Abstract
Despite growing concerns over the environmental impacts of pharmaceuticals, the use of Life Cycle Assessment (LCA) within the pharma-sector remains quite fragmentary. The aim of this paper is to present gaps and challenges, impeding a full adoption of LCA in the pharma-sector. A review of existing pharma-LCAs revealed a considerable degree of inconsistency and inhomogeneity in their methodological choices, highlighting the need for product category rules (PCRs) for the pharmaceutical industry to harmonize and facilitate the future use of LCA in that sector. Additionally, existing life cycle impact assessment (LCIA) methods fail to model several pharma-specific impact pathways (e.g. endocrine disruption). Preliminary thoughts on the development of pharma-PCRs and the inclusion of pharma-specific impact pathways into LCIA are presented, providing important stimulus for further research.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Product Category Rules (PCRs)
- Life Cycle Impact Assessment (LCIA)
- Pharma Sector
- Active Pharmaceutical Ingredients (APIs)
- Endocrine Disrupting Chemicals (EDCs)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A global rise in pharmaceutical consumption has come hand in hand with a parallel surge in environmental contamination with active pharmaceutical ingredients (APIs), their metabolites and transformation products [1, 2]. The eco-toxicological concerns regarding the presence of pharmaceuticals in surface waters, together with the pharmaceutical industry’s resource- and energy-intensive environmental profile, have prompted the sector to increasingly integrate green chemistry and green engineering principles into its production processes [3,4,5,6].
Despite some drug companies recognizing the merits of utilizing Life Cycle Assessment (LCA) to measure their progress towards ‘greener’ production, LCAs remain far from becoming common practice in the pharmaceutical industry [7, 8]. Furthermore, existing pharma-LCAs are quite inhomogeneous in multiple respects, e.g. the choice of functional unit or of impact categories. This, in turn, reduces the robustness of their results and their reproducibility and makes industry-wide insights with respect to typical environmental ‘hotspots’ or possible strategies for ‘greener’ drug designs practically futile. Naturally, the distinct goals of pharma-LCAs are partly responsible for the diversity of methodological choices among the studies. However, given the high degree of flexibility provided by the ISO 14040/14044 standards, it is fair to assume that even two studies of the same drug done by two different LCA-practitioners would show considerable disparity. It is exactly that realisation that has led over the last few years to the need—and consequently the parallel rise—of so-called ‘product category rules’ (PCRs), i.e. a set of harmonized rules on specific LCA modelling requirements for a given product category [9]. With the aim to develop PCRs for the pharma-sector, a three-year project, entitled ‘Development of a sector-specific environmental sustainability assessment approach for pharmaceutical products and processes’ (German abbreviation: SERUM), was launched last year at the Technische Universität Berlin.
The purpose of this paper is to present current gaps and challenges impeding a full adoption of LCA into the sustainability practices and product development processes of the pharma-sector and to provide preliminary recommendations on how to tackle some of those challenges. Following a short review of existing LCAs in the pharma-sector in Sect. 2, Sect. 3 will briefly describe the current state of Life Cycle Impact Assessment (LCIA) in pharma-LCAs and introduce some of the present constraints in modelling the toxicity of pharmaceutical compounds. Section 4 addresses early considerations on the way to developing PCRs for the pharmaceutical sector. Finally, a few concluding remarks and implications for future research within the LCA community will be presented.
2 Review of LCAs in the Pharmaceutical Industry
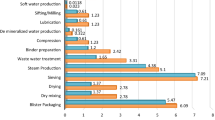
A comprehensive review on the state of LCA-application in the pharmaceutical industry, specifically human pharmaceuticals, was initially performed in order to identify common LCA-practices within the sector (e.g. choice of functional unit), regularly identified ‘Hotspots’ in the life cycle of drugs, as well as often encountered challenges and remaining gaps. Results of the review were meant to lay the ground for harmonized sector-specific rules—in the form of PCRs—and reveal thematic focal points for achieving greater LCA-application within the pharma-sector. Given the unique characteristics of the pharmaceutical industry (e.g. exceptionally high standards of cleanliness maintained during production) and the relatively young age of ‘green pharmacy’-practices, the focus of the literature review was on LCA case studies of human pharmaceutical products (i.e. APIs or final drug, incl. packaging) or pharmaceutical processes, performed in or after the year 2000. LCAs of precursor chemicals (e.g. enzymes) were only included if downstream application in the pharmaceutical industry is clearly intended. The search thus excluded LCAs in the broader field of green chemistry and in the healthcare sector in general (e.g. medical equipment). Using search terms such as “Life Cycle Assessment”, “LCA”, and “footprint” in combination with ‘pharmaceutical*’ or ‘fine chemical*’ on Google Scholar has so far yielded a notably limited number of ‘pure’ pharma-LCAs (<30 studies) which have been published in peer-reviewed journals. These LCAs were conducted for a myriad of purposes, including comparative assessments of different synthesis routes, processing modes (e.g. batch vs. continuous processing), drug formulations, varying dosages and packaging options.
The LCA-studies examined thus far have with very few exceptions all performed a cradle-to-gate analysis, while often criticizing the lack of sufficient data beyond the production phase. Figure 1 shows a generic product system of a pharmaceutical product and different possibilities to set the system boundaries. A full life-cycle perspective is crucial in the context of pharmaceuticals, because firstly outsourcing certain synthesis or formulation steps (and with that the ‘outsourcing’ of impacts) is quite prevalent in the sector. Secondly, the environmental burden of upstream processes (e.g. production of input chemicals and ‘background’ energy production) typically dwarfs impacts of the actual in-house synthetic processes [10, 11]. Furthermore, the use phase and end-of-life of a pharmaceutical product may as well bear considerable environmental impacts, especially if eco- and human-toxicological effects due to API-emissions into sewage systems (and eventually into surface waters) are to be included in the analysis (for further details see next section). Consequently, pharmaceutical corporations should strive towards complete cradle-to-grave analyses.
A closer examination of the existing pharma-LCAs quickly revealed that the individual studies are quite inhomogeneous in a number of respects: e.g. their choice of functional unit (FU), system boundaries setting, use of background databases and data quality, choice of impact assessment methods and the impact categories they consider. For instance, while Brunet et al. [12] set the FU at 20,840,000 kg of penicillin V produced over a time horizon of 20 years, De Soete et al. [13] chose 1 daily dosage of PREZISTA (anti-HIV medication) as their FU. The majority of reviewed studies though opted for 1 kg of API as FU. Similarly, while Wernet et al. [10] chose to assess sixteen impact categories (both at midpoint- and endpoint level) using 5 different impact assessment methods, Kim et al. [14] considered 5 impact categories using one method only.
In light of the different goals and scopes of the reviewed studies and the inherent uniqueness of individual APIs/drug formulations, a certain degree of methodological variation among pharma-LCAs is only logical, if not expedient. Nevertheless, lack of sufficient experience and guidance has led to large discrepancies in the application of LCA in the pharma-sector, often jeopardizing the coherence and reliability of pharma-LCAs. Consequently, there is an evident need for PCRs for the pharmaceutical industry to guide and facilitate future pharma-LCAs. Preliminary thoughts on pharma-PCRs are discussed in Sect. 4.
3 Life Cycle Impact Assessment (LCIA) in Pharma-LCAs
The LCIA phase of the reviewed pharma-LCAs was carried out using quite divergent impact categories and impact assessment methods. A streamlined LCA tool developed by the American Chemical Society Green Chemistry Institute (ACS-GCI) Pharmaceutical Roundtable (hereinafter ‘the Roundtable’) sets forth nine impact categories/indicators to be assessed in LCAs of drug synthesis routes [11]. Table 1 lists these nine impact categories, next to the top five assessed impact categories in the reviewed pharma-LCAs, as well as a preliminary selection of eight categories recognized by the authors as the most relevant for pharma-LCAs. The latter list was determined in consultation with the SERUM advisory committee, which comprises experts from academia, politics and the pharmaceutical industry.
Quite notably, impacts—especially toxicity-related impacts—which have been identified as relevant for the pharmaceutical industry within the SERUM project are not often considered in LCA studies nor recommended in the Roundtable’s streamlined tool. Given the desired functionality of pharmaceuticals—e.g. to kill rapidly dividing cells (anticancer), affect the action of neurotransmitter chemicals in the brain (antipsychotic), kill or inhibit microorganisms (antimicrobial)—and the growing body of literature providing pertinent evidence of potential unanticipated eco-toxicological effects of APIs (reviewed in [3, 15,16,17]), it is concerning that none of the existing pharma-LCAs considered impacts related to the presence of pharmaceutical residues in the environment.
The discrepancy between practice, recommendation and (perceived) relevance of the categories ‘human toxicity’ and ‘eco-toxicity’ for pharma-LCAs is largely the result of a number of methodological constraints on toxicity modelling within LCIA, the most prominent of which are:
-
(1)
Lack of characterization factors (CFs) for pharmaceutical compounds in existing toxicity models
-
(2)
Several impacts or impact pathways associated with pharmaceuticals and their toxic mode of action are neglected in current impact assessment methods.
In an attempt to address the first constraint and enhance the assessment of pharmaceuticals’ toxicity in LCIA, several studies have recently updated or calculated new CFs for APIs in the categories human toxicity, freshwater, marine or terrestrial eco-toxicity using mostly USEtox, but also EDIP97 and/or USES-LCA 2.0 [18,19,20]. Despite the mentioned efforts, the total number of covered pharmaceuticals within common toxicity models remains considerably low (at the current state of the authors’ knowledge below 100 compounds).
The second methodological constraint relates to a wide variety of missing, (pharma-specific) effects such as:
-
endocrine disruption mediated by exposure to endocrine disrupting chemicals (EDCs) (reviewed in [21]),
-
the development of antibiotic resistance [22] or
-
behavioural alterations such as change in feeding behaviour or predator avoidance due to wildlife-exposure to antidepressants (reviewed in [23]).
Such examples of more subtle and sub-lethal toxic effects with possibly significant repercussions on species’ ecological fitness and population-relevant endpoints are often not captured by endpoints that are typically assessed in regulatory ecotoxicology. Consequently, using toxicity data on traditional endpoints (e.g. mortality, growth and reproduction) as recommended by USEtox to calculate CFs for pharmaceutical compounds fails to reflect certain impact pathways of individual pharmaceuticals with specific toxic modes of action such as EDCs or antibiotics [24].
In an attempt to tackle the second methodological constraint, Larsen et al. [24] sought to integrate endocrine disruption into LCA by suggesting to use test results from fish laboratory tests with the endpoint sex ratio as effect data when estimating eco-toxicity characterization factors for estrogenic compounds [24]. Considering the complexity of the endocrine system—whether in humans or in wildlife—and the variety of EDCs’ mechanisms of action (e.g. (anti)estrogenic/(anti)androgenic or interference with thyroid hormone pathways) more endpoints will have to be eventually included to capture and map the ‘full’ array of EDCs and their potential toxic effects.
When seeking to include other missing pharma-specific impact pathways such as the development of antibiotic resistance, it is less likely that the same approach, i.e. to broaden the endpoints used to calculate effect factors, would suffice to reflect the specific mechanisms in which bacteria develop or transfer antibiotic resistant genes. Consequently, a new indicator and characterization model for the inclusion of antibiotic resistance into LCIA may need to be developed.
4 Product Category Rules (PCRs) for the Pharmaceutical Industry
PCRs are usually defined for a group of products which have an equivalent or similar function, making them largely comparable. Despite the compelling case for a harmonized framework in the form of PCRs to guide LCAs of pharmaceutical products, so far there has only been one PCR developed for vaccines [25]. On the basis of this PCR, Pfizer conducted and published an Environmental Product Declaration (EPD) for IMPROVAC, an immunological product used as alternative to physical castration in pig management [26]. However the EPD for IMPROVAC is no longer valid since 2015.
To develop new PCRs, there are no rules on how ‘narrow’ or ‘broad’ a product category should be defined. In other words, the ‘granularity’ of PCRs is entirely up to their developers. Based on feedback obtained in consultation with pharma-experts, developing PCRs at two different levels/granularities would prove practical for the pharmaceutical industry (see Fig. 2). First, a generic PCR for the pharmaceutical sector (‘horizontal rules’) should be developed, determining broad LCA-modelling provisions which capture some of the industry-wide characteristics (e.g. the importance of including treatment processes of solvent waste within the system boundaries). Such a generic “frame-PCR” is subject to a considerable degree of uncertainty/inaccuracy, as it intends to provide common modelling rules for quite distinct products of a given sector, while having to rely on numerous assumptions and a significantly simplified representation of the industry as a whole. Therefore, in a second step and in close alignment with the frame-PCR, specific PCRs (‘vertical rules’) should be cumulatively developed for:
-
(1)
pharmaceutical products categorized into different drug classes according to the international Anatomical Therapeutic Chemical (ATC) Classification System (product-PCRs) and
-
(2)
drug manufacturing processes (process-PCRs).
The product-PCRs are to be developed for the different drug classes available at the third level of the ATC-code, i.e. the different therapeutic/pharmacological subgroups of APIs, and would serve the objective of assessing drug alternatives according to the same harmonized scheme on the basis of their common functionality (therapeutic purpose) and pharmacological properties. Process-PCRs would guide LCAs which are primarily focused on process optimization. The existence of a generic ‘frame-PCR’ encircling product- and process-PCRs will prove particularly valuable at the early stages when PCRs have not yet been developed for all therapeutic/pharmacological subgroups of APIs.
A harmonized LCA reference framework for the pharmaceutical industry will inevitably guarantee better (qualitative) comparability of the results (especially within one therapeutic/pharmacological subgroup using the same product-PCR) and improve the reliability of future pharma-LCAs. Additionally, it paves the way for EPDs to establish themselves within the sector, with potentially significant social implications (e.g. doctors/consumers opting for ‘greener’ drug alternatives).
5 Conclusions and Outlook
Despite recent efforts of the pharmaceutical industry to integrate green chemistry and green engineering principles into their production processes and drug designs, the utilization of LCA to monitor and measure progress towards ‘greener’ pharmaceutical products remains far from common practice. A review of available pharma-LCAs revealed a considerable degree of inconsistency and inhomogeneity in their modelling choices, often leading to quite unreliable results. The problem is compounded by the fact that existing life cycle impact assessment methods fail to include a variety of pharma-specific impact pathways within their toxicity modelling (e.g. endocrine disruption or antibiotic resistance) and provide a noticeably limited number of characterization factors for pharmaceutical compounds.
The development of product category rules for pharmaceutical products and processes is regarded as a necessary development to harmonize, facilitate and expand the future use of LCA in the sector. Additionally, calculating new CFs for pharmaceutical compounds within established toxicity models or developing new characterization models that reflect pharma-specific toxicological effects is imperative to delivering a comprehensive and accurate quantification of the environmental impacts of human drugs.
Only when an applicable and robust LCA-based environmental sustainability assessment approach is adapted to the needs and specificities of the pharmaceutical industry can life cycle management establish itself within the sector and truly guide eco-innovation towards ‘green pharmacy’.
References
Taylor D, The Pharmaceutical Industry and the Future of Drug Development, In Pharmaceuticals in the Environment, Hester, R. E., Harrison, R. M., Eds., Issues in Environmental Science and Technology No. 41, The Royal Society of Chemistry, 2015, pp 1–33.
Christen V, Hickmann S, Rechenberg B, Fent K, Highly active human pharmaceuticals in aquatic systems: A concept for their identification based on their mode of action, Aquatic toxicology, Vol. 96, No. 3, 2010, pp 167–181.
Arnold, K. E, Brown A. R, Ankley G. T, Sumpter J. P, Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems, Phil. Trans. R. Soc. B, Vol. 369, 2014.
Caldwell D. J, Sources of Pharmaceutical Residues in the Environment and their Control, In Pharmaceuticals in the Environment, Hester, R. E., Harrison, R. M., Eds., Issues in Environmental Science and Technology No. 41, The Royal Society of Chemistry, 2015, pp 92–119.
Voulvoulis N, Barceló D, Verlicchi P, Pharmaceutical Residues in Sewage Treatment Works and their Fate in the Receiving Environment, In Pharmaceuticals in the Environment, Hester, R. E., Harrison, R. M., Eds., Issues in Environmental Science and Technology No. 41, The Royal Society of Chemistry, 2015, pp 120–179.
Jiménez-González C, Constable D. C, Ponder C. S, Evaluating the “greenness” of chemical processes and products in the pharmaceutical industry–a green metrics primer, Chemical Society reviews, Vol. 41, No. 4, 2012, pp 1485–1498.
Jiménez-González C, Overcash M. R, The evolution of life cycle assessment in pharmaceutical and chemical applications – a perspective, Green Chem., Vol. 16, No. 7, 2014, p 3392.
Mata T. M, Martins A. A, Neto B, Martins M. L, Salcedo, R, Costa C, LCA Tool for Sustainability Evaluations in the Pharmaceutical Industry, Chemical Engineering Transactions, Vol. 26, 2012.
Minkov N, Schneider L, Lehmann A, Finkbeiner M, Type III Environmental Declaration Programmes and harmonization of product category rules: Status quo and practical challenges, Journal of Cleaner Production, Vol. 94, 2015, pp 235–246.
Wernet G, Conradt S, Isenring H. P, Jiménez-González C, Hungerbühler K, Life cycle assessment of fine chemical production: A case study of pharmaceutical synthesis, Int J Life Cycle Assess, Vol. 15, No. 3, 2010, pp 294–303.
Jiménez-González C, Ollech C, Pyrz W, Hughes D, Broxterman Q. B, Bhathela N., Expanding the Boundaries: Developing a Streamlined Tool for Eco-Footprinting of Pharmaceuticals, Org. Process Res. Dev., Vol. 17, No. 2, 2013, pp 239–246.
Brunet R, Guillén-Gosálbez G, Jiménez L, Combined simulation–optimization methodology to reduce the environmental impact of pharmaceutical processes: Application to the production of Penicillin V, Journal of Cleaner Production, Vol. 76, 2014, pp 55–63.
De Soete W, Boone L, Willemse F, Meyer E, Heirman B, van Langenhove H, Dewulf J, Environmental resource footprinting of drug manufacturing: Effects of scale-up and tablet dosage, Resources, Conservation and Recycling, Vol. 91, 2014, pp 82–88.
Kim S, Jiménez-González C, Dale B. E, Enzymes for pharmaceutical applications—a cradle-to-gate life cycle assessment, Int J Life Cycle Assess, Vol. 14, No. 5, 2009, pp 392–400.
Taggart M. A, Richards N, Kinney C. A, Impacts of Pharmaceuticals on Terrestrial Wildlife, In Pharmaceuticals in the Environment, Hester, R. E., Harrison, R. M., Eds., Issues in Environmental Science and Technology No. 41, The Royal Society of Chemistry, 2015, pp 216–254.
Crane M, Watts C, Boucard T, Chronic aquatic environmental risks from exposure to human pharmaceuticals, Science of the Total Environment, Vol. 367, 2006, pp 23–41.
Santos L. H. M. L. M, Araujo A. N, Fachini A, Pena A, Delerue-Matos C, Montenegro M. C. B. S. M., Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment, Journal of hazardous materials, Vol. 175, 1–3, 2010, pp 45–95.
Alfonsín C, Hospido A, Omil F, Moreira M. T, Feijoo G, PPCPs in wastewater – Update and calculation of characterization factors for their inclusion in LCA studies, Journal of Cleaner Production, Vol. 83, 2014, pp 245–255.
Ortiz de García S, García-Encina P. A, Irusta-Mata R, The potential ecotoxicological impact of pharmaceutical and personal care products on humans and freshwater, based on USEtox™ characterization factors. A Spanish case study of toxicity impact scores, The Science of the total environment, Vol. 609, 2017, pp 429–445.
Muñoz I, José Gómez M, Molina-Díaz A, Huijbregts M. A, Fernández-Alba A. R, García-Calvo E, Ranking potential impacts of priority and emerging pollutants in urban wastewater through life cycle impact assessment, Chemosphere, Vol. 74, No. 1, 2008, pp 37–44.
Bergman Å, Heindel J. J, Jobling S, Kidd K. A, Zoeller R. T, Eds., State of the Science of Endocrine Disrupting Chemicals - 2012: An assessment of the state of the science of endocrine disruptors, WHO; UNEP, 2013.
Kümmerer K, Antibiotics in the aquatic environment–a review–part II, Chemosphere, Vol. 75, No. 4, 2009, pp 435–441.
Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M, Ecological effects of pharmaceuticals in aquatic systems–impacts through behavioural alterations, Phil. Trans. R. Soc. B, Vol. 369, No. 1656, 2014.
Larsen H. F, Olsen S. I, Hauschild M. Z, Laurent A, New sustainable concepts and processes for optimization and upgrading municipal wastewater and sludge treatment: Deliverable 4.2. Methodology for including specific biological effects and pathogen aspects into LCA, NEPTUNE Contract-No. 036845 (FP6 project), 2009.
International EPD System, Vaccines for human or veterinary medicine, whether or not put up as medicaments: Product group classification: UN CPC 35270, prepared by Life Cycle Engineering, 2011.
Pfizer, Environmental Product Declaration for IMPROVAC: EPD No.: S-P-00261, BUREAU VERITAS Certification No. SE00250-1, 2012 (accessed May 5, 2017).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Emara, Y., Siegert, MW., Lehmann, A., Finkbeiner, M. (2018). Life Cycle Management in the Pharmaceutical Industry Using an Applicable and Robust LCA-Based Environmental Sustainability Assessment Approach. In: Benetto, E., Gericke, K., Guiton, M. (eds) Designing Sustainable Technologies, Products and Policies. Springer, Cham. https://doi.org/10.1007/978-3-319-66981-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-66981-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66980-9
Online ISBN: 978-3-319-66981-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)