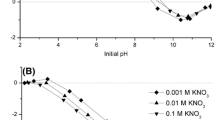
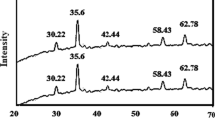
Abstract
Binder is indispensable in the production of iron ore pellets and the quality of pellets seems to be related to the interactions between binders and iron minerals. Humic acid is proved a suitable binder for iron ore pelletizing. In this paper, the interactions between humic acid and iron minerals were investigated by adsorption experiments and molecular dynamic simulations. Adsorption experimental results show that the adsorption of humic acid on hematite is easier than that on magnetite, and the maximum adsorption capacities of humic acid on hematite and magnetite reach 17.1, 7.39 mg/g, respectively. The results of adsorption isotherms indicate that the Langmuir model provides the best correlation of the experimental data, while the thermodynamics results demonstrate that the adsorption process is a spontaneous exothermic process. Molecular dynamic simulations reveal that the interaction between hematite and humic acid is stronger than that between humic acid and magnetite.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Chun T, Long H, Di Z, Wang P, Meng Q (2017) Influence of microwave heating on the microstructures of iron ore pellets with coal during reduction. Ironmak Steelmak 44(7):486–491
Savel’ev SG, Gubin GV, Stoikova YaA (2013) Prospects for the production of iron-ore pellets. Steel Transl 43(8):499–502
Bentonite RJS (1964) A challenge for Canadian industry. CIM Bull 57:648–652
Haas LA, Aldinger JA, Nigro JC (1989) Utilization of papermill sludges as binders for iron ore concentrate. Report of Investigations, United States Bureau of Mines, No. 9257
Huang YF, Zhang YB, Han GH et al (2010) Sodium-modification of ca-based bentonite via semidry process. J Cent South Univ T 17(6):1201–1206
Qiu G, Jiang T, Li H, Wang D (2003) Functions and molecular structure of organic binders for iron ore pelletization. Colloids Surf A 224(1–3):11–22
Inc. AS (2016) Materials studio release notes, release 8.0. Accelrys Software Inc., San Diego, CA
Han GH, Huang YF, Li G, Zhang YB, Jiang T (2014) Detailed adsorption studies of active humic acid fraction of a new binder on iron ore particles. Miner Process Extr Metall Rev 35(1):1–14
Zhou Y, Zhang Y, Li P, Li G, Jiang T (2014) Comparative study on the adsorption interactions of humic acid onto natural magnetite, hematite and quartz: effect of initial ha concentration. Powder Technol 251(1):1–8
Rappe AK, Casewit CJ, Colwell KS, Iii WAG, Skiff WM (1992) Uff, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114(25):10024–10035
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3):B864
Ozacar M, Sengil IA (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96(7):791–795
Ozacar M, Sengil IA (2003) Adsorption of reactive dyes on calcined alunite from aqueous solutions. J Hazard Mater 98(1–3):211–224
Hameed BH, Ahmad AA, Aziz N (2007) Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem Eng Sci 133(1–3):195–203
Xia S, Qiu M, Yu L, Liu F, Zhao H (2008) Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance. Corros Sci 50(7):2021–2029
Acknowledgements
The authors acknowledge the financial supports of the National Science Fund of China (No.51404213, No. 51404214, No. 51674225 and No. 51774252), Educational Commission of Henan Province of China (No. 17A450001, No. 18HASTIT011 and No. 18A450001) and the Development Fund for Outstanding Young Teachers of Zhengzhou University (No.1421324065).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Han, G., Su, S., Cao, Y., Huang, Y., Song, X. (2018). Research on the Interaction of Humic Acid with Iron Minerals. In: Li, B., et al. Characterization of Minerals, Metals, and Materials 2018 . TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72484-3_69
Download citation
DOI: https://doi.org/10.1007/978-3-319-72484-3_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72483-6
Online ISBN: 978-3-319-72484-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)