VILI without lengthening ECMO duration due to lung collapse.
Traditional ventilator management on ECMO has been a “lung-rest” strategy to limit VILI. Current ELSO guidelines suggest low rate, moderate PEEP (5–15 cmH2O) and low plateau pressure [25]. Few published studies describe mechanical ventilation practices while on ECMO in the pediatric population. A recent survey found most pediatric intensivists (87%) employ a strategy of “lung rest” while on ECMO [88].
According to a recent review, most adult patients have VT, FiO2, and plateau pressure significantly reduced after initiation of ECMO, while PEEP is minimally reduced from their pre-ECMO support, being maintained on average between 12 and 13 cmH2O [89]. In this review, the proportion of patients with ventilator settings considered to be injurious (defined as TV > 8 mL/kg, plateau pressure > 30 cmH2O, peak inspiratory pressure >35 cmH2O or FiO2 ≥ 0.8) decreased from 90% pre-ECMO to 18% after initiation of ECMO [89].
The goal of the historical practice of “lung rest” is to limit the risk of VILI, but this strategy often leads to lung collapse. In a recent survey of mechanical ventilation practices during ECMO, most pediatric intensivists (76%) target a PEEP of ≤10 cmH2O [88]. This level of PEEP in the setting of pARDS will frequently lead to total lung collapse and the need for re-recruitment of the lung later in the ECMO course. The need for re-recruitment may prolong the ECMO course and contribute to VILI. Given this concern, some intensivists no longer practice strict “lung rest” while on ECMO for ARDS [90]. The most popular new ventilation practice is an open-lung strategy on ECMO, similar to the adult ARDS low VT ventilation strategy [91].
While attempting aggressive lung recruitment in the acute inflammatory stage of ARDS is discouraged, as it can exacerbate VILI, maintaining lung recruitment with relatively high MAwP while on ECMO may be advisable. On conventional ventilation, this goal is achieved by maintaining high PEEP with a long inspiratory time. Airway pressure release ventilation (APRV) is a mode of ventilation, which utilizes a high distending pressure with brief intermittent releases of pressure to improve ventilation, and patients are able to spontaneously breathing during the periods of high distending pressure [92]. APRV can also be used to achieve a high MAwP without high plateau pressures, similarly to a high PEEP and long inspiratory time strategy with conventional ventilation, which maintains recruitment with potentially less risk of VILI. The ELSO recommendations regarding PEEP offer a wide acceptable range – 5–15 cmH2O [25]. The use of PEEP at the higher end of this range in neonates with respiratory failure has been shown to lead to shorter duration of ECMO [93, 94]. Similarly, in adults on V-V ECMO, higher PEEP during the first 3 days on ECMO has been associated with improved survival [95]. Conversely, higher PEEP over the whole ECMO course has been shown to be associated with increased mortality [96]. It is hard to reconcile these seemingly incongruous findings. However, one could hypothesize that PEEP later in the ECMO course is more related to patient factors than ventilator strategy. In other words, patients with improving lung disease will have PEEP decreased over the course on ECMO, while patients who do not improve and ultimately die will remain on higher PEEP throughout their course. Additionally, high PEEP in the first 3 days on ECMO is more likely to be a conscientious strategy of maintaining lung recruitment and less so reflective of the patient’s severity of lung disease.
The risks of maintaining a higher MAwP must be weighed against the risks of allowing lung collapse. High MAwP will cause elevated intrathoracic pressure which may impair hemodynamics due to impaired venous return. Elevated static pressures, like those used in an open-lung ventilation strategy or in APRV, do not to contribute significantly to VILI [97]. There are also risks to a lung-rest approach resulting in complete lung collapse. Hemodynamically, lung collapse will lead to increased right ventricular afterload by way of increased pulmonary vascular resistance. On V-V ECMO, lung collapse will also lead to severe pulmonary venous desaturation, and systemic arterial saturations will suffer. Additionally, there is emerging evidence of the damaging effects of recurrent atelectasis, often called atelectrauma [8, 98, 99]. If there is complete lung collapse, the lungs will need to be re-recruited later in the ECMO course, which often can only be accomplished with high driving pressure, which is the biggest contributor to VILI [97]. Lastly, the time necessary to re-recruit the lungs may prolong the ECMO course. Neonatal data shows that higher PEEP (i.e.,12–14 cmH2O vs 4–6 cmH2O) leads to shorter ECMO runs and fewer complications [93, 94]. Identifying the ideal PEEP for a patient is a multifactorial decision that includes evaluation of lung expansion on chest x-ray, hemodynamics, oxygenation, lung compliance, and other factors. In a recent study, the use of electrical impedance tomography for adult patients with respiratory failure on ECMO showed that an optimal PEEP of 15 cmH2O best balanced overdistension and lung collapse [100].
The one consistent finding from the limited data published about mechanical ventilation for adults on ECMO is that higher driving pressure (peak inspiratory pressure minus PEEP) or plateau pressure is associated with increased mortality [89, 101, 102]. Limiting peak or plateau pressure is advisable for adults on ECMO as it is for any adult with ARDS. However, the upper limit of what is reasonable for driving and plateau pressures in children has not been well established in pARDS and might be higher than in adults [17, 18, 103].
Secretion Clearance on ECMO
Invasive procedures on ECMO are avoided whenever possible due to the increased risk of hemorrhage associated with anticoagulation. Bronchoscopy, however, is an invasive procedure that may be necessary for some patients supported on ECMO. Though there are only a few reports of bronchoscopy on ECMO in the literature [98,99,100,101], it is commonly implemented in clinical practice. Routine bronchoscopy of patients on ECMO is performed in 55% of pediatric ECMO centers and 76% of adult centers [90]. While the benefits of bronchoscopy on ECMO have not been established in the literature, they are often seen at the bedside in patients with thick respiratory secretions that cannot be mobilized through traditional suctioning techniques. In the limited published data on pediatric bronchoscopies on ECMO to date, minor bleeding episodes occur in up to 35% of patients in some report, the risk of significant pulmonary hemorrhage is 0–1.5% [104,105,106,107]. Bronchoscopy is therefore feasible and relatively safe in patients on ECMO, with a small risk of pulmonary hemorrhage.
HFPV is a combination of high-frequency ventilation and conventional mechanical ventilation principles. HPFV consists of high-frequency sub-physiological tidal volumes superimposed on low-frequency conventional tidal volume ventilation [108]. One institution published their experience with the use of high-frequency percussive ventilation (HFPV) and increased frequency of bronchoscopy to target secretion clearance for pediatric respiratory ECMO. They showed an increase in ECLS-free days when compared to historical controls [106]. It is not clear if the benefit was due to HFPV, increased bronchoscopies, the combination of the two, or other changes to practice that were not measured. The use of HFPV and frequent bronchoscopy promotes secretion clearance and is most likely to be beneficial in diseases that cause excessive secretions such as bronchiolitis.
Extubation on ECMO
The practice of extubation on ECMO has becoming increasingly utilized in the past several years. In a 2015 survey, only 10% of centers reported extubating patients on ECMO, while a 2017 survey reported 41% of centers extubate patients [90, 91]. ECMO is employed to allow for oxygenation and ventilation without the risk of VILI. It has been suggested, therefore, that the best way to limit VILI would be to remove the “V” or ventilator. Extubating patients on V-V ECMO has been shown to be feasible and safe in selected patients [90, 109]. In one retrospective study, extubation on ECMO was associated with improved survival [109]. If extubation is feasible, there is the benefit of reducing sedation once the noxious stimulus that is the endotracheal tube is removed. Patients who are extubated and not sedated will also be able to participate in more aggressive rehabilitation while on ECMO. Not all patients will be candidates for extubation on ECMO. Younger patients, for instance, may require paralysis or heavy sedation to prevent mechanical complications, obviating any possibility of extubation. It should also be noted that extubated patients might require reintubation for procedures including bronchoscopy and decannulation from ECMO.
Recommendations
Maintenance of lung aeration with modest ventilator settings may be advantageous to their recovery. Lung aeration will facilitate higher arterial oxygen saturations (SaO2), allow for some oxygenation if there is an emergency requiring separation from the circuit, and possible shorten ECMO course by eliminating the time needed to re-recruit collapsed lung. Our usual strategy is to maintain lung aeration with high PEEP (10–14 mmHg) or APRV when possible. Due to our concerns that strict adherence to the historical “lung-rest” settings with low driving pressure (e.g.. 10 cmH2O) and complete lung collapse may not be optimal and may prolong the ECMO course, we often use slight higher MAwP (15–20 mmHg) and driving pressure (12–18 mmHg) than historical “lung rest.” On the other hand, when there is complete lung collapse despite these moderate MAwP, we do not advise trying to re-recruit alveoli in the acute inflammatory phase of pARDS.
Some patients will have complete lung collapse in the face of high MAwP. In patients with lung collapse and who are likely to have prolonged ECMO courses, extubation is an option. Patients that are most likely to be able to be extubated and benefit from extubation are school-aged or older children in whom a short ECMO run (<7 days) is not expected. Further, to successfully extubate patients on ECMO, the circuit must be able support oxygenation without the aid of mechanical ventilation, which requires high flow rates without significant recirculation issues.
We support aggressive airway clearance with bronchoscopy for pediatric respiratory ECMO, particularly in patients with disease processes that lead to mucous plugging. Bronchoscopy is likely most beneficial when performed early, such as within the first week on ECMO, with the goal of lung recruitment when the inflammatory stage of pARDS is resolving. The need for further bronchoscopy can be determined by the secretion burden noted during the first bronchoscopy and the subsequent clinical course. Diagnostic bronchoscopy can also be performed to assess for infectious complications. Routine reduction or interruption anticoagulation for bronchoscopy is not suggested due to low risk of significant bleeding.
Case Presentation
After cannulation for venovenous ECMO, the 2-year-old child with influenza and Staphylococcus pneumonia was managed with a high PEEP strategy (i.e.,10–12 mmHg) and modest driving pressure (i.e., pressure above PEEP 12–16 mmHg). Daily chest x-rays demonstrated modest lung aeration. During the initial portion of her ECMO course, she was receiving only relatively small tidal volumes (~2–3 mL/kg) with this approach. Over the next 2 weeks, with the assistance of an aggressive pulmonary toilet that included multiple bronchoscopies, her tidal volumes gradually increased to 6–7 mL/kg, and she tolerated weaning of ECMO support. She was successfully decannulated after 20 days of ECMO support with no detectable complications.
Centralization of ECMO and the Effect of Center Volume
The relationship between ECMO volume and the quality of ECMO care delivered at individual centers are active topics of discussion and study. There are theoretical benefits of centralization of this highly complex care. ECMO is relatively rare, even in the largest centers, and centralization of care allows for a concentration of expertise. ECMO requires a team approach between ECMO technicians, bedside nurses, respiratory therapists, surgeons, intensivists, and other medical personnel. All members of the team must have an understanding of the special needs of a patient on ECMO. Centers that perform a high volume of ECMO develop an institutional knowledge that can only develop over many years and many ECMO runs.
There have been multiple studies done to evaluate if center volumes affect outcomes. Children treated at sites with 20–49 ECMO runs per year and greater than 50 ECMO runs per year (pediatric and neonatal combined) have been shown to have lower odds of mortality compared to centers with less than 20 runs per year [110]. The single cut-point that produced the most significant difference in mortality was 22 cases per year [110]. In another study, there was a strong relationship between center volume and mortality for pediatric cardiac patients [111]. Lastly, when survival was assessed based on age-specific center ECMO volumes, neonatal and adult volumes were significantly associated with survival, and there was a nonsignificant trend toward improved survival in higher-volume centers for pediatric patients [112]. The effect of center volume is particularly difficult to answer concerning pediatric respiratory ECMO. A medium-to-large sized pediatric ECMO program may only have five respiratory cases per year, and few centers perform more than ten pediatric respiratory ECMO runs annually. A recent international position paper on ECMO for acute respiratory failure in adults argues for centralization of ECMO and a minimum of 20 ECMO cases per year, with 12 of them being for respiratory support [113]. This recommendation is based on the pediatric data discussed above and expert opinion.
In the CESAR trial, adult patients with severe ARDS were randomized to staying at community hospitals or being transferred to a center capable of ECMO [5]. Patients who were transferred had improved survival (63% vs. 47%), even though only 75% of transferred patients were cannulated for ECMO [5]. Therefore, early transfer from centers that do not provide ECMO can be recommended for patients with severe ARDS with high OI. Volume alone however does not ensure quality care; education, training, and ongoing quality improvement must be combined with clinical expertise. It is recommended that all personnel caring for patients on ECMO are familiar with the circuit function and potential complications [26]. Given the relative rarity of ECMO, particularly pediatric respiratory ECMO, hands-on clinical experience can be limited. Simulation is a tool that can increase familiarity to ECMO and its complications. Simulation has been shown to be a beneficial educational tool in ECMO [114, 115]. A simulation program for all personnel caring for ECMO patients should be pursued, especially at low-volume centers, to augment orientation and ongoing learning.
In summary, though the minimum number of cases needed to provide adequate ECMO care is not clear, center volume likely plays a role in the quality of ECMO care and outcomes. Furthermore, in pediatrics, there may be indication specific and age specific minimums since cardiac, neonatal, and pediatric respiratory ECMO have distinct differences that require different knowledge and skill sets. Centralization of ECMO is likely beneficial to patients.
Prolonged ECMO and Lung Transplantation
Long-Term ECMO
The average for duration of ECMO support for pediatric respiratory failure is slightly less than 13 days [1]. However, there are some patients who require ECMO longer for lung recovery. In a study of prolonged ECMO published from the ELSO database in 2012, pediatric respiratory ECMO runs lasted ≥21 days in 12% of cases [116]. ECMO continues to be employed for increasingly sicker and more complex patients, which has likely led to more prolonged ECMO courses [24]. The current rate of prolonged ECMO may therefore be higher than 12%.
Children on ECMO for more than 21 days for acute respiratory failure have significantly worse survival, 38%, compared to 61% for less than 14 days and 53% for 2–3 weeks [116] (Fig. 2.1). Survival in pediatric respiratory ECMO patients steadily decreases as length of ECMO increases from 5 days to 37 days, and there is a late steep drop in survival after approximately 48 days on ECMO [116]. From 1993 to 2007, there were no survivors of the nine patients supported on ECMO for 52 days or more in the ELSO database [116]. More recently, there have been many cases reported of long-term respiratory ECMO in both children and adults with good outcomes, including lung transplant or late lung recovery after up to 265 days of ECMO [116,117,118,119,120,121]. These reports are remarkable as, historically, it has been thought that if native lung function was not improving or restored at 2–4 weeks of ECMO support, the chance of recovery was remote [119]. As evidenced by these case reports of successful prolonged ECMO, there may be previously underappreciated regenerative capacity of the lungs. The paradigm for lung recovery may be more similar to recovery of kidney function after acute kidney injury, where the chances of recovery of native function decrease over time, but there is still potential for return to normal function in some patients even weeks to months after injury.
Survival of patients to discharge as a function of days on ECMO (n = 3160). (Reproduced with permission from Brogan et al. [116])
The decision of when lung recovery becomes remote is difficult. There is a paucity of data to help physicians prognosticate which patients on prolonged ECMO may recover. Despite the lack of many survivors after 8 weeks on ECMO, reports of successful outcomes beyond this time frame suggest there should be no absolute cutoff for length of ECMO duration in pediatric respiratory failure. It is therefore imperative to carefully assess patients on prolonged ECMO support in regard to proximate cause of respiratory failure, premorbid lung function, potential for lung recovery, presence of other organ dysfunctions, and the goals of the patient or decision-makers.
ECMO as a Bridge to Lung Transplant
Patients who are on prolonged ECMO without recovery of native function may be candidates for lung transplant. The evaluation process for lung transplantation should be considered for patients on ECMO for greater than 3 weeks without a trajectory toward improvement or early in the ECMO course for patients with known progressive lung diseases such as CF. While consensus guidelines state that ECLS is a relative contraindication to lung transplantation, 1.5% of lung transplant recipients are bridged to transplant on ECMO [122, 123]. Patients bridged to lung transplant on ECMO may have worse short-term survival, but adult lung transplant recipients who are spontaneously breathing on ECMO have similar 3-year survival to patients on no support and better survival than patients on mechanical ventilation alone or on ECMO with mechanical ventilation [123, 124].
Considerations on Prolonged ECMO
When a patient is supported on prolonged ECMO, other important considerations are rehabilitation and tracheostomy. Tracheostomy on ECMO is not common practice in pediatrics, with only 13% of pediatric centers reporting performing tracheostomies in these patients [90]. The placement of tracheostomy while on ECMO is more frequently performed in adults [90]. To our knowledge, the largest report of tracheostomy on ECMO at a pediatric institution contains nine patients ages 7–25 years [125]. Two of nine survived without lung transplantation, and four survived with lung transplantation. All patients had decreased sedation needs within 72 h. The biggest concern with performing tracheostomy on ECMO is bleeding due to anticoagulation. Practices for anticoagulation vary from holding of anticoagulation around the time of procedure to varying reductions in heparin dosing [90, 125]. In two adult case series of 168 ECMO patients in total, minor bleeding after tracheostomy was common (approximately 30%), while major bleeding occurred in only 2% and 8% of cases. No deaths were attributed to complications of tracheostomy [126, 127]. If tracheostomy is to be considered, the timing of tracheostomy remains a difficult decision. The average time on ECMO to tracheostomy in the pediatric study of nine patients was 10 days [125]. Older pediatric patients with longer than average expected ECMO durations or those on ECMO as a bridge to lung transplant are most likely to benefit. These patients may be able to tolerate decreases in sedation and actively participate in rehabilitation due to developmental stage. These patients are also the same subset of children that may benefit from extubation on ECMO. Tracheostomy is a higher risk than extubation due to bleeding complications but would be preferred when the ECMO circuit cannot support oxygenation without positive-pressure ventilation.
The other major consideration for patients on prolonged ECMO is rehabilitation. Patients on ECMO often have multiple risk factors for ICU-related neuromuscular weakness including corticosteroids, neuromuscular blockade, systemic inflammation, immobility, hyperglycemia, and multiorgan failure [128]. Physical therapy with mobilization is common on ECMO, but ambulation of patients on ECMO is rare [125, 129, 130]. Ambulation on ECMO is a difficult process but can be accomplished safely with a coordinated team approach and either extubation or tracheostomy to eliminate the precarious endotracheal tube.
Children on ECMO as a bridge to lung transplantation who participate in rehabilitation have shorter length of ventilation after transplant and shorter length of ICU stay compared to those who do not participate in rehabilitation [130]. Additionally, patients ambulated on ECMO have lower hospital costs than those who do not ambulate [131]. In a survey of 208 adult ECMO centers, major barriers to ambulation identified included concerns about hemodynamic instability, hypoxemia, and femoral cannulation [132]. In pediatrics, these barriers are present, but there is additional concern for cannula movement. Pediatric patients have a smaller margin for error in the location of cannula, especially when a bicaval cannula is being used for V-V-ECMO.
We suggest physical therapy in all patients on ECMO for acute respiratory failure if oxygenation and hemodynamics tolerate it. Patients who are likely to be on prolonged ECMO, if able to be extubated or has a tracheostomy in place, may benefit from more aggressive rehabilitation such as ambulation.
Termination of Extracorporeal Support
ECMO is a resource-intense, high-risk technological modality that can provide extended respiratory support for children with respiratory failure. Within this framework, ECMO serves as a bridge to a destination for each child it supports. Potential pathways include bridge to a diagnosis, bridge to recovery, and bridge to lung transplantation. Making diagnostic determinations and outlining criteria for lung transplantation are typically concrete decisions within the medical team’s grasp. Likewise, the development of a catastrophic complication (e.g., massive hemorrhagic stroke) on ECMO renders the decision to terminate ECMO support, in most instances, straightforward. But in the absence of such a complication, our ability to predict pulmonary recovery is fraught with difficulty and uncertainty. Much of this complexity is related to the tremendous amount of heterogeneity within our patient population in the pediatric ICU, including diagnosis, age, comorbidities, and many of the other factors discussed in the preceding sections. Decision-making around prolonged ECMO support is challenging due to these limitations, and the clinical team may find uncertainty as to whether pulmonary recovery is still possible or if continuing ECMO now reached the point of futility. A multidisciplinary discussion of prognosis and probability of lung recovery should be initiated by 3 weeks of support, when the chance of survival begins to decline [116]. Current recommendations in the ethics literature suggest a careful analysis for potentially inappropriate care ECMO when respiratory support reaches 2–3 months [133].
Importantly, the potential for non-recovery and the possibility of termination of ECMO support should be introduced and communicated to the child’s family during the initial informed consent process prior to commencement of ECMO. However, several issues make true informed consent difficult, if not sometimes impossible, during these conversations such as complex and unfamiliar technology, uncertainty regarding diagnosis and prognosis, limited time for discussion due to acuity of the clinical situation, and a sense of urgency conveyed by the medical team [134]. The specific necessity of withdrawal of ECMO support in cases of futility is recommended as a standard component of the consent form for ECMO [133]. Second, P-PREP [135] and Ped-RESCUER [136] are recently validated prediction models that help pediatric intensivists estimate the mortality risk for children with respiratory failure at the time of initiation of ECMO (Table 2.2). Both models consider patient diagnosis, several pre-ECMO variables, and accompanying comorbidities. While not perfect tools, P-PREP and Ped-RESCUERS can provide the clinical team guidance when counseling families regarding their child’s risk of dying on ECMO.
Conclusion
In summary, ECMO continues to be an available modality to support children with the most severe forms of hypoxemic and hypercarbic respiratory failure. Ideal candidates for ECMO are those patients with reversible lung injury or those whom could be considered for lung transplantation in the event of non-recovery. V-V ECMO is the ideal extracorporeal modality to support these patients if left ventricular function is not depressed. Single-site cannulation utilizing double-lumen ECMO catheters is becoming the standard technique by which to obtain vascular access for V-V ECMO. Once on ECMO, ideal strategies for mechanical ventilation include a moderate-to-high degree of PEEP to maintain alveolar recruitment while minimizing plateau pressures to avoid further lung injury. As clinical experience with ECMO for pediatric respiratory failure continues to increase, we will have a better understanding of the optimal means of using this technology as well as its limitations.
Take-Home Points
-
ECMO candidacy evaluation requires holistic approach to the patient, including evaluation of the cause of respiratory failure, the trajectory of disease, and comorbidities.
-
Venovenous ECMO support is the preferred configuration for pediatric respiratory failure when feasible.
-
The goal of mechanical ventilation on ECMO is to limit ventilator-induced injury; however, the best strategy is yet to be determined.
-
Prolonged ECMO for respiratory failure in children has an increased risk of mortality, with the risk consistently rising after the first few weeks on ECMO.
-
Center ECMO volumes may play a role in patient outcomes, but these are likely age- and disease-specific. Centers with low ECMO volumes should use other methods to maintain competency, such as simulation.
References
ECMO. Registry of the Extracorporeal Life Support Organization (ELSO). Ann Arbor: Extracorporeal Life Support Organization; 2017.
O’Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84(6):957–63.
UK Collaborative ECMO Trail Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348(9020):75–82.
Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev. 2008;3:CD001340.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63.
Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379–89.
Barbaro RP, Xu Y, Borasino S, Truemper EJ, Watson RS, Thiagarajan RR, et al. Does extracorporeal membrane oxygenation improve survival in pediatric acute respiratory failure? Am J Respir Crit Care Med. 2018;197:1177.
Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37(4):633–46.
Rimensberger PC, Cheifetz IM, Pediatric Acute Lung Injury Consensus Conference Group. Ventilatory support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S51–60.
Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001.
Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124(1):87–95.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–6.
Bhalla AK, Belani S, Leung D, Newth CJ, Khemani RG. Higher dead space is associated with increased mortality in critically ill children. Crit Care Med. 2015;43(11):2439–45.
Yehya N, Bhalla AK, Thomas NJ, Khemani RG. Alveolar dead space fraction discriminates mortality in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17(2):101–9.
Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S23–40.
Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–11.
Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317–23.
Khemani RG, Conti D, Alonzo TA, Bart RD 3rd, Newth CJ. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med. 2009;35(8):1428–37.
Raj SSJ, Rigby M. Factors associated with survival during high-frequency oscillatory ventilation in children. J Pediatr Intensive Care. 2015;4(3):146–55.
Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–37.
Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470–6.
Zinter MS, Orwoll BE, Spicer AC, Alkhouli MF, Calfee CS, Matthay MA, et al. Incorporating inflammation into mortality risk in pediatric acute respiratory distress syndrome. Crit Care Med. 2017;45(5):858–66.
Domico MB, Ridout DA, Bronicki R, Anas NG, Cleary JP, Cappon J, et al. The impact of mechanical ventilation time before initiation of extracorporeal life support on survival in pediatric respiratory failure: a review of the Extracorporeal Life Support Registry. Pediatr Crit Care Med. 2012;13(1):16–21.
Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Crit Care Med. 2011;39(2):364–70.
General Guidelines for all ECLS Cases. Extracorporeal Life Support Organization. 2013;1.3:24.
Dalton HJ, Macrae DJ, Pediatric Acute Lung Injury Consensus Conference Group. Extracorporeal support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S111–7.
Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402–14.
Santschi M, Randolph AG, Rimensberger PC, Jouvet P, Pediatric Acute Lung Injury Mechanical Ventilation I, Pediatric Acute Lung I, et al. Mechanical ventilation strategies in children with acute lung injury: a survey on stated practice pattern. Pediatr Crit Care Med. 2013;14(7):e332–7.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Barnes T, Zochios V, Parhar K. Re-examining permissive hypercapnia in ARDS: a narrative review. Chest. 2017;154(1):185–95.
Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(4):456–63.
Hebbar KB, Petrillo-Albarano T, Coto-Puckett W, Heard M, Rycus PT, Fortenberry JD. Experience with use of extracorporeal life support for severe refractory status asthmaticus in children. Crit Care. 2009;13(2):R29.
Hayes D Jr, Kopp BT, Preston TJ, Kirkby S, Tobias JD, Papadimos TJ, et al. Survival of patients with cystic fibrosis on ECMO: analysis of the Extracorporeal Life Support Organization Registry. Int J Clin Exp Med. 2014;7(5):1370–2.
Daoud O, Augustin P, Mordant P, Lasocki S, Al-Attar N, Maury JM, et al. Extracorporeal membrane oxygenation in 5 patients with bronchial fistula with severe acute lung injury. Ann Thorac Surg. 2011;92(1):327–30.
Dolgner A, Bain J, Peterson-Carmichael SL, Turner DA, Rehder KJ. Extracorporeal membrane oxygenation for refractory air leak in a child presenting with bacterial tracheitis. Respir Care. 2014;59(10):e163–5.
Raake J, Johnson B, Seger B, Manning PB, Eghtesady P, Boesch P, et al. Extracorporeal membrane oxygenation, extubation, and lung-recruitment maneuvers as rescue therapy in a patient with tracheal dehiscence following slide tracheoplasty. Respir Care. 2011;56(8):1198–202.
Hoetzenecker K, Klepetko W, Keshavjee S, Cypel M. Extracorporeal support in airway surgery. J Thorac Dis. 2017;9(7):2108–17.
Sapru A, Flori H, Quasney MW, Dahmer MK, Pediatric Acute Lung Injury Consensus Conference Group. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S6–22.
Askegard-Giesmann JR, Besner GE, Fabia R, Caniano DA, Preston T, Kenney BD. Extracorporeal membrane oxygenation as a lifesaving modality in the treatment of pediatric patients with burns and respiratory failure. J Pediatr Surg. 2010;45(6):1330–5.
Skarda D, Henricksen JW, Rollins M. Extracorporeal membrane oxygenation promotes survival in children with trauma related respiratory failure. Pediatr Surg Int. 2012;28(7):711–4.
Fortenberry JD, Meier AH, Pettignano R, Heard M, Chambliss CR, Wulkan M. Extracorporeal life support for posttraumatic acute respiratory distress syndrome at a children’s medical center. J Pediatr Surg. 2003;38(8):1221–6.
Scalzo AJ, Weber TR, Jaeger RW, Connors RH, Thompson MW. Extracorporeal membrane oxygenation for hydrocarbon aspiration. Am J Dis Child. 1990;144(8):867–71.
Kimura D, Shah S, Briceno-Medina M, Sathanandam S, Haberman B, Zhang J, et al. Management of massive diffuse alveolar hemorrhage in a child with systemic lupus erythematosus. J Intensive Care. 2015;3:10.
Zulian F, Martinez Toledo MM, Amigoni A, Martini G, Agosto C, Pettenazzo A. Successful use of extracorporeal membrane oxygenation for severe interstitial lung disease in a child with dermatomyositis. Intensive Care Med. 2007;33(9):1663–6.
Lopez-Fernandez Y, Azagra AM, de la Oliva P, Modesto V, Sanchez JI, Parrilla J, et al. Pediatric acute lung injury epidemiology and natural history study: incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40(12):3238–45.
Halasa NB, Barr FE, Johnson JE, Edwards KM. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics. 2003;112(6 Pt 1):1274–8.
Sawal M, Cohen M, Irazuzta JE, Kumar R, Kirton C, Brundler MA, et al. Fulminant pertussis: a multi-center study with new insights into the clinico-pathological mechanisms. Pediatr Pulmonol. 2009;44(10):970–80.
Surridge J, Segedin ER, Grant CC. Pertussis requiring intensive care. Arch Dis Child. 2007;92(11):970–5.
Rowlands HE, Goldman AP, Harrington K, Karimova A, Brierley J, Cross N, et al. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics. 2010;126(4):e816–27.
Stewart DL, Cook LN, Rabalais GP. Successful use of extracorporeal membrane oxygenation in a newborn with herpes simplex virus pneumonia. Pediatr Infect Dis J. 1993;12(2):161–2.
Bonacchi M, Di Lascio G, Harmelin G, Pasquini A, Peris A, Sani G. Extracorporeal membrane oxygenation for refractory, life-threatening, and herpes simplex virus 1-induced acute respiratory distress syndrome. Our experience and literature review. Am J Emerg Med. 2012;30(6):1014.e3–e10.
Prodhan P, Wilkes R, Ross A, Garcia X, Bhutta AT, Rycus P, et al. Neonatal herpes virus infection and extracorporeal life support. Pediatr Crit Care Med. 2010;11(5):599–602.
Pluim T, Halasa N, Phillips SE, Fleming G. The morbidity and mortality of patients with fungal infections before and during extracorporeal membrane oxygenation support. Pediatr Crit Care Med. 2012;13(5):e288–93.
Creech CB, Johnson BG, Bartilson RE, Yang E, Barr FE. Increasing use of extracorporeal life support in methicillin-resistant Staphylococcus aureus sepsis in children. Pediatr Crit Care Med. 2007;8(3):231–5; quiz 47.
Bridges BC, Hardison D, Pietsch J. A case series of the successful use of ECMO, continuous renal replacement therapy, and plasma exchange for thrombocytopenia-associated multiple organ failure. J Pediatr Surg. 2013;48(5):1114–7.
Kawai Y, Cornell TT, Cooley EG, Beckman CN, Baldridge PK, Mottes TA, et al. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med. 2015;16(4):366–74.
Moler FW, Palmisano J, Custer JR. Extracorporeal life support for pediatric respiratory failure: predictors of survival from 220 patients. Crit Care Med. 1993;21(10):1604–11.
Pranikoff T, Hirschl RB, Steimle CN, Anderson HL 3rd, Bartlett RH. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med. 1997;25(1):28–32.
Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–95.
Nance ML, Nadkarni VM, Hedrick HL, Cullen JA, Wiebe DJ. Effect of preextracorporeal membrane oxygenation ventilation days and age on extracorporeal membrane oxygenation survival in critically ill children. J Pediatr Surg. 2009;44(8):1606–10.
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–68.
Fleming GM, Sahay R, Zappitelli M, King E, Askenazi DJ, Bridges BC, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med. 2016;17(12):1157–69.
Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. 2017;18(12):1126–35.
Gow KW, Heiss KF, Wulkan ML, Katzenstein HM, Rosenberg ES, Heard ML, et al. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: the extracorporeal life support experience. Crit Care Med. 2009;37(4):1308–16.
Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. 2003;31(5):1299–305.
Lamas A, Otheo E, Ros P, Vazquez JL, Maldonado MS, Munoz A, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. 2003;29(1):91–6.
Keenan HT, Bratton SL, Martin LD, Crawford SW, Weiss NS. Outcome of children who require mechanical ventilatory support after bone marrow transplantation. Crit Care Med. 2000;28(3):830–5.
Rowan CM, Loomis A, McArthur J, Smith LS, Gertz SJ, Fitzgerald JC, et al. High-frequency oscillatory ventilation use and severe pediatric ARDS in the pediatric hematopoietic cell transplant recipient. Respir Care. 2017;63:404.
Morris SH, Haight AE, Kamat P, Fortenberry JD. Successful use of extracorporeal life support in a hematopoietic stem cell transplant patient with diffuse alveolar hemorrhage. Pediatr Crit Care Med. 2010;11(1):e4–7.
Liao WI, Tsai SH, Chiu SK. Successful use of extracorporeal membrane oxygenation in a hematopoietic stem cell transplant patient with idiopathic pneumonia syndrome. Respir Care. 2013;58(2):e6–10.
Wolfson RK, Kahana MD, Nachman JB, Lantos J. Extracorporeal membrane oxygenation after stem cell transplant: clinical decision-making in the absence of evidence. Pediatr Crit Care Med. 2005;6(2):200–3.
Gow KW, Wulkan ML, Heiss KF, Haight AE, Heard ML, Rycus P, et al. Extracorporeal membrane oxygenation for support of children after hematopoietic stem cell transplantation: the Extracorporeal Life Support Organization experience. J Pediatr Surg. 2006;41(4):662–7.
Di Nardo M, Locatelli F, Palmer K, Amodeo A, Lorusso R, Belliato M, et al. Extracorporeal membrane oxygenation in pediatric recipients of hematopoietic stem cell transplantation: an updated analysis of the Extracorporeal Life Support Organization experience. Intensive Care Med. 2014;40(5):754–6.
Brogan TVL, Lequier L, Lorusso R, MacLaren G, Peek G. Extracorporeal life support: the ELSO red book. 5th ed. Ann Arbor: Extracorporeal Life Support Organization; 2017.
Teele SA, Salvin JW, Barrett CS, Rycus PT, Fynn-Thompson F, Laussen PC, et al. The association of carotid artery cannulation and neurologic injury in pediatric patients supported with venoarterial extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2014;15(4):355–61.
Rollins MD, Hubbard A, Zabrocki L, Barnhart DC, Bratton SL. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg. 2012;47(1):68–75.
Pettignano R, Fortenberry JD, Heard ML, Labuz MD, Kesser KC, Tanner AJ, et al. Primary use of the venovenous approach for extracorporeal membrane oxygenation in pediatric acute respiratory failure. Pediatr Crit Care Med. 2003;4(3):291–8.
Skinner SC, Iocono JA, Ballard HO, Turner MD, Ward AN, Davenport DL, et al. Improved survival in venovenous vs venoarterial extracorporeal membrane oxygenation for pediatric noncardiac sepsis patients: a study of the Extracorporeal Life Support Organization registry. J Pediatr Surg. 2012;47(1):63–7.
Rehder KJ, Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for neonatal and pediatric respiratory failure: an evidence-based review of the past decade (2002–2012). Pediatr Crit Care Med. 2013;14(9):851–61.
Zamora IJ, Shekerdemian L, Fallon SC, Olutoye OO, Cass DL, Rycus PL, et al. Outcomes comparing dual-lumen to multisite venovenous ECMO in the pediatric population: the Extracorporeal Life Support Registry experience. J Pediatr Surg. 2014;49(10):1452–7.
Subramanian S, Vafaeezadeh M, Parrish AR, McMullan DM. Comparison of wire-reinforced and non-wire-reinforced dual-lumen catheters for venovenous ECMO in neonates and infants. ASAIO J. 2013;59(1):81–5.
Javidfar J, Brodie D, Wang D, Ibrahimiye AN, Yang J, Zwischenberger JB, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91(6):1763–8; discussion 9.
Teman NR, Haft JW, Napolitano LM. Optimal endovascular methods for placement of bicaval dual-lumen cannulae for venovenous extracorporeal membrane oxygenation. ASAIO J. 2013;59(4):442–7.
Fallon SC, Shekerdemian LS, Olutoye OO, Cass DL, Zamora IJ, Nguyen T, et al. Initial experience with single-vessel cannulation for venovenous extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Crit Care Med. 2013;14(4):366–73.
Lazar DA, Cass DL, Olutoye OO, Kim ES, Welty SE, Fernandes CJ, et al. Venovenous cannulation for extracorporeal membrane oxygenation using a bicaval dual-lumen catheter in neonates. J Pediatr Surg. 2012;47(2):430–4.
Jarboe MD, Gadepalli SK, Church JT, Arnold MA, Hirschl RB, Mychaliska GB. Avalon catheters in pediatric patients requiring ECMO: placement and migration problems. J Pediatr Surg. 2018;53(1):159–62.
Conrad SA, Grier LR, Scott LK, Green R, Jordan M. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med. 2015;43(5):1010–5.
Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11(6):956–61.
Marhong JD, Munshi L, Detsky M, Telesnicki T, Fan E. Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensive Care Med. 2015;41(6):994–1003.
Jenks CL, Tweed J, Gigli KH, Venkataraman R, Raman L. An international survey on ventilator practices among extracorporeal membrane oxygenation centers. ASAIO J. 2017;63(6):787–92.
Camporota L, Nicoletti E, Malafronte M, De Neef M, Mongelli V, Calderazzo MA, et al. International survey on the management of mechanical ventilation during ECMO in adults with severe respiratory failure. Minerva Anestesiol. 2015;81(11):1170–83, 77 p following 83.
Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, et al. Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
Keszler M, Ryckman FC, McDonald JV Jr, Sweet LD, Moront MG, Boegli MJ, et al. A prospective, multicenter, randomized study of high versus low positive end-expiratory pressure during extracorporeal membrane oxygenation. J Pediatr. 1992;120(1):107–13.
Keszler M, Subramanian KN, Smith YA, Dhanireddy R, Mehta N, Molina B, et al. Pulmonary management during extracorporeal membrane oxygenation. Crit Care Med. 1989;17(6):495–500.
Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med. 2015;43(3):654–64.
Modrykamien AM, Hernandez OO, Im Y, Walters RW, Schrader CL, Smith LE, et al. Mechanical ventilation in patients with the acute respiratory distress syndrome and treated with extracorporeal membrane oxygenation: impact on hospital and 30 day postdischarge survival. ASAIO J. 2016;62(5):607–12.
Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41(4):1046–55.
Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004;32(1):168–74.
Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174(3):279–89.
Franchineau G, Brechot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet JL, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(4):447–57.
Chiu LC, Hu HC, Hung CY, Chang CH, Tsai FC, Yang CT, et al. Dynamic driving pressure associated mortality in acute respiratory distress syndrome with extracorporeal membrane oxygenation. Ann Intensive Care. 2017;7(1):12.
Serpa Neto A, Schmidt M, Azevedo LC, Bein T, Brochard L, Beutel G, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis : mechanical ventilation during ECMO. Intensive Care Med. 2016;42(11):1672–84.
de Jager P, Burgerhof JG, van Heerde M, Albers MJ, Markhorst DG, Kneyber MC. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies. Crit Care Med. 2014;42(12):2461–72.
Kamat PP, Popler J, Davis J, Leong T, Piland SC, Simon D, et al. Use of flexible bronchoscopy in pediatric patients receiving extracorporeal membrane oxygenation (ECMO) support. Pediatr Pulmonol. 2011;46(11):1108–13.
Prentice E, Mastropietro CW. Flexible bronchoscopy for children on extracorporeal membrane oxygenation for cardiac failure. Pediatr Crit Care Med. 2011;12(4):422–5.
Yehya N, Dominick CL, Connelly JT, Davis DH, Minneci PC, Deans KJ, et al. High-frequency percussive ventilation and bronchoscopy during extracorporeal life support in children. ASAIO J. 2014;60(4):424–8.
Karlson KH Jr, Pickert CB, Schexnayder SM, Heulitt MJ. Flexible fiberoptic bronchoscopy in children on extracorporeal membrane oxygenation. Pediatr Pulmonol. 1993;16(4):215–8.
Lucangelo U, Fontanesi L, Antonaglia V, Pellis T, Berlot G, Liguori G, et al. High frequency percussive ventilation (HFPV). Principles and technique. Minerva Anestesiol. 2003;69(11):841–8, 8–51.
Bataillard A, Hebrard A, Gaide-Chevronnay L, Martin C, Durand M, Albaladejo P, et al. Extubation in patients undergoing extracorporeal life support. Int J Artif Organs. 2017;40(12):696–700.
Freeman CL, Bennett TD, Casper TC, Larsen GY, Hubbard A, Wilkes J, et al. Pediatric and neonatal extracorporeal membrane oxygenation: does center volume impact mortality? Crit Care Med. 2014;42(3):512–9.
Karamlou T, Vafaeezadeh M, Parrish AM, Cohen GA, Welke KF, Permut L, et al. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145(2):470–5.
Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901.
Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–96.
Chan SY, Figueroa M, Spentzas T, Powell A, Holloway R, Shah S. Prospective assessment of novice learners in a simulation-based extracorporeal membrane oxygenation (ECMO) education program. Pediatr Cardiol. 2013;34(3):543–52.
Zakhary BM, Kam LM, Kaufman BS, Felner KJ. The utility of high-fidelity simulation for training critical care fellows in the management of extracorporeal membrane oxygenation emergencies: a randomized controlled trial. Crit Care Med. 2017;45(8):1367–73.
Brogan TV, Zabrocki L, Thiagarajan RR, Rycus PT, Bratton SL. Prolonged extracorporeal membrane oxygenation for children with respiratory failure. Pediatr Crit Care Med. 2012;13(4):e249–54.
Wiktor AJ, Haft JW, Bartlett RH, Park PK, Raghavendran K, Napolitano LM. Prolonged VV ECMO (265 days) for ARDS without technical complications. ASAIO J. 2015;61(2):205–6.
Moon SM, Lee H, Moon JH, Kim HK, Park JE, Byeon S, et al. Prolonged maintenance of VV ECMO for 104 days with native lung recovery in acute respiratory failure. ASAIO J. 2016;62(2):e15–7.
Rosenberg AA, Haft JW, Bartlett R, Iwashyna TJ, Huang SK, Lynch WR, et al. Prolonged duration ECMO for ARDS: futility, native lung recovery, or transplantation? ASAIO J. 2013;59(6):642–50.
Iacono A, Groves S, Garcia J, Griffith B. Lung transplantation following 107 days of extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2010;37(4):969–71.
Gupta P, McDonald R, Chipman CW, Stroud M, Gossett JM, Imamura M, et al. 20-year experience of prolonged extracorporeal membrane oxygenation in critically ill children with cardiac or pulmonary failure. Ann Thorac Surg. 2012;93(5):1584–90.
Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014 – an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15.
Schechter MA, Ganapathi AM, Englum BR, Speicher PJ, Daneshmand MA, Davis RD, et al. Spontaneously breathing extracorporeal membrane oxygenation support provides the optimal bridge to lung transplantation. Transplantation. 2016;100(12):2699–704.
Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145(4):1065–70; discussion 70–1.
Schwartz SP, Bonadonna D, Hartwig MG, Cheifetz IM. Bedside tracheostomy on pediatric ICU subjects supported by extracorporeal membrane oxygenation. Respir Care. 2017;62:1447.
Kruit N, Valchanov K, Blaudszun G, Fowles JA, Vuylsteke A. Bleeding complications associated with percutaneous tracheostomy insertion in patients supported with venovenous extracorporeal membrane oxygen support: a 10-year institutional experience. J Cardiothorac Vasc Anesth. 2017;32:1162.
Braune S, Kienast S, Hadem J, Wiesner O, Wichmann D, Nierhaus A, et al. Safety of percutaneous dilatational tracheostomy in patients on extracorporeal lung support. Intensive Care Med. 2013;39(10):1792–9.
Zorowitz RD. ICU-acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest. 2016;150(4):966–71.
Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18(1):R38.
Turner DA, Rehder KJ, Bonadonna D, Gray A, Lin S, Zaas D, et al. Ambulatory ECMO as a bridge to lung transplant in a previously well pediatric patient with ARDS. Pediatrics. 2014;134(2):e583–5.
Bain JC, Turner DA, Rehder KJ, Eisenstein EL, Davis RD, Cheifetz IM, et al. Economic outcomes of extracorporeal membrane oxygenation with and without ambulation as a bridge to lung transplantation. Respir Care. 2016;61(1):1–7.
Marhong JD, DeBacker J, Viau-Lapointe J, Munshi L, Del Sorbo L, Burry L, et al. Sedation and mobilization during venovenous extracorporeal membrane oxygenation for acute respiratory failure: an international survey. Crit Care Med. 2017;45(11):1893–9.
Ramanathan K, Cove ME, Caleb MG, Teoh KL, Maclaren G. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth. 2015;29(1):229–33.
Peetz AB, Sadovnikoff N, O’Connor MF. Is informed consent for extracorporeal life support even possible? AMA J Ethics. 2015;17(3):236–42.
Bailly DK, Reeder RW, Zabrocki LA, Hubbard AM, Wilkes J, Bratton SL, et al. Development and validation of a score to predict mortality in children undergoing extracorporeal membrane oxygenation for respiratory failure: pediatric pulmonary rescue with extracorporeal membrane oxygenation prediction score. Crit Care Med. 2017;45(1):e58–66.
Barbaro RP, Boonstra PS, Paden ML, Roberts LA, Annich GM, Bartlett RH, et al. Development and validation of the pediatric risk estimate score for children using extracorporeal respiratory support (Ped-RESCUERS). Intensive Care Med. 2016;42(5):879–88.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Friedman, M., Hobson, M. (2019). Extracorporeal Membrane Oxygenation for Acute Pediatric Respiratory Failure. In: Mastropietro, C., Valentine, K. (eds) Pediatric Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-319-96499-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-96499-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96498-0
Online ISBN: 978-3-319-96499-7
eBook Packages: MedicineMedicine (R0)