Abstract
Lung transplantation has become a viable treatment option for end-stage lung disease. Common indications for lung transplantation are chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, cystic fibrosis, alpha-1 antitrypsin deficiency, and pulmonary arterial hypertension. Either single or bilateral lung transplantation can be performed, but bilateral lung recipients appear to have a better median survival than single lung recipients. Complications after lung transplantation are common and may have nonspecific clinical and radiologic manifestations. The time point at which these complications occur relative to the date of transplant is crucial in formulating a differential diagnosis and recognizing them accurately. Significant advances in imaging techniques and recognition of air trapping in exhalation images and other patterns /distribution of parenchymal abnormalities have led to routine use of HRCT for diagnostic evaluation in patients manifesting respiratory decline in the lung transplant recipient.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Lung transplantation is a viable treatment option for end-stage lung disease. Common indications for lung transplantation are chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, cystic fibrosis, alpha-1 antitrypsin deficiency, and pulmonary arterial hypertension (PAH). Other less common indications include end-stage sarcoidosis, lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis/granulomatosis (PLCH/G), and other interstitial lung diseases. Either single or bilateral lung transplantation can be performed. Bilateral transplantation is required for patients with suppurative lung diseases including cystic fibrosis and most patients with PAH. While suitable outcomes may be achieved with single lung transplantation for other diagnoses, there is a trend in recent years for use of bilateral transplantation, largely related to a suggestion of better long-term survival seen in registry data [1]. Specifically, according to the 2014 International Society for Heart and Lung Transplantation (ISHLT) registry report, the median survival for all adult recipients is 5.7 years, but bilateral lung recipients appear to have a better median survival than single lung recipients (7 versus 4.5 years, respectively) [1]. Overall survival after lung transplantation has improved due to refinement in surgical technique, improvement in organ procurement and preserving techniques, and advances in medical management and immunosuppression. The most common cause of mortality in first 30 days after lung transplantation is primary graft dysfunction. Infection is the most common cause of mortality in the first 6 months and chronic rejection thereafter [1].
Complications after lung transplantation are common and may have nonspecific clinical and radiologic manifestations. In addition, frequently, several of these complications may coexist and complicate their presentation. In order to reduce mortality and morbidity among lung transplant recipients, it is important to recognize these complications accurately and in time. The time point at which these complications occur relative to the date of transplant is crucial in formulating a differential diagnosis and recognizing them accurately.
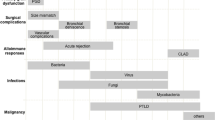
In this chapter, we will describe the radiologic manifestations of various pulmonary complications occurring after lung transplantation. Complications are classified chronologically into groups based on the time from transplant: immediate (<24 h), early (24 h to 1 week), intermediate (8 days to 2 months), primary late (2–4 months), and secondary late (≥4 months) (Table 19.1).
Immediate Complications (<24 h)
Donor-Recipient Size Mismatch
Size mismatch between the donor lung and recipient thoracic cavity may result in mechanical complications. Most centers accept a size difference ranging from 10 to 25% [2]. If the donor lung is too large for recipient thoracic cavity, atelectasis and impaired ventilation may result. Atelectasis may be evident on immediate postoperative radiograph. On the other hand, when a small allograft lung is used in single lung transplantation for emphysema, the hyperexpanded native lung may compress the allograft, resulting in restrictive pulmonary function (Fig. 19.1a, b). Native lung volume reduction surgery performed at the time of, or after, transplantation may help prevent this complication [3].
(a, b) A 73-year-old woman status post left lung transplant for severe COPD. CT chest obtained 11 years after transplantation (a) showed hyperinflation of native right lung herniating to left hemithorax. The patient had restrictive pulmonary impairment and developed squamous cell lung cancer in the right lower lobe (not shown), for which she underwent right lower lobectomy. Post lobectomy CT (b) showed an improvement in hyperinflation of the right lung
Lung torsion is a rare but serious complication that can occur due to discrepancy in size between the recipient’s enlarged thoracic cavity and donor’s small lung. Imaging will show torsion of the hilar structures including vasculature and airway. It can lead to collapse of a lobe or entire lung due to airway compromise or expansible consolidated lobe due to hemorrhagic infarction . Once lung torsion is suspected, immediate surgery is indicated to avoid death from parenchymal necrosis.
Hyperacute Rejection
Hyperacute rejection is caused by the presence of preformed antibodies to donor organ-specific human leukocyte antigen (HLA) or ABO antigen. ABO matching and improved antibody detection and crossmatch techniques have made hyperacute rejection very rare after lung transplantation. When present, it can lead to acute alveolar damage resulting in graft dysfunction within minutes to hours after graft reperfusion [4]. On chest radiograph, hyperacute rejection manifests as diffuse homogeneous opacification of the entire allograft. Management options include aggressive immunosuppression, plasmapheresis, or urgent retransplantation [4].
Early Complications (24 h to 1 Week)
Primary Graft Dysfunction (Ischemic Reperfusion Injury or Reperfusion Edema)
Primary graft dysfunction is a form of non-cardiogenic pulmonary edema that usually occurs by postoperative day 1, peaks in severity by day 4, and generally improves by day 7. It is diagnosed after excluding left heart failure, fluid overload, infection, and acute transplant rejection. The pathogenesis of primary graft dysfunction is multifactorial including interruption of lymphatic and bronchial circulation, donor organ ischemia, surgical trauma, denervation of donor lung, and decreased surfactant production [5].
Chest radiography and CT manifestations are nonspecific and include perihilar and lower lobe predominant ground-glass opacity, and/or consolidation, interlobular septal thickening, and peribronchial air-space opacities (Figs. 19.2 and 19.3).
Pleural Complications
Pleural complications —including pleural effusion, hemothorax, pneumothorax, and empyema—occur in 22–34% of patients after transplantation [6, 7]. Existing pleural abnormalities before lung transplantation can predispose to pleural complications. For instance, pleural infection or inflammation can result from end-stage lung diseases for which lung transplantation is required such as cystic fibrosis. Invasive procedures required to diagnose (wedge biopsy) or treat (pleurodesis or pleural drain for pneumothorax or pleural effusion) end-stage lung disease may result in pleural abnormalities. Pneumothorax is the most common pleural complication [7] (Fig. 19.4a, b). Airway anastomotic complications should be suspected if pneumothorax persists or enlarges after the early postoperative period (>7 days after transplantation). Pleural effusion is also a common complication secondary to increased capillary permeability and impaired lymphatic drainage of the allograft lung during the early postoperative period (Fig. 19.5a, b). Pleural fluid in the immediate postoperative period is often hemorrhagic and becomes progressively less hemorrhagic by 7 days. Pleural effusion usually resolves by 2 weeks. Persistent or enlarging pleural effusion should raise suspicion for empyema or left heart failure. Hemothorax usually results from surgical complications (Fig. 19.6a, b). Persistent air leak, empyema, and hemothorax are associated with increased mortality and should be promptly recognized and managed [7, 8].
(a, b) A 69-year-old man with single orthotropic lung transplant (SOLT) in 2015 for IPF. CT obtained 4 days after transplantation shows pneumomediastinum (black arrows in a), pneumothorax (curved arrow in b), pneumopericardium (arrowheads in b), and subcutaneous emphysema (white arrow in a). The bronchial anastomosis was intact. Right chest tube is in place. Note fibrosis in native left lung
(a, b) A 33-year-old woman with BOLT in 2015 for pulmonary arterial hypertension. CT obtained 2 days after transplantation shows high density material in right pleural space (arrowheads in a and b), consistent with hemothorax. Consolidation in both lower lobes was presumably due to aspiration (arrows)
Intermediate Complications (8 Days to 2 Months) and Primary Late Complications (2–4 Months)
Acute Rejection
Acute rejection usually occurs after the second postoperative week and is classically caused by cell-mediated immune response. Approximately 80% of acute rejections occur in first 3 months, and 50% of patients have at least one episode of acute rejection in the first postoperative year [9, 10]. Repeated episodes of acute rejection are considered a risk factor for the development of chronic rejection [9, 11].
When present, radiographic manifestations are nonspecific and similar to primary graft dysfunction including ground-glass opacities, consolidation, interlobular septal thickening, and pleural effusion (Figs. 19.7a, b, 19.8, and 19.9a, b). Radiographic improvement within 48 h of administration of intravenous corticosteroids favors the diagnosis of acute rejection [9].
A 69-year-old woman status post left lung transplant for chronic hypersensitivity pneumonitis. CT obtained 4 months after transplantation showed patchy ground-glass opacities in the allograft. Although a nonspecific pattern, the transbronchial biopsies revealed A2B0 acute cellular rejection. Note fibrosis in the native right lung
Airway Anastomotic Complications
Airway anastomotic complications include bronchial dehiscence, bronchial stenosis, or bronchomalacia. Reported incidence of airway complications is approximately 15%, but there has been a decrease in the airway complication rate due to the refinement in surgical technique, improvement in immunosuppression, and donor organ preservation technique [12]. The most important factor predisposing to airway complication is donor bronchus ischemia, due to disruption of the native bronchial circulation. Recurrent infection and rejection are some other contributing factors.
Bronchial dehiscence usually occurs in the first 2–4 weeks after transplantation affecting 2–3% patients [9]. CT usually shows extraluminal foci of air in perianastomotic region, and occasionally bronchial defects can be visualized (Figs. 19.10a–c, 19.11a, b, and 19.12a, b). Indirect signs include persistent or enlarging air leak, pneumothorax, or pneumomediastinum. Bronchial dehiscence may resolve spontaneously or progress to bronchial stenosis.
(a–c) A 57-year-old woman with BOLT in 2015 for alpha-1 antitrypsin deficiency. CT obtained 6 weeks after transplantation showed small pocket of extra-luminal air (arrows in a and b) adjacent to right mainstem bronchus, consistent with bronchial anastomotic dehiscence. This was confirmed on bronchoscopy that shows necrotic plaque on medial right mainstem bronchus (arrow in c)
Bronchial stenosis and bronchomalacia are late complications usually occurring at least 2–4 months after transplantation. Bronchial stenosis occurs in 10% of subjects and is usually seen at the anastomotic site or distally [13]. CT shows bronchial irregularity and focal stenosis (Figs. 19.13a–d and 19.14a, b). Management includes resection of the granulation tissue and balloon dilation with or without stenting. Bronchomalacia or transient airway narrowing can be diagnosed with bronchoscopy or CT. Inspiratory and expiratory CT images are invaluable in the diagnosis of bronchomalacia (Fig. 19.15a, b). A diagnosis is suggested when there is more than 50 (liberal) to 70% (conservative) dynamic narrowing of the airway or lunate shape of the airway on expiration.
(a–d) A 69-year-old man with BOLT in 2009 for IPF. Axial MinIP (a) shows anastomotic narrowing of right mainstem bronchus . Coronal reformation (b) shows narrowing of the left mainstem bronchus distal to anastomosis. Bronchoscopic images confirming narrowing of the right (c) and left (d) mainstem bronchi
Vascular Anastomotic Complications
Vascular anastomotic complications are uncommon. Pulmonary artery stenosis is more common than pulmonary vein stenosis. It can occur in the early or late postoperative period [14]. Contrast-enhanced CT angiogram is the best available noninvasive modality to assess narrowing or occlusion of the anastomotic site. This may cause pulmonary infarction more readily due to the absence of bronchial artery circulation in the early postoperative period. Treatment options include angioplasty and stenting.
Infections
Infection is the most common complication and a leading cause of morbidity and mortality after lung transplantation. Patients are at increased risk of infection because of immunosuppression, impaired mucociliary clearance, absence of cough reflex, disruption of lymphatic drainage, and direct communication between the allograft and the atmosphere.
Infectious risks vary based on factors which include the time elapsed since transplantation. Bacterial infections are the most common cause of pneumonia with incidence highest in the first 4 weeks after lung transplantation [15]. Gram-negative organisms such as Klebsiella species and Pseudomonas aeruginosa are common causative agents. Infection by gram-positive organisms including Staphylococcus aureus is also observed. During the first postoperative month, 35–70% recipients develop bacterial pneumonia [16]. Imaging features are similar to the non-transplant population and include lobar or multifocal consolidation, ground-glass opacity, nodules, and pleural effusion [13, 15, 17] (Fig. 19.16a, b).
Fungal infections are caused by Aspergillus and less commonly by the Candida species. Pneumocystis pneumonia is uncommon these days because of routine chemoprophylaxis. Fungal infections can occur at any time but usually peak between 10 and 60 days after transplantation. These are less common but carry higher mortality compared to bacterial or viral infections. Patterns of fungal infection include angioinvasive pulmonary infection, ulcerative tracheobronchitis, and bronchial anastomosis infection [16]. CT in angioinvasive fungal infection shows ill-defined nodules or mass-like consolidation with surrounding ground-glass opacity (halo sign) indicating hemorrhage [13, 15] (Fig. 19.17a, b). On CT tracheobronchitis shows diffuse central airway wall thickening and surrounding mediastinal fat stranding. Bronchial anastomosis infection can cause bronchial dehiscence and show small foci of extra-luminal air adjacent to the airways.
Cytomegalovirus (CMV ) is the most common opportunistic infection and the second most common cause of pneumonia in lung transplantation patients. CMV pneumonia usually occurs between 1 and 6 months from transplantation [13]. CMV-seronegative recipients who receive CMV-seropositive donor lungs are at increased risk for developing primary infection. CT shows varying patterns including ground-glass opacity, centrilobular nodules with tree-in-bud pattern, consolidation, and interlobular septal thickening [13, 17] (Fig. 19.18a, b). In addition to CMV, other community acquired viruses such as adenovirus, respiratory syncytial virus (RSV), and influenza and parainfluenza viruses may infect lung transplant recipients. Viral infections can predispose lung transplant recipients to obliterative bronchiolitis [18, 19], a manifestation of chronic rejection.
Secondary Late Complications (>4 Months)
Chronic Rejection
Chronic rejection is a clinicopathologic syndrome most often characterized by obliterative bronchiolitis, affecting 50% of lung transplant recipients at 5 years [20], and the term bronchiolitis obliterans syndrome ( BOS) is used to describe the clinical situation. It is a major factor limiting the long-term survival in lung transplant recipients. It usually occurs 6 months after transplantation, and risk factors include prior episodes of recurrent acute rejection or infections, particularly CMV pneumonia. Obliterative bronchiolitis is the most common histologic pattern seen in BOS and manifests as eosinophilic hyaline fibrosis in respiratory bronchiolar walls with luminal occlusion [20]. Due to patchy distribution of the disease, transbronchial biopsy may be negative. In such cases diagnosis is made clinically on the basis of otherwise unexplained, persistent decrease in FEV1 indicative of small airway disease. Pulmonary function tests classically show an obstructive pattern, but combined obstruction and restriction may be seen [21]. High-resolution CT shows bronchial wall thickening, bronchiectasis, and heterogeneous areas of hyperlucent (dark) interspersed with adjacent normal lung parenchyma (mosaic attenuation) that is accentuated in exhalation images. Thus, it is very important to obtain paired inspiratory and expiratory HRCT images when chronic rejection is suspected clinically. On expiration HRCT, normal lung parenchyma becomes greyer, and hyperlucent (pathologic) lung remains dark indicating air trapping (Figs. 19.19a, b and 19.20a, b). Thus, the differentiation between normal and abnormal lung becomes more pronounced on expiration CT [13, 22].
Organizing Pneumonia pattern
Organizing pneumonia pattern similar to patients with cryptogenic organizing pneumonia occurs in 10–28% of patients after lung transplantation and may be associated with both acute and chronic rejection [15, 23] or may represent infection. It is characterized by the presence of inflammatory granulation tissues within the alveoli, alveolar sacs, and ducts. It responds rapidly to high-dose corticosteroids. Bronchoscopy is often done to exclude infection before starting on high-dose corticosteroids. CT shows peripheral, peribronchovascular, or perilobular consolidation (Fig. 19.21). Frequently, peripheral arc-like consolidation with central ground-glass opacity (reverse halo/atoll sign) is seen [15, 24].
Post-transplant Lymphoproliferative Disorders (PTLD)
Post-transplant lymphoproliferative disorder is a spectrum of disease varying from benign lymphoid hyperplasia to high-grade lymphoma. It typically manifests within 1 year after transplantation but can be seen from 1 month to several years after transplantation. PTLD risk is increased significantly in Epstein-Barr virus (EBV) seronegative recipients receiving lungs from seropositive donors. The other major risk factor is augmented immunosuppression. Incidence varies between 2.8 and 6% at 1 year after transplantation [25, 26]. It is more common with lung transplantation than with other solid organ transplantation. The majority of cases of PTLD is of B cell origin and associated with EBV primary infection. A much smaller proportion of PTLDs are of T cell origin, Hodgkin’s type, or, rarely, plasma cell type such as multiple myeloma. These categories are more likely to be EBV-negative. EBV-associated PTLD presents early (within 1 year), usually has a benign course and is intrathoracic, whereas EBV-negative PTLD presents late (median onset 50–60 months post-transplant), generally more aggressive and extrathoracic. Early disease responds to antiviral therapy and reduction in immunosuppression. Late disease generally requires chemotherapy and radiation therapy [25]. CT shows solitary or multiple nodules with peripheral and basal predominant distribution (Figs. 19.22a, b and 19.23a, b). Mass-like consolidation and mediastinal and hilar lymphadenopathy are also commonly seen. Rarely, interlobular septal thickening can be seen. Pleural and chest wall masses with or without pleural effusion and/or pericardial effusion have been described [27, 28]. Biopsy is often needed to differentiate PTLD from infection [29, 30].
(a, b) A 23-year-old woman with BOLT in 2010 for CF. CT shows mass-like consolidation in left upper lobe (arrow in a). CT-guided biopsy was obtained, and histopathology was consistent with post-transplant lymphoproliferative disorder (PTLD). CT also shows bronchiectasis (arrow in b) and air trapping, consistent with chronic lung allograft dysfunction
Upper Lobe Fibrosis
Upper lobe fibrosis is uncommon and occurs 1–4 years after transplantation. The exact cause for this is unknown but is described as a component of chronic allograft dysfunction (CLAD) demonstrating restrictive physiology hypothesized to be a variant of chronic rejection [21, 31]. Clinically, patients present with restrictive allograft syndrome (RAS), which is marked by restrictive pulmonary function testing and often demonstrates a more aggressive and treatment-refractory course compared to obliterative bronchiolitis. CT shows interlobular septal thickening , traction bronchiectasis, volume loss, and architectural distortion [31] (Fig. 19.24a, b).
Recurrence of Primary Disease
Recurrence of primary disease in transplanted lungs is uncommon with reported incidence of 1% [32]. Sarcoidosis is the most common disease recurring after transplantation reported in approximately 35% of patients [32, 33]. Other diseases that can recur include lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (PLCH), pulmonary alveolar proteinosis (PAP), and multifocal adenocarcinoma (previously bronchioloalveolar carcinoma). Recurrence of primary disease has been reported as early as 2 weeks and as late as 2 years after transplantation. The radiologic manifestations are similar to primary disease [32].
Transbronchial Biopsy Associated Complications
Bronchoscopy and transbronchial biopsy are frequently performed after lung transplantation to aide in the diagnosis of the various aforementioned complications [34]. Transbronchial biopsy itself can lead to various complications including pneumothorax, hemothorax, and pulmonary hemorrhage. Solid and cavitary nodules with surrounding ground-glass opacity may be an incidental finding in the allograft up to 1 month as a result of transbronchial biopsy in the corresponding location, and thus such a finding must be interpreted with caution and not confused with a fungal infection [35]. The temporal relationship to biopsy and location of nodules at the known biopsy sites helps distinguish these nodules from infection.
Summary
A wide spectrum of complications with overlapping radiologic findings may occur after lung transplantation. Familiarity with radiologic appearance of these complications in correlation with clinical manifestations and time course since transplantation is very helpful in the management of these patients. The threshold for performing CT should be low to detect infections in this immunocompromised population at an early stage. CT protocols should be tailored to suspected complications such as performing inspiration and expiration CT when chronic allograft dysfunction is suspected and performing CT angiogram when vascular complications are suspected. Accurate tailoring of CT protocols increases the yield of imaging and is useful in appropriate retrieval of specimens/samples from abnormal airways and pulmonary parenchyma in identifying the specific pathology. Follow-up imaging is essential and is guided by patient’s clinical course and pulmonary function tests.
References
Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1009–24.
Frost AE. Donor criteria and evaluation. Clin Chest Med. 1997;18(2):231–7.
Kuno R, Kanter KR, Torres WE, Lawrence EC. Single lung transplantation followed by contralateral bullectomy for bullous emphysema. J Heart Lung Transplant. 1996;15(4):389–94.
Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest. 1996;110(2):559–62.
Kundu S, Herman SJ, Winton TL. Reperfusion edema after lung transplantation: radiographic manifestations. Radiology. 1998;206(1):75–80.
Herridge MS, de Hoyos AL, Chaparro C, Winton TL, Kesten S, Maurer JR. Pleural complications in lung transplant recipients. J Thorac Cardiovasc Surg. 1995;110(1):22–6.
Ferrer J, Roldan J, Roman A, Bravo C, Monforte V, Pallissa E, et al. Acute and chronic pleural complications in lung transplantation. J Heart Lung Transplant. 2003;22(11):1217–25.
Arndt A, Boffa DJ. Pleural space complications associated with lung transplantation. Thorac Surg Clin. 2015;25(1):87–95.
King-Biggs MB. Acute pulmonary allograft rejection. Mechanisms, diagnosis, and management. Clin Chest Med. 1997;18(2):301–10.
Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult lung and heart-lung transplantation report-2009. J Heart Lung Transplant. 2009;28(10):1031–49.
Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Semin Respir Crit Care Med. 2006;27(5):521–33.
Alvarez A, Algar J, Santos F, Lama R, Aranda JL, Baamonde C, et al. Airway complications after lung transplantation: a review of 151 anastomoses. Eur J Cardiothorac Surg. 2001;19(4):381–7.
Ng YL, Paul N, Patsios D, Walsham A, Chung TB, Keshavjee S, et al. Imaging of lung transplantation: review. AJR Am J Roentgenol. 2009;192(3 Suppl):S1–13, quiz S4–9.
Clark SC, Levine AJ, Hasan A, Hilton CJ, Forty J, Dark JH. Vascular complications of lung transplantation. Ann Thorac Surg. 1996;61(4):1079–82.
Krishnam MS, Suh RD, Tomasian A, Goldin JG, Lai C, Brown K, et al. Postoperative complications of lung transplantation: radiologic findings along a time continuum. Radiographics. 2007;27(4):957–74.
Avery RK. Infections after lung transplantation. Semin Respir Crit Care Med. 2006;27(5):544–51.
Collins J, Muller NL, Kazerooni EA, Paciocco G. CT findings of pneumonia after lung transplantation. AJR Am J Roentgenol. 2000;175(3):811–8.
Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11(10):2190–6.
Collins J. Imaging of the chest after lung transplantation. J Thorac Imaging. 2002;17(2):102–12.
Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310.
Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–33.
Lee ES, Gotway MB, Reddy GP, Golden JA, Keith FM, Webb WR. Early bronchiolitis obliterans following lung transplantation: accuracy of expiratory thin-section CT for diagnosis. Radiology. 2000;216(2):472–7.
Chaparro C, Chamberlain D, Maurer J, Winton T, Dehoyos A, Kesten S. Bronchiolitis obliterans organizing pneumonia (BOOP) in lung transplant recipients. Chest. 1996;110(5):1150–4.
Arakawa H, Kurihara Y, Niimi H, Nakajima Y, Johkoh T, Nakamura H. Bronchiolitis obliterans with organizing pneumonia versus chronic eosinophilic pneumonia: high-resolution CT findings in 81 patients. AJR Am J Roentgenol. 2001;176(4):1053–8.
Reams BD, McAdams HP, Howell DN, Steele MP, Davis RD, Palmer SM. Posttransplant lymphoproliferative disorder: incidence, presentation, and response to treatment in lung transplant recipients. Chest. 2003;124(4):1242–9.
Paranjothi S, Yusen RD, Kraus MD, Lynch JP, Patterson GA, Trulock EP. Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transplant. 2001;20(10):1054–63.
Carignan S, Staples CA, Muller NL. Intrathoracic lymphoproliferative disorders in the immunocompromised patient: CT findings. Radiology. 1995;197(1):53–8.
Dodd GD III, Ledesma-Medina J, Baron RL, Fuhrman CR. Post-transplant lymphoproliferative disorder: intrathoracic manifestations. Radiology. 1992;184(1):65–9.
Scarsbrook AF, Warakaulle DR, Dattani M, Traill Z. Post-transplantation lymphoproliferative disorder: the spectrum of imaging appearances. Clin Radiol. 2005;60(1):47–55.
Rappaport DC, Chamberlain DW, Shepherd FA, Hutcheon MA. Lymphoproliferative disorders after lung transplantation: imaging features. Radiology. 1998;206(2):519–24.
Konen E, Weisbrod GL, Pakhale S, Chung T, Paul NS, Hutcheon MA. Fibrosis of the upper lobes: a newly identified late-onset complication after lung transplantation? AJR Am J Roentgenol. 2003;181(6):1539–43.
Collins J, Hartman MJ, Warner TF, Muller NL, Kazerooni EA, McAdams HP, et al. Frequency and CT findings of recurrent disease after lung transplantation. Radiology. 2001;219(2):503–9.
Kazerooni EA, Jackson C, Cascade PN. Sarcoidosis: recurrence of primary disease in transplanted lungs. Radiology. 1994;192(2):461–4.
Boehler A, Vogt P, Zollinger A, Weder W, Speich R. Prospective study of the value of transbronchial lung biopsy after lung transplantation. Eur Respir J. 1996;9(4):658–62.
Kazerooni EA, Cascade PN, Gross BH. Transplanted lungs: nodules following transbronchial biopsy. Radiology. 1995;194(1):209–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Ahuja, J., Kapnadak, S.G., Pipavath, S. (2018). Imaging of Lung Transplantation. In: Raghu, G., Carbone, R. (eds) Lung Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-91184-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-91184-7_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91182-3
Online ISBN: 978-3-319-91184-7
eBook Packages: MedicineMedicine (R0)