Abstract
It has been argued that solid-state physics was born, as a separate field, with the publication, in 1940, of Frederick Seitz’s book, Modern Theory of Solids [82].
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
The viscosity of glass is typically greater than 1013 poise and it is disordered.
- 2.
It might be of interest to some students to start off with advice on a career. One author of this book has written two articles on this topic. See:
1. James D. Patterson, “An Open Letter to the Next Generation,” Physics Today, 57, 56 (2004)
2. James D. Patterson, “Ten Mistakes for Physicists to Avoid,” APS News, January 2012 (Volume 21, Number 1).
- 3.
See Anderson [1.1].
- 4.
The Born–Haber cycle starts with (say) NaCl solid. Let U be the energy needed to break this up into Na+ gas and Cl− gas. Suppose it takes EF units of energy to go from Cl− gas to Cl gas plus electrons, and EI units of energy are gained in going from Na+ gas plus electrons to Na gas. The Na gas gives up heat of sublimation energy S in going to Na solid, and the Cl gas gives up heat of dissociation D in going to Cl2 gas. Finally, let the Na solid and Cl2 gas go back to NaCl solid in its original state with a resultant energy W. We are back where we started and so the energies must add to zero: U − EI + EF − S − D − W = 0. This equation can be used to determine U from other experimental quantities.
- 5.
A much more sophisticated approach to the binding of metals is contained in the pedagogical article by Tran and Perdew [1.26]. This article shows how exchange and correlation effects are important and discusses modern density functional methods (see Chap. 3).
- 6.
More accurately, one thinks of the electron states as being combinations formed from s and p states to form sp3 hybrids. A very simple discussion of this process as well as the details of other types of bonds is given by Moffatt et al. [1.17].
- 7.
Note that the application starts on the right so 3 → 1 → 2, for example.
- 8.
One can get around the requirement of having an infinite crystal and still preserve translational symmetry by using periodic boundary conditions. These will be described later.
- 9.
See Shechtman et al. [1.21].
- 10.
See Levine and Steinhardt [1.15]. See also Steinhardt and Ostlund [1.22].
- 11.
For example, Shull and Smart in 1949 used elastic neutron diffraction to directly demonstrate the existence of two magnetic sublattices on an antiferromagnet.
- 12.
Diffraction of electrons was originally demonstrated by Davisson and Germer in an experiment clearly showing the wave nature of electrons.
- 13.
A particularly clear discussion of these topics is found in Brown and Forsyth [1.4]. See also Kittel [1.13, Chaps. 2 and 19]
- 14.
- 15.
See, e.g., Ghatak and Kothari [1.9].
- 16.
See Maradudin et al. [1.16]
Author information
Authors and Affiliations
Corresponding author
Problems
Problems
-
1.1.
Show by construction that stacked regular pentagons do not fill all two-dimensional space. What do you conclude from this? Give an example of a geometrical figure that when stacked will fill all two-dimensional space.
-
1.2.
Find the Madelung constant for a one-dimensional lattice of alternating, equally spaced positive and negative charged ions.
-
1.3.
Use the Evjen counting scheme [1.19] to evaluate approximately the Made-lung constant for crystals with the NaCl structure.
-
1.4.
Show that the set of all rational numbers (without zero) forms a group under the operation of multiplication. Show that the set of all rational numbers (with zero) forms a group under the operation of addition.
-
1.5.
Construct the group multiplication table of D4 (the group of three dimensional rotations that map a square into itself).
-
1.6.
Show that the set of elements (1, −1, i, −i) forms a group when combined under the operation of multiplication of complex numbers. Find a geometric group that is isomorphic to this group. Find a subgroup of this group. Is the whole group cyclic? Is the subgroup cyclic? Is the whole group Abelian?
-
1.7.
Construct the stereograms for the point groups 4(C4) and 4 mm(C4v). Explain how all elements of each group are represented in the stereogram (see Table 1.3).
-
1.8.
Draw a bcc (body-centered cubic) crystal and draw in three crystal planes that are neither parallel nor perpendicular. Name these planes by the use of Miller indices. Write down the Miller indices of three directions, which are neither parallel nor perpendicular. Draw in these directions with arrows.
-
1.9.
Argue that electrons should have energy of order electron volts to be diffracted by a crystal lattice.
-
1.10.
Consider lattice planes specified by Miller indices (h, k, l) with lattice spacing determined by d(h, k, l). Show that the reciprocal lattice vectors G(h, k, l) are orthogonal to the lattice plane (h, k, l) and if G(h, k, l) is the shortest such reciprocal lattice vector then
$$ d\left( {h,k,l} \right) = \frac{2\pi }{{\left| {\varvec{G}\left( {h,k,l} \right)} \right|}}. $$ -
1.11.
Suppose a one-dimensional crystal has atoms located at nb and αmb where n and m are integers and α is an irrational number. Show that sharp Bragg peaks are still obtained.
-
1.12.
Find the Bragg peaks for a grating with a modulated spacing. Assume the grating has a spacing
$$ d_{n} = nb + \varepsilon b\,\sin \left( {2\pi knb} \right), $$where ε is small and kb is irrational. Carry your results to first order in ε and assume that all scattered waves have the same geometry. You can use the geometry shown in the figure of this problem. The phase φn of scattered wave n at angle θ is
$$ {{\upvarphi }}_{n} = \frac{2\pi }{\lambda }d_{n} \sin \theta , $$where λ is the wavelength. The scattered intensity is proportional to the square of the scattered amplitude, which in turn is proportional to
$$ E \equiv \left| {\sum\limits_{0}^{N} {\exp \left( {{\text{i}}\varphi_{n} } \right)} } \right| $$for N + 1 scattered wavelets of equal amplitude.
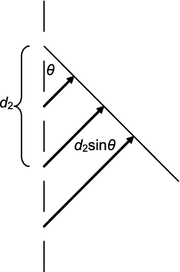
-
1.13.
Find all Bragg angles less than 50° for diffraction of X-rays with wavelength 1.5 angstroms from the (100) planes in potassium. Use a conventional unit cell with structure factor.
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Patterson, J.D., Bailey, B.C. (2018). Crystal Binding and Structure. In: Solid-State Physics. Springer, Cham. https://doi.org/10.1007/978-3-319-75322-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-75322-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75321-8
Online ISBN: 978-3-319-75322-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)


