Abstract
A new method of dispersion of single-wall carbon nanotubes (SWNT) in aqueous solution using supramolecular compounds is proposed in this chapter. The described system consists of SWNT overlaid by Congo red. SWNT are formed from a rolled layer of graphene, providing a large surface area for binding compounds with planar, aromatic structures (including drugs). Congo red is able to associate with proteins in the form of supramolecular, ribbon-like structures, and may bind various drugs by intercalation.
The study reveals strong interactions between Congo red and the surface of SWNT. The authors’ aim was to explain the mechanism driving this interaction. Spectral analysis of the SWNT-CR complex, effects of sonication on CR binding, microscopic imaging and molecular modelling analyses are all discussed. Results indicate that binding of supramolecular Congo red to the surface of nanotubes is based on face-to-face stacking. Having attached itself to the surface of a nanotube, a dye molecule may attract other similarly oriented molecules, giving rise to a protruding supramolecular appendage. This explains the high affinity of CR for nanotubes and the resulting system’s capability to bind drugs.
Analysis of complexes formed by SWNT-CR with the model drug (DOX) and with other planar compounds (EB, TY) indicates that it may be possible to construct complexes capable of binding multiple compounds simultaneously.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Single-walled carbon nanotubes
- Supramolecular compounds
- Bis-azo dye
- Congo red
- Chemical container
- Drug delivery system
- Shortenng of carbon nanotubes
- Pi-pi stacking
- Face-to-face stacking
7.1 Methods of Dispersing Carbon Nanotubes – The Search for Optimal Dispersion Methods
Carbon nanotubes (CNT) are used in electronics [1], composite materials research [2, 3], catalysis [4], textiles [5] and many other areas. A particularly interesting is their use in the field of biomedicine : in biosensors [6, 7], medical diagnostics [8, 9], transplantology [10, 11], tissue engineering [12] or in pharmacology – including the field of targeted therapies [13,14,15,16].
In studies on the use of CNT in biomedicine it is necessary to obtain their suspension in water media. CNT are most often found not in the form of single fibres, but in the form of bundles stabilized by van der Waals interactions and it is very difficult to evenly distribute them in the liquid phase. The purpose of research is thus to find the compounds that provide effective dispersion of carbon nanotubes and simultaneously bind other compounds, including some drugs . Numerous reports concern both covalent or non-covalent modifications of CNT [17].
The covalent modification based on the introduction of functional groups into the graphene surface of carbon nanotubes is an effective way to increase CNT dispersion. This may, however, lead to changes in the physicochemical properties of CNT and create defects in their structure [18,19,20].
Non-covalent modification is considered a less invasive method. Because of the fact, that there is no heating or acidic environment involved, virtually no damage is done to the structure of nanotubes [8, 21]. Among non-covalent CNT modifications one can distinguish: interactions with amphiphilic molecules – surfactants (e.g. SDS – sodium dodecyl sulphate, SDBS – sodium dodecylbenzenesulphonate , CTAB – cetyltrimethylammonium bromide , DDAB – dimethyldioctadecyl -ammonium bromide, Triton , Pluronic [22]). The most effective interactions between CNTs and surfactants were observed for surfactants containing a benzene ring in the structure (e.g. SDBS), due to the presence of strong pi-pi stacking interactions between the phenyl ring and the graphene nanotube surface [23].
Other compounds used for the noncovalent functionalization and dispersing of CNT include: ionic liquids (ILs) – which form bucky gels , polymers – especially those with aromatic rings in their structure [24], other compounds with an aromatic ring [25] (for example ammonium salts containing pyrene rings [26]) and or deoxycholic acid sodium salts [27].
7.2 The Interaction of Congo Red with Carbon Nanotubes
CR , a bis-azo dye , similarly to the carbon nanotube dispersing compounds mentioned above, has aromatic rings in its structure. It is therefore possible to form a non-covalent pi-pi interaction between CR molecules and the graphene surface of carbon nanotubes. Reports can be found in literature on the interaction of CR with carbon nanotubes [28,29,30,31] and the surface of graphene oxide [32, 33].
Hu C. et al. [28] describe a complex of single-walled carbon nanotubes (SWNT) with CR (SWNT-CR ), with water solubility of up to 3.5 mg/mL. The described complex was formed by prolonged grinding of SWNT and CR in the agate mortar, followed by drying. In this case CR probably interacts with the surface of nanotubes as single molecules bound to the nanotube surface via pi-pi interactions. The described method enables nanotubes dispersion, however the results may not be reproducible and the CR binding effectiveness is relatively low.
In another paper [31] authors point to the possibility of using CNT for adsorbing dyes from aqueous solutions – in post-production wastes containing textile dyes. CR was used as a model compound in these studies and the results show an effective binding of CR by carbon nanotubes (with a maximum adsorption of 500 mg/g).
The SWNT dispersion method that was used in our studies is based on the sonication of SWNT with CR solution and is much simpler and reproducible than the above described method [34]. It leads to the formation of complexes between graphene surfaces of carbon nanotubes and supramolecular ribbon-like structures created by CR molecules. An attempt was made to explain the mechanism of interaction between the ribbon-like supramolecular compounds like CR and the single-walled carbon nanotubes. The results indicate a very efficient binding of CR by SWNT (up to 35 mg CR per 1 mg of SWNT) (Fig. 7.1).
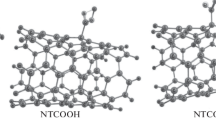
In contrast to the method proposed by Hu et al. the samples are not dried, which allows for efficient binding of CR – in the form of large supramolecular structures – to the SWNT surface. A model of SWNT-CR complex has been proposed based on a “face to face” interaction between CR and the carbon nanotube surface. In this model, the SWNT play a role of a scaffolding to the surface of which CR molecules are bound in the form of supramolecular, ribbon-like structures that protrude from the surface of the carbon nanotube. The nanotube-CR interaction involves the pi-pi stacking between the benzene rings (Fig. 7.2), as in the case of aforementioned surfactants .
Model of interaction between single CR molecule (left) and supramolecular CR (right) with the SWNT surface (based on calculations presented in [30])
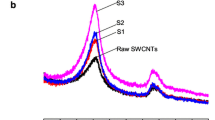
The supramolecular character of CR bound to the carbon nanotube surface is indicated by the results obtained during the analysis of SWNT-CR complexes formed in solutions of various ionic strengths. The properties of CR as a supramolecular system depend on the ionic strength of the solution [35]. If the ionic strength is low, then the ability of CR to form a supramolecular structure is also low, as a result of the lack of shielding of the negatively charged sulphonic groups by Na+ ions. In contrast, at high salt concentration the supramolecular structure is stabilized. In order to explain if single CR molecules are involved in the SWNT-CR interaction or whether the supramolecular structures are required, an analysis of the SWNT-CR interaction in solutions of various ionic strengths was performed. All samples were prepared in the 0.05 M Tris/HCl pH7.4 buffer. The significant differences in CR binding between samples to which 0.145 M NaCl was added and the NaCl-free samples were observed (Fig. 7.3).
Additional information concerning the mechanism of CR - nanotube interaction is provided by the birefringence of CR -nanotube complexes observed using polarized light microscopy. This phenomenon, which is typical for the amyloid containing preparations stained with CR , proves that the CR molecules bound to the fibres are ordered [36]. The presence of “apple-green” birefringence in histopathological samples is a diagnostic criterion for amyloidosis. In the case of the SWNT-CR complexes the birefringence also demonstrates the ordering of CR molecules bound to the carbon nanotube surface (Fig. 7.4) and supports the supramolecular, ribbon-like character of CR interacting with the carbon nanotube.
Samples containing carbon nanotubes sonicated with highly concentrated CR (above 5 mg/mL), presented gel-like properties. CR in these samples was bound to SWNT strongly enough not to be removed during filtration through the PTFE membrane (non-bonded CR can pass freely through this membrane) (Fig. 7.5A). SWNT-CR complexes in these samples slowly sedimented at the bottom of the test tube but could be easily dispersed even by gentle mixing (Fig. 7.5C). The formation of such big, sedimenting complexes can be explained by the presence of CR ribbons, which protrude from the surface of nanotubes and cross-link them to form stable gels, as in the case of bucky gels resulting from the interaction between CNT and ionic liquids (room-temperature ionic liquids , RILs) [24, 37]. In the case of solutions containing a lower concentration of CR the resulting solution is clearer, but also tends to separate over a longer period of time (Fig. 7.5B).
(A) SWNT-CR complexes – filtration on PTFE membrane ; (B) SWNT-CR complex obtained from 1 mg of SWNT and 2 mg CR , after filtration, suspended in 1 mL of the buffer; (C) SWNT-CR complex obtained from 1 mg of SWNT and 5 mg CR , after filtration, suspended in 1 mL of the buffer; the dashed line indicates the sedimentation of the gel-like complex
Spectral analyses have shown changes in the CR spectrum after binding to SWNT, demonstrating the interaction between CR and carbon nanotubes (Fig. 7.6). This effect is related to the change in the electron structure of the CR molecule after binding to carbon nanotubes and is explained by the transition of the CR molecule to the quinoid form [34, 38]. A similar phenomenon is described in the case of binding CR to the surface of graphene oxide [32]. According to the mechanism of the interaction between CR and SWNT described in our paper, not all molecules in the CR ribbon-like structure adhere directly to the surface of the nanotube, while the observed spectral effect – the shift of the absorbance maximum from 490 to 510 nm – suggests that all CR molecules (not only those that directly adhere to the nanotube surface) participate in the transfer of electrons (and switch to the quinoid form) [34].
The SWNT-CR complexes were studied using a variety of microscopic techniques. All analyses have shown that CR binding to the surface of the carbon nanotubes significantly increased their diameter. Depending on the amount of CR added per portion of single-walled carbon nanotubes more or less uniformly loaded nanotubes were obtained [34]. The TEM , SEM and AFM images of the SWNT-CR complexes confirm that the interaction between CR and carbon nanotube surface is based on the “face to face” stacking . A comparison of transmission microscopy images of a free carbon nanotube (Fig. 7.7A) and a CR -loaded nanotube (Fig. 7.7B) shows a sixfold increase in nanotube diameter after CR binding. The image shows that addition of highly concentrated CR (10 mg/mL) leads to an uniform covering of the carbon nanotube with CR . This can be interpreted as a large number of short, “face to face” bound CR “ribbons”, attached side by side to the carbon nanotube.
TEM micrographs of (A) a single SWNT, without CR ; (B) a single SWNT functionalized with CR (obtained from 1 mg of SWNT and 10 mg CR , after filtration, suspended in 1 mL of the buffer) – CR layers evenly distributed at the surface of a carbon nanotube are visible (dark lines indicate the thickness of CR layer)
The presence of supramolecular CR ribbons that protrude from the surface of carbon nanotubes was confirmed by the results obtained using the ternary complex, composed of CR , Titan yellow (TY) and silver ions (CR /TY/Ag) (see Chap. 6). Titan Yellow-silver ion complex (TY/Ag) intercalates between CR molecules within a supramolecular ribbon. This system was developed because CR molecules do not complex metal ions and thus cannot be directly used for contrasting in TEM images [39, 40]. Thanks to the use of silvercontrast, the binding sites of the ternary complex have been made visible. EDS (energy dispersive spectroscopy ) analysis of chemical composition, indicated the presence of silver and sulphur in these sites, which confirms the presence of both CR and TY/Ag. Electronically dense silver ions are visible primarily in places of higher CR /TY loading. Contrasting with silver ions also allowed to confirm the mechanism of supramolecular CR ribbons interaction with carbon nanotubes – silver is not directly attached to the carbon nanotube surface, but is bound to the dye that forms a sheath around the nanotube (Fig. 7.8).
Complexes of SWNT-CR contrasted by intercalated Titan Yellow -silver ion complexes (obtained from 1 mg of SWNT and 5 mg CR/TY/Ag (1:1:0.8 molar ratio), after removal of dyes and Ag excess by filtration). The EDS analysis indicates the presence of CR -TY-Ag complexes in highly loaded places (indicated by arrows)
The images obtained by scanning electron microscopy (Fig. 7.9) and atomic force microscopy [34] indicate an increase in carbon nanotube diameter after CR binding to its surface. Unevenly distribution of dye on nanotubes is seen on Fig. 7.9B.
The SWNT-CR complexes were also analysed by molecular modelling , which has shown various possibilities of nanotube-CR interaction depending on nanotube diameter [30]. In the case of narrow nanotubes, CR partially retains the supramolecular ribbon structure, adhering to the outer nanotube surface, while some of the molecules are adsorbed individually on the surface (as shown in Fig. 7.2). In the case of wider nanotubes, individual CR molecules adsorb to the nanotube surface and supramolecular ribbon-like structur e of CR is lost. The geometry of the wide nanotube increases the contact surface between CR molecule and the nanotube which allows to ascribe the dominant role in the creation of the system’s equilibrium structure to the pi-pi stacking . Nanotubes of smaller diameter have a smaller side wall area that is characterized by a greater curvature. For this reason, partial preservation of the supramolecular ribbon structure of CR is preferable. The molecular modelling results show a tendency for CR to group into ribbons, while simultaneously leaving a portion of the nanotube surface exposed, which was also observed in microscopic images. These results were also confirmed by analysis of the radial distribution functions (rdf) between CR and SWNT [30].
7.3 The Incorporation of Other Compounds by SWNT-CR Complexes – Examples of Possible Biomedical Use
A particularly interesting feature of carbon nanotube-CR complex is its ability to bind other molecules. CNT, as hollow structures, are excellent high volume lightweight containers, in which other molecules can be enclosed while their large surface allows for efficient adsorption of many compounds. CR (and similar compounds) that create ribbon-like supramolecular structures can bind numerous polyaromatic planar molecules that intercalate into supramolecular CR ribbon. Compounds that can be bound this way include e.g. antineoplastic drug doxorubicin, Titan yellow , rhodamine B . The combination SWNT and CR creates a “chemical container ” characterized by significantly increased capacity and the ability to bind different molecules simultaneously. It could be used as a drug carrier , which limits the drug’s toxicity and allows for the targeted delivery [41].
An interesting phenomenon of breaking carbon nanotubes resulting from the interaction with mixed supramolecular systems was observed. Mixed supramolecular systems (CR/EB and CR-DOX) interact with carbon nanotubes causing them to stiffen and break (Fig. 7.10). Dispersion of nanotubes by CR is not accompanied by their shortening. Similar dye – EB shows much lower nanotube dispersing capability and does not interact with their surface [42]. The effect of nanotube breaking can be explained by the mechanical weakening of the nanotube in the area where a large mixed supramolecular system is attached. This phenomenon can also be explained by changes in the electron structure of the nanotube and the supramolecular system bound to it.
The dispersion of carbon nanotubes based on their interaction with CR is simple, efficient and reproducible. Supramolecular systems (both pure CR and mixed) allow not only to disperse carbon nanotubes, but can also bind other compounds through intercalation . As carriers of different compounds (e.g. fluorescent dyes, drugs , metal ions ), these complexes present an interesting alternative to the currently used systems. They can be used in diagnostics as well as targeted delivery system s for drugs or metal ions.
References
Avouris P, Chen Z, Perebeinos V (2007) Carbon-based electronics. Nat Nanotechnol 2(10):605–615
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes-the route toward applications. Science 297(5582):787–792
Díez-Pascual AM, Ashrafi B, Naffakh M et al (2011) Influence of carbon nanotubes on the thermal, electrical and mechanical properties of poly(ether ether ketone)/glass fiber laminates. Carbon N Y 49(8):2817–2833
Guo D-J, Li H-L (2005) High dispersion and electrocatalytic properties of palladium nanoparticles on single-walled carbon nanotubes. J Colloid Interface Sci 286(1):274–279
Liu Y, Wang X, Qi K et al (2008) Functionalization of cotton with carbon nanotubes. J Mater Chem 18(29):3454
Besteman K, Lee J-O, Wiertz FGM et al (2003) Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett 3(6):727–730
Liu Y, Wang M, Zhao F et al (2005) The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens Bioelectron 21:984–988
Chen RJ, Bangsaruntip S, Drouvalakis KA et al (2003) Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci U S A 100(9):4984–4989
Ji S, Liu C, Zhang B et al (2010) Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta Rev Cancer 1806(1):29–35
Zanello LP, Zhao B, Hu H et al (2006) Bone cell proliferation on carbon nanotubes. Nano Lett 6(3):562–567
Pok S, Vitale F, Eichmann SL et al (2014) Biocompatible carbon nanotube–chitosan scaffold matching the electrical conductivity of the heart. ACS Nano 8(10):9822–9832
Aliev AE, Oh J, Kozlov ME et al (2009) Giant-stroke, superelastic carbon nanotube aerogel muscles. Science 323(5921):1575–1578
Chen J, Chen S, Zhao X et al (2008) Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J Am Chem Soc 130(49):16778–16785
Heister E, Neves V, Tîlmaciu C et al (2009) Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon N Y 47(9):2152–2160
Bhirde AA, Patel V, Gavard J et al (2009) Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3(2):307–316
Wong BS, Yoong SL, Jagusiak A et al (2013) Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev 65(15):1964–2015
Pastorin G (2011) Carbon nanotubes: from bench chemistry to promising biomedical applications. Pan Stanford Pub, Singapore
Bahr JL, Tour JM, Jakab E et al (2002) Covalent chemistry of single-wall carbon nanotubes. J Mater Chem 12(7):1952–1958
Prato M, Kostarelos K, Bianco A (2008) Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res 41(1):60–68
Akasaka T, Nagase S, Wudl F (2010) Chemistry of nanocarbons. Wiley, Chichester
Backes C, Hirsch A (2010) Noncovalent functionalization of carbon nanotubes in chemistry of nanocarbons. Wiley, Ltd, Chichester, pp 1–48
Wang H (2009) Dispersing carbon nanotubes using surfactants. Curr Opin Colloid Interface Sci 14(5):364–371
Islam MF, Rojas E, Bergey DM et al (2003) High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett 3(2):269–273
Tunckol M, Durand J, Serp P (2012) Carbon nanomaterial–ionic liquid hybrids. Carbon N Y 50(12):4303–4334
Tomonari Y, Murakami H, Nakashima N (2006) Solubilization of single-walled carbon nanotubes by using polycyclic aromatic ammonium amphiphiles in water—strategy for the design of high-performance solubilizers. Chem Eur J 12(15):4027–4034
Bluemmel P, Setaro A, Popeney CS et al (2010) Dispersion of carbon nanotubes using an azobenzene derivative. Phys Status Solidi 247(11–12):2891–2894
Haggenmueller R, Rahatekar SS, Fagan JA et al (2007) Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir 24(9):5070–5078
Hu C, Chen Z, Shen A et al (2006) Water-soluble single-walled carbon nanotubes via noncovalent functionalization by a rigid, planar and conjugated diazo dye. Carbon N Y 44(3):428–434
Mishra AK, Arockiadoss T, Ramaprabhu S (2010) Study of removal of azo dye by functionalized multi walled carbon nanotubes. Chem Eng J 162(3):1026–1034
Panczyk T, Wolski P, Jagusiak A et al (2014) Molecular dynamics study of Congo red interaction with carbon nanotubes. RSC Adv 4(88):47304–47312
Szlachta M, Wójtowicz P (2013) Adsorption of methylene blue and Congo red from aqueous solution by activated carbon and carbon nanotubes. Water Sci Technol 68(10):2240–2248
Barkauskas J, Stankevičienė I, Dakševič J et al (2011) Interaction between graphite oxide and Congo red in aqueous media. Carbon N Y 49(15):5373–5381
Du Q, Sun J, Li Y et al (2014) Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem Eng J 245:99–106
Jagusiak A, Piekarska B, Pańczyk T et al (2017) Dispersion of single-wall carbon nanotubes with supramolecular Congo red – properties of the complexes and mechanism of the interaction. Beilstein J Nanotechnol 8(1):636–648
Skowronek M, Stopa B, Konieczny L et al (1998) Self-assembly of Congo Red-A theoretical and experimental approach to identify its supramolecular organization in water and salt solutions. Biopolymers 46(5):267–281
Spólnik P, Król M, Stopa B et al (2011) Influence of the electric field on supramolecular structure and properties of amyloid-specific reagent Congo red Eur. Biophys J 40(10):1187–1196
Fukushima T, Kosaka A, Ishimura Y et al (2003) Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 300(5628):2072–2074
Pigorsch E, Elhaddaoui A, Turrell S (1994) Spectroscopic study of pH and solvent effects on the structure of Congo red and its binding mechanism to amyloid-like proteins. Spectrochim Acta Part A Mol Spectrosc 50(12):2145–2152
Chłopaś K, Jagusiak A, Konieczny L et al (2015) The use of Titan yellow dye as a metal ion binding marker for studies on the formation of specific complexes by supramolecular Congo red. Bio-Algorithms Med-Syst 11(1):9–17
Rybarska J, Konieczny L, Jagusiak A et al (2017) Silver ions as EM marker of congo red ligation sites in amyloids and amyloid-like aggregates. Acta Biochim Pol 64(1):161–169
Stopa B, Piekarska B, Jagusiak A et al (2011) Acta Biochim Pol 58(Suppl. 2):282. Supramolecular Congo red as a potential drug carrier. Properties of Congo red-doxorubicin complexes in Proceedings of the 2nd Congress of Biochemistry and Cell Biology 46th Meeting of the Polish Biochemical Society and 11st Conference of the Polish Cell Biology Society, Kraków, 2011
Jagusiak A, Piekarska B, Chłopaś K et al (2016) Shortening and dispersion of single-walled carbon nanotubes upon interaction with mixed supramolecular compounds. Bio-Algorithms Med-Syst 12(3):123–132
Acknowledgements
We acknowledge the financial support from the National Science Centre, Poland (grant no. 2016/21/D/NZ1/02763) and from the project Interdisciplinary PhD Studies “Molecular sciences for medicine” (co-financed by the European Social Fund within the Human Capital Operational Programme) and Ministry of Science and Higher Education (grant no. K/DSC/001370).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Jagusiak, A., Piekarska, B., Chłopaś, K., Bielańska, E. (2018). Congo Red Interactions with Single-Walled Carbon Nanotubes. In: Roterman, I., Konieczny, L. (eds) Self-Assembled Molecules – New Kind of Protein Ligands. Springer, Cham. https://doi.org/10.1007/978-3-319-65639-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-65639-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65638-0
Online ISBN: 978-3-319-65639-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)