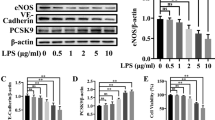
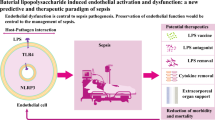
Abstract
In sepsis, endothelial dysfunction is a crucial driver known to limit the survival rate of affected patients. For this, ROS-mediated signaling plays an important role in endothelial communication and functionality. In the management of sepsis, polyunsaturated fatty acids (PUFA) have received increasing attention regarding their anti-inflammatory potential neglecting the oxidative properties of these substances. Therefore, in the present study we examined the capacity of PUFA to interfere with the expression of major ROS-producing enzymes, as well as endothelial ROS production itself. The human microvascular endothelial cells TIME (ATCC number: CRL-4025) were used. Cells were cultured in medium enriched with LNA (C18:3n3), EPA (C20:5n3), DHA (C22:6n3), LA (C18:2n6), or AA (C20:4n6) in concentrations of 15 μM totaling 144 h. Stimulation of cells was performed in the last 24 h of fatty acid supplementation by addition of the cytokines TNF-α + IL-1β + IFN-γ (5 ng/ml each). Gene expression of eNOS, COX-2, and NOX-4 was evaluated by qPCR. ROS synthesis was analyzed by means of a flow cytometry-based rhodamine 123 assay. Cytokine stimulation was found to differentially affect gene expression of major ROS synthesizing enzymes: eNOS was decreased whereas COX-2 and NOX-4 were increased. As a consequence, cytokine stimulation had no effect on rhodamine accumulation in endothelial cells. PUFA supplementation alone did not affect the gene expression of eNOS, COX-2, and NOX-4. Nevertheless, an increasing action of PUFA on the stimulation-induced reduction in eNOS expression was found. More importantly, the number of rhodamine positive endothelial cells almost doubled following enrichment with the PUFA EPA, DHA or AA. This effect was independent of the stimulation status of the cells but seemed to be related to the number of double bonds of a supplemented fatty acid. Our data warrant further studies to ensure that increased endothelial cell oxidative stress is not boosted by PUFA in septic patients.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aird WC (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101(10):3765–3777. https://doi.org/10.1182/blood-2002-06-1887
Cepinskas G, Wilson JX (2008) Inflammatory response in microvascular endothelium in sepsis: role of oxidants. J Clin Biochem Nutr 42(3):175–184
Calder PC (2008) The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids 79(3–5):101–108. https://doi.org/10.1016/j.plefa.2008.09.016
Wanten GJA, Calder PC (2007) Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 85(5):1171–1184
Cao S, Ren J, Sun L et al (2011) Fish oil-supplemented parenteral nutrition prolongs survival while beneficially altering phospholipids’ fatty acid composition and modulating immune function in rat sepsis. Shock 36(2):184–190. https://doi.org/10.1097/SHK.0b013e31821e4f8b
Drummond GR, Sobey CG (2014) Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25(9):452–463. https://doi.org/10.1016/j.tem.2014.06.012
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510(7503):92–101. https://doi.org/10.1038/nature13479
Acknowledgments
This work was supported by the Wilhelm-Roux-Program, Martin Luther University Halle-Wittenberg. We thank Prof. Dr. Barbara Seliger from the Institute of Medical Immunology, Martin Luther University Halle-Wittenberg for providing access to the FacsCalibur.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Trommer, S., Leimert, A., Bucher, M., Schumann, J. (2018). Polyunsaturated Fatty Acids Induce ROS Synthesis in Microvascular Endothelial Cells. In: Thews, O., LaManna, J., Harrison, D. (eds) Oxygen Transport to Tissue XL. Advances in Experimental Medicine and Biology, vol 1072. Springer, Cham. https://doi.org/10.1007/978-3-319-91287-5_63
Download citation
DOI: https://doi.org/10.1007/978-3-319-91287-5_63
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91285-1
Online ISBN: 978-3-319-91287-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)