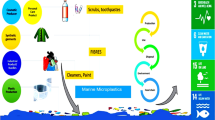
Abstract
In recent years, interest in the environmental occurrence and effects of microplastics (MPs) has shifted towards our inland waters, and in this chapter we provide an overview of the issues that may be of concern for freshwater environments. The term ‘contaminant of emerging concern’ does not only apply to chemical pollutants but to MPs as well because it has been detected ubiquitously in freshwater systems. The environmental release of MPs will occur from a wide variety of sources, including emissions from wastewater treatment plants and from the degradation of larger plastic debris items. Due to the chemical makeup of plastic materials, receiving environments are potentially exposed to a mixture of micro- and nano-sized particles, leached additives, and subsequent degradation products, which will become bioavailable for a range of biota. The ingestion of MPs by aquatic organisms has been demonstrated, but the long-term effects of continuous exposures are less well understood. Technological developments and changes in demographics will influence the types of MPs and environmental concentrations in the future, and it will be important to develop approaches to mitigate the input of synthetic polymers to freshwater ecosystems.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Anthropogenic activity has resulted in the deposition of a complex combination of materials in lake sediments, including synthetic polymers (plastics) that differ greatly from the Holocene signatures. Accordingly, plastics are considered one indicator of the Anthropocene [1]. Plastic has for some time been known to be a major component of riverine pollution [2,3,4,5,6], and plastic degradation products have been noted as a potential issue for soil environments [7]. However, up until recently the main focus of research on plastic pollution has been the marine environment. To highlight this, a literature search on Thomson Reuters’ ISI Web of Science returns 1,228 papers containing the term ‘microplastic*’, of which only a subset of 45 publications (3.7%) contains the term ‘freshwater’. This has started to change in recent years, and attention is now also been directed towards both the terrestrial [8, 9] and freshwater environments [8, 10, 11]. These publications point out the lack of knowledge for freshwater and terrestrial environments in terms of the occurrence and impacts of plastics debris.
Monitoring studies have quantified microscopic plastics debris, so-called microplastics (MPs), in freshwater systems, including riverine beaches, surface waters and sediments of rivers, lake, and reservoirs [12,13,14,15,16,17,18,19]. Although far less data is available compared to marine systems, these studies highlight that MP is ubiquitous and concentrations are comparable [20]. Alongside the monitoring data, ecotoxicological studies have mainly explored MP ingestion by various species and their effects on life history parameters [21,22,23,24]. While the majority of studies used primary microspheres of polyethylene (PE) and polystyrene (PS) at high concentrations [25] over short-term exposures, there is some evidence that MPs may pose a risk to freshwater ecosystems [26]. In addition, there is concern that long-term exposure may lead to bioaccumulation of submicron particles with wider implications for environmental health [27,28,29].
This chapter provides an overview of MPs and the issues, which may be of concern to freshwater environments. The first section provides a background to the topic of discussion by describing and defining plastic materials, MPs, emerging contaminants. Subsequent sections then discuss the potential input, fate and transportation, effects, and potential risk management options for plastics and MPs in freshwater environments.
2 Plastics and Microplastics: An Overview
In this section, some context to the topic of environmental MPs is given by (1) providing a brief historical overview of the development of plastic materials, (2) describing the complex chemical composition of plastic material, and (3) defining MPs as a contaminants of emerging concern.
2.1 A Brief Overview of Plastic Development
The creation of new synthetic chemicals combined with the engineering capabilities of mass production has made plastics one of the most popular materials in modern times. Today’s major usage of plastic materials can be traced back to the 1800s with the development of rubber technology. One of the key breakthroughs in this area was the discovery of vulcanisation of natural rubber by Charles Goodyear [30]. Throughout the 1800s a number of attempts were made to develop synthetic polymers including polystyrene (PS) and polyvinyl chloride (PVC), but at this time these materials were either too brittle to be commercially viable or would not keep their shape. The first synthetic polymer to enter mass production was Bakelite, a phenol-formaldehyde resin, developed by the Belgian chemist Leo Baekeland in 1909 [31]. Later, around the 1930s the modern forms of PVC, polyethylene terephthalate (PET), polyurethane (PUR), and a more processable PS were developed [32]. The early 1950s saw the development of high-density polyethylene (HDPE) and polypropylene (PP; Table 1). In the 1960s, advances in the material sciences led to the development of plastic materials produced other from natural resources [34], such as the bacterial fermentation of sugars and lipids, and include polyhydroxyalkanoates (PHA), polylactides (PLA), aliphatic polyesters, and polysaccharides [35]. PLA is on the verge of entering into bulk production, while PHA production is between pilot plant and commercial stage [36, 37].
2.2 Describing Plastic Materials
Plastics are processable materials based on polymers [38], and to make them into materials fit for purpose, they are generally processed with a range of chemical additives (Table 2). These compounds are used in order to adjust the materials properties and make them suitable for their intended purpose. Therefore, within polymer classifications plastic materials can still differ in structure and performance depending on the type and quantity of additives they are compounded with. More recently, technological advances have seen the development of new applications of elements based on nanoscales that are now producing plastic nanocomposites. The plastics industry is expected to be a major field for nanotechnology innovation. It is estimated that by 2020, the share of nanocomposites among plastics in the USA will be 7% [39]. These nanocomposites include materials that are reinforced with nano-fillers (nano-clay and nano-silica) for weight reduction, carbon nanotubes (CNTs) for improved mechanical strength, and nano-silver utilised as an antimicrobial agent in plastic food packaging materials.
2.3 Microplastics as Contaminants of Emerging Concern
The term ‘microplastics’ commonly refers to plastic particles whose longest diameter is <5 mm and is the definition used by most authors. It has been suggested that the term microplastics be redefined as items <1 mm to include only particles in the micrometer size range [40, 41], and the term ‘mesoplastic’ introduced to account for items between 1 and 2,500 mm [42]. Lambert et al. [8] described macroplastics as >5 mm, mesoplastics as ≤5 to >1 mm, microplastics as ≤1 mm to >0.1 μm, and nanoplastics as ≤0.1 μm. However, the upper limit of 5 mm is generally accepted because this size is able to include a range of small particles that can be readily ingested by organisms [42].
Generally, MPs are divided into categories of either primary or secondary MPs. Primary MPs are manufactured as such and are used either as resin pellets to produce larger items or directly in cosmetic products such as facial scrubs and toothpastes or in abrasive blasting (e.g. to remove lacquers). Compared to this deliberate use, secondary MPs are formed from the disintegration of larger plastic debris.
MPs have undoubtedly been present in the environment for many years. For instance, Carpenter et al. [43], Colton et al. [44], and Gregory [45] reported on marine plastics in the 1970s, but they have not been extensively studied particularly in the context of freshwater systems. As research focused on the issue more intensively since the early 2000s, MPs are considered contaminants of emerging concern [8, 10, 46].
3 Sources of Plastics and Microplastics into the Freshwater Environment
Plastics will enter freshwater environments from various sources through various routes. On land littering is an important environmental and public issue [47, 48] and is a matter of increasing concern in protected areas where volumes are influenced by visitor density; consequently, measures are now needed to reduce and mitigate for damage to the environment [49]. In addition, waste management practices in different regions of the world also vary, and this may be a more important source in one geographical region compared to another [8]. As with bulk plastic items, MPs can enter the environment by a number of pathways, and an important route in one geographical region may be less important in another. For example, primary MPs used in consumer cosmetics are probably more important in affluent regions [8]. MPs have several potential environmental release pathways: (1) passage through WWTPs, either from MP use in personal care products or release of fibres from textiles during the washing of clothes, to surface waters, (2) application of biosolids from WWTPs to agricultural lands [50], (3) storm water overflow events, (4) incidental release (e.g. during tyre wear), (5) release from industrial products or processes, and (6) atmospheric deposition of fibres (discussed further in Dris et al. [51]). Plastic films used for crop production are considered an important agricultural emission, and their use is thought to be one of the most important sources of plastic contamination of agricultural soils [52,53,54]. There advantages include conserve of moisture, thereby reducing irrigation; reduce weed growth and increase soil temperature which reduces competition for soil nutrients and reduces fertiliser costs, thereby improving crop yields; and protect against adverse weather conditions [7, 55]. However, weathering can make them brittle and difficult to recover resulting in disintegration of the material, and when coupled with successive precipitation events, the residues and disintegrated particles can be washed into the soil where they accumulate [7, 55, 56]. Other sources exist and include emissions from manufacturing and constructions sites. Automotive tyre wear particles may also release large volumes of synthetic particles. These tyre wear particles are recognised as a source of Zn to the environment, with anthropogenic Zn concentrations that are closely correlated to traffic density [57]. The sources and emission routes of nanoplastics are also discussed in Rist and Hartmann [58].
4 Occurrence in Freshwater Systems
The isolation of MPs in environmental matrices can be highly challenging particularly when dealing with samples high in organic content such as sediments and soils. Likewise, the spectroscopic identification of synthetic polymers is complicated by high pigment contents and the weathering of particles and fibres. Accordingly, the detection and analytical confirmation of MPs require access to sophisticated equipment (e.g. micro-FTIR and micro-Raman; discussed further in Klein et al. [20]). Recent monitoring studies have established that – similar to marine environments – MPs are ubiquitously found in a variety of freshwater matrices. Reported MP concentrations in surface water samples of the Rhine river (Germany) average 892,777 particles km−2 with a peak concentration of 3.9 million particles km−2 [15]. In river shore sediments the number of particles ranged from 228 to 3,763 and 786 to 1,368 particles kg−1 along the rivers Rhine and Main (Germany), respectively [19]. High surface water concentrations are reported at the Three Gorges Dam, China (192–13,617 particles km−2), which are attributed to a lack of wastewater treatment facilities in smaller towns, as well as infrastructure issues when dealing with recycling and waste disposal [14]. These studies may underestimate the actual MP concentrations because their separation and identification are based on visual observation methods (e.g. Reddy et al. [59]) and may exclude those in the submicron size ranges. The environmental occurrence and sources of MPs in freshwater matrices in an African, Asian, and European context are further discussed in Dris et al. [51], Wu et al. [60], Khan et al. [61], respectively.
5 Fate and Transport in Freshwater Systems
Once MPs are released or formed in the freshwater environment, they will undergo fate and transportation processes. In the following section, these processes are discussed.
5.1 Environmental Transportation
Many plastic materials that enter the environment will not remain stationary. Instead they will be transported between environmental compartments (e.g. from land to freshwater and from freshwater to marine environments), with varying residence times in each. For example, the movement from land to river systems will depend upon prevailing weather conditions, distance to a specific river site, and land cover type. The collection of plastic litter at roadside habitats is easily observed, and the regular grass cutting practices of road verges in some countries means that littered items are quickly disintegrated by mowing equipment [8]. The movement of MPs from land to water may then occur through overland run-off or dispersion (via cutting action) to roadside ditches. The movement of bulk plastics and MPs within the riverine system will be governed by its hydrology (e.g. flow conditions, daily discharge) and the morphology (e.g. vegetation pattern) at a specific river site that will have a large effect upon the propagation of litter because of stranding and other watercourse obstructions such as groynes and barrages [2]. Compared to larger plastics, MPs may also be subject to different rates of degradation as they will be transported and distributed to various environment compartments at quicker rates than macroplastics. The formation of MP-associated biofilms has been investigated for LDPE in marine setting [62]. Transport to sediments and the formation of biofilms over the surface of MPs may also limit rates of degradation as this removes exposure to light. The modelling of MP fate and transportation in freshwaters is discussed further in Kooi et al. [63], while MP-associated biofilm are discussed in Harrison et al. [64].
5.2 Environmental Persistence and Degradation
The majority of our current understanding regarding plastic degradation processes is derived from laboratory studies that often investigate a single mechanism such as photo-, thermal, or bio-degradation [65]. There is limited information on the degradation of plastics under environmentally relevant conditions where a number of degradation mechanisms occur at together. Where information is available these studies have tended to focus on weight loss, changes in tensile strength, breakdown of molecular structure, and identification of specific microbial strains to utilise specific polymer types. The degradation processes are defined in accordance with the degradation mechanism under investigation (e.g. thermal degradation) and the experimental result generated. In contrast, particle formation rates are often not investigated. This is important because polymers such as PE do not readily depolymerise and generally decompose into smaller fragments. These fragments then further disintegrate into increasingly smaller fragments eventually forming nanoplastics [66,67,68].
The prediction of plastic fragmentation rates is not a simple process. Kinetic fragmentation models have been investigated in the mathematics and physics literatures, and the kinetics of polymer degradation has been researched extensively in the polymer science literature. These models describe the distribution of fragment sizes that result from breakup events. These processes can be expressed by rate equations that assume each particle is exposed to an average environment, mass is the unit used to characterise a particle, and the size distribution is taken to be spatially uniform [69, 70]. These processes can be described linearly (i.e. particle breakup is driven only by a homogeneous external agent) or nonlinearly (i.e. additional influences also play a role), and particle shape can be accounted for by averaging overall possible particle shape [69]. The models used to describe these degradation process are often frequently complicated, but as a general rule focus on chain scission in the polymer backbone through (a) random chain scission (all bonds break with equal probability) characterised by oxidative reactions; (b) scission at the chain midpoint dominated by mechanical degradation; (c) chain-end scission, a monomer-yielding depolymerisation reaction found in thermal and photodecomposition processes; and (d) in terms of inhomogeneity (different bonds have different breaking probability and dispersed throughout the system) [71,72,73]. The estimation of degradation half-lives has also been considered for strongly hydrolysable polymers through the use of exponential decay eqs. [65, 74, 75]. However, the applicability of modelling the exponential decay of more chemically resistant plastics requires greater investigation [74].
Important variables that will influence MP degradation and fragmentation are environmental exposure conditions, polymer properties such as density and crystallinity (Table 3), and the type and quantity of chemical additives. Molecular characteristics that generally counteract degradation are the complexity of the polymer structure and the use of structural features that are not easy to biodegrade. Here, crystallinity is an important polymer property because the crystalline region consists of more ordered and tightly structured polymer chains. Crystallinity affects physical properties such as density and permeability. This in turn affects their hydration and swelling behaviour, which affects accessibility of sorption sites for microorganisms. Stabilisers such as antioxidants and antimicrobial agents act to prolong the life of plastics, whereas biological ingredients act to decompose the plastic in shorter time frames.
Overall, environmental degradation processes will involve MP fragmentation into increasingly smaller particles including nanoplastics, chemical transformation of the plastic fragments, degradation of the plastic fragments into non-polymer organic molecules, and the transformation/degradation of these non-polymer molecules into other compounds [65]. The environmental degradation of plastic materials is also further discussed in Klein et al. [20].
5.3 Interactions with Other Compounds
The sorption of hydrophobic pollutants to MPs is considered an important environmental process, because this will affect the mobility and bioavailability of these pollutants. It is well known that MPs in marine environments concentrate persistent organic pollutants (POPs) such as DDT, PCBs, and dioxins [78,79,80]. In addition, Ashton et al. [81] also found concentrations of metals in composite plastic pellet samples retrieved from the high tide line along a stretch of coastline in Southwest England. To investigate whether the metals were in fact associated with nonremovable fine organic matter associated with the pellet samples, new polypropylene pellets were suspended in a harbour for 8 weeks and were found to accumulate metals from the surrounding seawater, from low of 0.25 μg g−1 for Zn to a high of 17.98 μg g−1 for Fe [81]. So far, little data is available on freshwater and terrestrial ecosystems, which will have a pollutant makeup very different to that found in marine environments. In the freshwater environment MPs are likely to co-occur with other emerging contaminants such as pharmaceuticals, personal care products, flame retardants, and other industrial chemicals, which enter the environment as parts of complex solid and liquid waste streams.
Sorption processes will occur through physical and chemical adsorption as well as pore-filling processes. Physical adsorption is the reversible sorption to surfaces of the polymer matrix and does not involve the formation of covalent bonds. Chemical adsorption involves chemical reactions between the polymer surface and the sorbate. This type of reaction generates new chemical bonds at the polymer surface and may depend on how aged the polymer surface is. These processes can be influenced by changes in pH, temperature, and ionic strength of the localised environment [82]. Pore-filling occurs when hydrophobic pollutants enter the polymer matrix and will be dependent on the pore diameter of a particular polymer structure and the molecular size of the chemical. Here, pollutants with lower molecular weights will more easily move through a polymer matrix with larger pores.
Adsorption kinetics will depend on polymer type, polymer characteristics such as density and crystallinity, the surrounding environment, and the pollutants present. For instance, the sorption and diffusion of hydrophobic contaminants are most likely to take place in the amorphous area of a plastic material, because the crystalline region consists of more ordered and tightly structured polymer chains. Polymers that have structures with short and repeating units, a high symmetry, and strong interchain hydrogen bonding will have a lower sorption potential. A good example is low-density polyethylene (LDPE) and high-density polyethylene (HDPE; Table 3). LDPE contains substantial concentrations of branches that prevent the polymer chains from been easily stacked side by side. This results in a low crystallinity and a density of 0.90–0.94 g cm−3 [83]. Whereas, HDPE consists primarily of linear unbranched molecules and is chemically the closest in structure to pure polyethylene. The linearity HDPE has a high degree of crystallinity and higher density of 0.94–0.97 g cm−3 [83]. LDPE is often used for passive sampling devices to determine dissolved polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls (PCB), and other hydrophobic organic compounds in aquatic environments [84,85,86,87,88]. Batch sorption experiments were also used to determine PAH sorption to LDPE and HDPE pellets, and LDPE was identified to exhibit higher diffusion coefficients than HDPE meaning shorter equilibrium times for low-density polymers [89]. This indicates that PE is of interest from an environmental viewpoint because of its high sorption capacity. In addition, particle size will influence the sorption parameters because the higher surface to volume ratio of smaller particles will shorten diffusion times. Isolating and quantifying the sorption mechanisms for all polymer types in use today will be challenging, because sorption behaviour may differ within polymer classification depending on the type and quantity of additive compounds the polymer is compounded with and the effects that this may have on polymer crystallinity and density. These issues are discussed in further detail in Scherer et al. [26] and Rist and Hartmann [58] in relation to MP and nanoplastics, respectively.
An interesting question is to what extent does irreversible sorption play a role? Some evidence in the pesticides literature suggests that a proportion of pesticides bind irreversibly soils [90, 91]. The study of sorption equilibrium isotherms is an important step in investigating the sorption processes that exist between different polymer types and co-occurring hydrophobic contaminants. This will make it possible to identify the sorption and diffusion relationships between case study co-occurring contaminants and MPs. Another interesting question is to what extent sorbed chemicals become bioavailable in the water column due to the continued breakdown and degradation of the MPs, or due to changes in environmental conditions, such as changes in pH, temperature, or system chemistry.
6 Effects of Plastics and Microplastics on Freshwater Ecosystems
Once in the aquatic environment, the mobility and degradation of plastics will generate a mixture of parent materials, fragmented particles of different sizes, and other non-polymer degradation products. Accordingly, biota will be exposed to a complex mixture of plastics and plastic-associated chemicals that changes in time and space.
6.1 Uptake and Biological Effects
MPs may be taken up from the water column and sediment by a range of organisms. This can occur directly through ingestion or dermal uptake most importantly through respiratory surfaces (gills). Previous investigations on freshwater zooplankton have included Bosmina coregoni that did not differentiate between PS beads (2 and 6 μm) and algae when exposed to combinations of both [92]. The same study also found that Daphnia cucullata, when exposed to PS beads (2, 6, 11, and 19 μm) in combination with algae cells of the same size, was observed to exhibit similar filtering rates for the three smaller size classes but preferred alga over the larger beads [92]. Rosenkranz et al. [93] demonstrated that D. magna ingests nano (20 nm) and micro (1 μm) PS beads. The authors note that both types of PS beads were excreted to some extent, but the 20 nm beads were retained to a greater degree within the organism.
The extent to which organisms are exposed to physical stress because of MP uptake depends on particle size, because particles larger than sediment or food particles may be harder to digest [94]. In addition, particle shape is also an important parameter, because particles with a more needle-like shape may attach more readily to internal and external surfaces. The indirect effects of MPs may include physical irritation, which may depend on MP size and shape. Smaller more angular particles may be more difficult to dislodge than smooth spherical particles and cause blockage of gills and digestive tract. In a recent study, the chronic effects of MP exposure to D. magna were evaluated [21]. Exposure to secondary MPs (mean particle size 2.6 μm) caused elevated mortality, increased inter-brood period, and decreased reproduction but only at very high MP levels (105,000 particles L−1). In contract, no effects were observed in the corresponding primary MP (mean particle size 4.1 μm) [21].
There is some evidence suggesting that a trophic transfer of MP may occur, for instance, from mussels to crabs [27]. The blue mussel Mytilus edulis was exposed to 0.5 μm PS spheres (ca. 1 million particles mL−1) and fed to crabs (Carcinus maenas). The concentration of microspheres in the crab haemolymph was reported to be the highest after 24 h (15,033 particles mL−1) compared to 267 residual particles mL−1 after 21 days, which is 0.027% of the concentration fed to the mussels. Another study has demonstrated the potential of MP transfer from meso- to macro-zooplankton, using PS microspheres (10 μm) at much lower concentrations of 1,000, 2,000, and 10,000 particles mL−1 [28]. Because excretion rates are unavailable and MP uptake is often defined as particles present in the digestive tract (i.e. the outside and not the tissues of an organism), it is so far not clear whether the trophic transfer of MP also results in a bioaccumulation or biomagnification. However, it is clear that MP will certainly be transferred from the prey to the predator and that this can – in certain situations – be retained for longer periods in the body of the latter.
An open question is to what extent the organisms consume naturally occurring microparticles and how the effects compare to MPs (for a more in-depth discussion on this topic see Scherer et al. [26]). This is important because naturally occurring particles are an important component of aquatic ecosystems and particle properties, such as concentration, particle size distribution, shape, and chemical composition, as well as duration of exposure plays a strong role in determining their interactions with aquatic communities [95].
Overall, an understanding of the relationships between cellular level responses and population level impacts will be important in order to determine the broader implications for ecosystem functioning. Points to be assessed concern both the biological aspects (molecular target, affected endpoints) and the particle aspects such as MP physical and chemical characteristics. The bioavailability of the MPs and the penetration of submicron MPs into the cells are factors to take into consideration.
6.2 Effects of Leaching Chemicals
The environmental effects of residual starting substances and monomers, non-intentionally added substances (impurities, polymerisation byproducts, breakdown products), catalysts, solvents, and additives leaching from plastic materials are not easy to assess [96]. The mixture composition and concentration of leachable compounds depend on the physical, chemical, and biological conditions of receiving environments. The leaching of water-soluble constituents from plastic products using deionised water is considered a useful method for profiling environmental hazards posed by plastics [97, 98]. Lithner et al. used such leachates in a direct toxicity testing approach to assess their acute toxicity to D. magna [97, 98]. For instance, with a liquid to solid (L/S) ratio of 10 and 24 h leaching time, leachates from polyvinyl chloride (PVC), polyurethane (PUR), and polycarbonate (PC) were the most toxic with EC50 values of 5–69 g plastic L−1 [98]. Higher L/S ratios and longer leaching times resulted in leachates from plasticised PVC and epoxy resin products to be the most toxic at (EC50 of 2–235 g plastic L−1) [99]. In a recent study, Bejgarn et al. [99] investigated the leachates from plastic that were ground to a power and had undergone artificial weathering, using a L/S of 10 and a 72 h leaching time, to the marine harpacticoid copepod Nitocra spinipes. Here, leachates from different PVC materials differed in their toxicity, with the toxicity of leachate from PVC packaging increasing after artificial weathering; whereas the leachate from PVC garden hose material decreased after artificial weathering [99]. This study also showed that the leachable PVC constitutes were a complex mixture of substances, and interestingly mass fragments containing chlorine were not identified. There are many challenges associated with the characterisation of such leachates owing to the potential diversity of physicochemical properties that chemical migrants and breakdown products may have. A test protocol for the identification of migration products from food contact materials has been developed that combines LC-TOF-MS and GC-MS techniques that generate accurate mass and predicted formulae to screen for volatile, semi-volatile, and non-volatile substances [100, 101].
Overall, the L/S ratio of plastic material used in these studies is higher than that typically identified during environmental monitoring studies. However, this type of screening when applied to materials manufactured from hazardous monomers and additives could facilitate the identification of compounds of interest so that they can be effectively replaced.
6.3 Biological Effects of Sub-micrometer Plastics
Depending on their use, plastic materials can contain compounds such as antimicrobial agents and nanomaterials that may be toxic to organisms such as bacteria and fungi that play a critical role in ecosystem functioning. It is possible that a combination of microscopic particles, leached additives, and other degradation products may cause subtle effects towards aquatic and terrestrial organisms that are difficult to identify in current testing methodologies. The formation of plastic particles in the submicron and nanometer size range during degradation is highly likely [8, 40, 66, 102, 103]. Engineered nanoparticles (ENPs) are able to cross cell membranes and become internalised, and the uptake of ENPs is size dependent with uptake occurring by endocytosis or phagocytosis [104]. Once inside the cell ENPs are stored inside vesicles and mitochondria and able to exert a response [104]. Cellular responses include oxidative stress, antioxidant activity, and cytotoxicity [105]. In terms of toxicity assessments, there is a need to understand the molecular and cellular pathways and the kinetics of absorption, distribution, metabolism, and excretion mechanisms that may be unique to MPs in the nano-size range. Desai et al. [106] showed that 100 nm particles of a polylactic polyglycolic acid copolymer had a tenfold higher intracellular uptake in an in vitro cell culture when compared to 10 μm particles made of the same material. ENPs have also been shown to produce cytotoxic, genotoxic, inflammatory, and oxidative stress responses in mammalian and fish systems [107]. A literature review by Handy et al. [108] highlighted the gills, gut, liver, and brain as possible target organs in fish, as well as a range of toxic effects including oxidative stress, cellular pathologies consistent with tumour formation in the liver, some organ specific iono-regulatory disturbances, and vascular injury. Taking into account the complex chemical makeup of some plastics and the ability to sorb co-occurring contaminants, experimental investigation of these endpoints for MPs seems to be merited. There are many lessons to be learned from the growing literature on the biological effects of ENPs, and these are discussed in more detail in Rist and Hartmann [58].
7 Considerations for Assessing Environmental Risks
In most countries chemical risk assessments rely on mass concentrations of substances of interest as an exposure and effect metric. In the nano-literature the mass concentrations of particles predicted to be emitted have been used to assess the risks of ENPs [109, 110]. These approaches assume particles are evenly distributed with no transfer between different environmental compartments. This approach was further developed by Gottschalk et al. [111] who used transfer coefficients to model emission flows between the different compartments used in their model, as well as the inclusion of sedimentation rates. Such modelling approaches (further discussed in Kooi et al. [63]) could be used to assess the environmental fate of primary MPs where emissions to the environment are distributed across a geographical region proportional to population density and consumption rates, assuming that the route of enter into the environment depends on the use of the MP. However, this type of approach requires extensive information on primary MP production levels, industrial applications and uses, levels in consumer products, fate in wastewater treatment, discharges to landfill, and environmental fate and distribution modelling to perform a meaningful exposure assessment. An exposure assessment for secondary MPs will require monitoring data, but this is hindered as the size ranges reported in field studies are generally constrained by the sampling techniques used [42].
The problems of using mass concentrations as an effect metric are similar to those discussed in the context of ENPs in that biological effects might not be mass dependent but dependent on physical and chemical properties of the substance in question [112, 113]. Consequently, when estimating the hazards presented by MP properties such as size, shape, polymer density, surface area, chemical composition of the parent plastic, and the chemical composition of sorbed co-occurring contaminants may need to be considered [114]. However, when considering secondary MPs information on some of these properties may be unavailable. This lack of information makes it difficult to identify the key characteristics, or combinations of characteristics, that may be responsible for hazards in the environment.
The assessment of MPs based on their chemical composition also presents a considerable challenge, because chemically MPs can be considered as a mixture. A simplified example of a risk assessment for polyurethane (PUR) based on its chemical composition is provided in Table 4. PUR flexible foam is used for mattresses and car seats and is made by combining three monomers and can consist of up to 18% flame retardant content [117]. An example risk assessment based on predicted environmental concentration (PEC)/predicted no effect concentration (PNEC) ratios for all components of the mixture are then used to calculate a risk quotient (RQ; Table 4). The RQ for this particular example is less than one; however, this type of assessment does not account for potential negative effects caused by physical irritation of solid particles. In this case it becomes clear that risk assessment for MPs as with ENPs holds specific challenges (see Brennholt et al. [118] for an in-depth discussion of the regulatory challenges).
The different particles sizes of MPs in environmental systems will present different risks to organisms living in those systems. For example, small plankton feeding fish species may encounter MPs from the nanoscale through to MPs 5 mm or greater. The fish may avoid larger particles but small particles may be ingested while feeding. For filter feeding organisms the upper size boundary will depend on the size of particles that a particular organism will naturally ingest. The risk assessment of MPs could therefore be based on particle size. A simplified hypothetical case is presented in Box 1 that draws on an example given by Arvidsson [119]. This approach assumes that there is information on harm-related thresholds of MPs based on size classes and particle concentration for the most sensitive species in that particles size range. However, the use of particle size for defining environmental risk may not be that straight forward, because MPs are not monodispersed in the environment. Additionally, as described by Hansen, [120] when discussing ENPs it remains unclear whether a ‘no effect threshold’ can be established, what the best hazard descriptor(s) are, and what are the most relevant endpoints.
Box 1: A hypothetical case for the risk assessment for MPs based on particles size
A lake has a PEC of MPs ≤5 mm at 10,000 particles/L and it is assumed that the PNEC of these particles is 1,000 particles/L. Furthermore, it is assumed that 1% of the PEC consists of particles <1 mm, assuming that the lower boundary is the same; the RQ is then determined by the upper boundary of the particles size as given below:
Risk or no risk is then determined by the setting of the upper boundary.
8 Concluding Thoughts
In this chapter, we have provided a brief overview of the environmental challenges associated with MP in freshwater systems and refer the readers to the appropriate chapters of this book for more detailed information. Overall, the environmental inputs in different geographical regions may vary depending on per capita consumption of consumer plastics, population demographics [121], and the capability of infrastructure to deal with waste materials. Environmental concentrations may change in the long term (whether positivity or negative) because of urbanisation, population increase, and technological developments. A better understanding of the environmental exposure in different geographical regions will identify those areas where mitigation actions and options will be the most effective. Future work should focus on better understanding the environmental fate and ecological impacts of MPs. Such an understanding should ultimately allow the development of new modelling approaches to assess transport of MPs in soil, sediments, and the water column. Little is also known about the long-term, subtle effects of MP exposure and sensitive endpoints (e.g. oxidative stress) need to be identified that integrate particle as well as chemical toxicity. Finally, although science is far from understanding the ecological implications of freshwater MPs, technological innovation, societal action, and political interventions need to be taken to mitigate the plastics pollution, which will – in case of inaction – certainly increase over the years to come.
References
Waters CN, Zalasiewicz J, Summerhayes C, Barnosky AD, Poirier C, Galuszka A, Cearreta A, Edgeworth M, Ellis EC, Ellis M, Jeandel C, Leinfelder R, McNeill JR, Richter D, Steffen W, Syvitski J, Vidas D, Wagreich M, Williams M, Zhisheng A, Grinevald J, Odada E, Oreskes N, Wolfe AP (2016) The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351(6269):aad2622
Balas CE, Williams AT, Simmons SL, Ergin A (2001) A statistical riverine litter propagation model. Mar Pollut Bull 42(11):1169–1176. doi:10.1016/S0025-326X(01)00133-3
Williams AT, Simmons SL (1999) Sources of riverine litter: The River Taff, South Wales, UK. Water Air Soil Pollut 112(1–2):197–216
Williams AT, Simmons SL (1997) Movement patterns of riverine litter. Water Air Soil Pollut 98(1):119–139. doi:10.1007/bf02128653
Williams AT, Simmons SL (1996) The degradation of plastic litter in rivers: implications for beaches. J Coast Conserv 2(1):63–72. doi:10.1007/bf02743038
Tudor DT, Williams AT (2004) Development of a ‘matrix scoring technique’ to determine litter sources at a Bristol channel beach. J Coast Conserv 10(1–2):119–127. doi:10.1652/1400-0350(2004)010[0119:doamst]2.0.co;2
Klemchuk PP (1990) Degradable plastics – a critical-review. Polym Degrad Stab 27(2):183–202
Lambert S, Sinclair CJ, Boxall ABA (2014) Occurrence, degradation and effects of polymer-based materials in the environment. Rev Environ Contam Toxicol 227:1–53. doi:10.1007/978-3-319-01327-5_1
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46(12):6453–6454. doi:10.1021/es302011r
Wagner M, Scherer C, Alvarez-Muñoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T, Rodriguez-Mozaz S, Urbatzka R, Vethaak A, Winther-Nielsen M, Reifferscheid G (2014) Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur 26(1):1–9. doi:10.1186/s12302-014-0012-7
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82. doi:10.1016/j.watres.2015.02.012
Zbyszewski M, Corcoran PL (2011) Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut 220(1–4):365–372. doi:10.1007/s11270-011-0760-6
Biginagwa FJ, Mayoma BS, Shashoua Y, Syberg K, Khan FR (2016) First evidence of microplastics in the African Great Lakes: recovery from Lake Victoria Nile perch and Nile tilapia. J Great Lakes Res 42(1):146–149. doi:10.1016/j.jglr.2015.10.012
Zhang K, Gong W, Lv J, Xiong X, Wu C (2015) Accumulation of floating microplastics behind the three Gorges Dam. Environ Pollut 204:117–123. doi:10.1016/j.envpol.2015.04.023
Mani T, Hauk A, Walter U, Burkhardt-Holm P (2015) Microplastics profile along the Rhine River. Sci Rep 5:17988. doi:10.1038/srep17988
Bartsch WM, Axler RP, Host GE (2015) Evaluating a Great Lakes scale landscape stressor index to assess water quality in the St. Louis River area of concern. J Great Lakes Res 41(1):99–110. doi:10.1016/j.jglr.2014.11.031
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181. doi:10.1016/j.envpol.2014.02.006
Castañeda RA, Avlijas S, Simard MA, Ricciardi A (2014) Microplastic pollution in St. Lawrence River sediments. Can J Fish Aquat Sci 71(12):1767–1771. doi:10.1139/cjfas-2014-0281
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-main area in Germany. Environ Sci Technol 49(10):6070–6076. doi:10.1021/acs.est.5b00492
Klein S, Dimzon IK, Eubeler J, Knepper TP (2017) Analysis, occurrence, and degradation of microplastics in the aqueous environment. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_3 (in this volume)
Ogonowski M, Schur C, Jarsen A, Gorokhova E (2016) The effects of natural and anthropogenic microparticles on individual Fitness in Daphnia magna. PLoS One 11(5):e0155063
Booth AM, Hansen BH, Frenzel M, Johnsen H, Altin D (2016) Uptake and toxicity of methyl methacrylate-based nanoplastic particles in aquatic organisms. Environ Toxicol Chem 35(7):1641–1649
Greven AC, Merk T, Karagoz F, Mohr K, Klapper M, Jovanovic B, Palic D (2016) Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ Toxicol Chem 35(12):3093–3100
Green DS, Boots B, Sigwart J, Jiang S, Rocha C (2016) Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ Pollut 208:426–434. doi:10.1016/j.envpol.2015.10.010
Phuong NN, Zalouk-Vergnoux A, Poirier L, Kamari A, Châtel A, Mouneyrac C, Lagarde F (2016) Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ Pollut 211:111–123. doi:10.1016/j.envpol.2015.12.035
Scherer C, Weber A, Lambert S, Wagner M (2017) Interactions of microplastics with freshwater biota. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer Nature, Heidelberg. doi:10.1007/978-3-319-61615-5_8 (in this volume)
Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.) Environ Pollut 177:1–3
Setälä O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83. doi:10.1016/j.envpol.2013.10.013
Murray F, Cowie PR (2011) Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar Pollut Bull 62(6):1207–1217. doi:10.1016/j.marpolbul.2011.03.032
Stevenson K, Stallwood B, Hart AG (2008) Tire rubber recycling and bioremediation: a review. Biorem J 12(1):1–11. doi:10.1080/10889860701866263
Vlachopoulos J, Strutt D (2003) Polymer processing. Mater Sci Technol 19(9):1161–1169. doi:10.1179/026708303225004738
Brandsch J, Piringer O (2008) Characteristics of plastic materials. In: Plastic packaging. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 15–61. doi:10.1002/9783527621422.ch2
Lambert S (2013) Environmental risk of polymers and their degradation products. PhD thesis, University of York
Lambert S (2015) Biopolymers and their application as biodegradable plastics. In: Kalia CV (ed) Microbial factories: biodiversity, biopolymers, bioactive molecules, vol 2. Springer India, New Delhi, pp 1–9. doi:10.1007/978-81-322-2595-9_1
Reddy CSK, Ghai R, Rashmi KVC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87(2):137–146. doi:10.1016/S0960-8524(02)00212-2
Mohan SR (2016) Strategy and design of innovation policy road mapping for a waste biorefinery. Bioresour Technol 215:76–83. doi:10.1016/j.biortech.2016.03.090
Amulya K, Jukuri S, Venkata Mohan S (2015) Sustainable multistage process for enhanced productivity of bioplastics from waste remediation through aerobic dynamic feeding strategy: process integration for up-scaling. Bioresour Technol 188:231–239. doi:10.1016/j.biortech.2015.01.070
Baner AL, Piringer O (2007) Preservation of quality through packaging. In: Plastic packaging materials for food. Wiley-VCH Verlag GmbH, Weinheim, pp 1–8. doi:10.1002/9783527613281.ch01
Roes L, Patel MK, Worrell E, Ludwig C (2012) Preliminary evaluation of risks related to waste incineration of polymer nanocomposites. Sci Total Environ 417:76–86. doi:10.1016/j.scitotenv.2011.12.030
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. doi:10.1021/es201811s
GESAMP (2015) Sources, fate and effects of microplastics in the marine environment: a global assessment. Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection Reports and studies 90
Carpenter E, Anderson SJ, Miklas HP, Peck BB, Harvey GR (1972) Polystyrene spherules in coastal waters. Science 178(4062):749. doi:10.1126/science.178.4062.749
Colton JB, Knapp FD, Burns BR (1974) Plastic particles in surface waters of Northwestern Atlantic. Science 185(4150):491–497. doi:10.1126/science.185.4150.491
Gregory MR (1977) Plastic pellets on New-Zealand beaches. Mar Pollut Bull 8(4):82–84. doi:10.1016/0025-326x(77)90193-x
Sutherland WJ, Clout M, Cote IM, Daszak P, Depledge MH, Fellman L, Fleishman E, Garthwaite R, Gibbons DW, De Lurio J, Impey AJ, Lickorish F, Lindenmayer D, Madgwick J, Margerison C, Maynard T, Peck LS, Pretty J, Prior S, Redford KH, Scharlemann JPW, Spalding M, Watkinson AR (2010) A horizon scan of global conservation issues for 2010. Trends Ecol Evol 25(1):1–7. doi:10.1016/j.tree.2009.10.003
Seco Pon JP, Becherucci ME (2012) Spatial and temporal variations of urban litter in Mar del Plata, the major coastal city of Argentina. Waste Manag 32(2):343–348. doi:10.1016/j.wasman.2011.10.012
Njeru J (2006) The urban political ecology of plastic bag waste problem in Nairobi, Kenya. Geoforum 37(6):1046–1058
Cierjacks A, Behr F, Kowarik I (2012) Operational performance indicators for litter management at festivals in semi-natural landscapes. Ecol Indic 13(1):328–337. doi:10.1016/j.ecolind.2011.06.033
Nizzetto L, Langaas S, Futter M (2016) Pollution: Do microplastics spill on to farm soils? Nature 537(7621):488–488. doi:10.1038/537488b
Dris R, Gasperi J, Tassin B (2017) Sources and fate of microplastics in urban areas: a focus on Paris Megacity. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_4 (in this volume)
Xu G, Wang QH, Gu QB, Cao YZ, Du XM, Li FS (2006a) Contamination characteristics and degradation behavior of low-density polyethylene film residues in typical farmland soils of China. J Environ Sci Health Part B 41(2):189–199. doi:10.1080/03601230500365069
Brodhagen M, Peyron M, Miles C, Inglis DA (2015) Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl Microbiol Biotechnol 99(3):1039–1056. doi:10.1007/s00253-014-6267-5
Kyrikou I, Briassoulis D (2007) Biodegradation of agricultural plastic films: a critical review. J Polym Environ 15(2):125–150. doi:10.1007/s10924-007-0053-8
Liu EK, He WQ, Yan CR (2014) ‘White revolution’ to ‘white pollution’ – agricultural plastic film mulch in China. Environ Res Lett 9(9):091001. doi:10.1088/1748-9326/9/9/091001
Xu J, Wang P, Guo WF, Dong JX, Wang L, Dai SG (2006b) Seasonal and spatial distribution of nonylphenol in Lanzhou Reach of Yellow River in China. Chemosphere 65(9):1445–1451. doi:10.1016/j.chemosphere.2006.04.042
Councell TB, Duckenfield KU, Landa ER, Callender E (2004) Tire-wear particles as a source of zinc to the environment. Environ Sci Technol 38(15):4206–4214. doi:10.1021/es034631f
Rist SE, Hartmann NB (2017) Aquatic ecotoxicity of microplastics and nanoplastics - lessons learned from engineered nanomaterials. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_2 (in this volume)
Reddy MS, Basha S, Adimurthy S, Ramachandraiah G (2006) Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuar Coast Shelf Sci 68(3–4):656–660. doi:10.1016/j.ecss.2006.03.018
Wu C, Zhang K, Xiong X (2017) Microplastic pollution in inland waters focusing on Asia. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_5 (in this volume)
Khan FR, Mayoma BS, Biginagwa FJ, Syberg K (2017) Microplastics in inland African waters: presence, sources and fate. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_6 (in this volume)
Harrison JP, Schratzberger M, Sapp M, Osborn AM (2014) Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol 14(232):014–0232. doi:10.1186/s12866-014-0232-4
Kooi M, Besseling E, Kroeze C, van Wezel AP, Koelmans AA (2017) Modeling the fate and transport of plastic debris in freshwaters: review and guidance. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_7 (in this volume)
Harrison JP, Hoellein TJ, Sapp M, Tagg AS, Ju-Nam Y, Ojeda JJ (2017) Microplastic-associated biofilms: a comparison of freshwater and marine environments. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_9 (in this volume)
Lambert S, Sinclair CJ, Bradley EL, Boxall ABA (2013) Effects of environmental conditions on latex dergadation in aquatic systems. Sci Total Environ 447:225–234. doi:10.1016/j.scitotenv.2012.12.067
Lambert S, Wagner M (2016a) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145:265–268. doi:10.1016/j.chemosphere.2015.11.078
Lambert S, Wagner M (2016b) Formation of microscopic particles during the degradation of different polymers. Chemosphere 161:510–517. doi:10.1016/j.chemosphere.2016.07.042
Gigault J, Pedrono B, Maxit B, Ter Halle A (2016) Marine plastic litter: the unanalyzed nano-fraction. Environ Sci Nano 3(2):346–350. doi:10.1039/c6en00008h
Cheng Z, Redner S (1990) Kinetics of fragmentation. J Phys A Math Gen 23(7):1233
Redner S (1990) Statistical theory of fragmentation. In: Charmet JC, Roux S, Guyon E (eds) Disorder and fracture, NATO ASI series, vol 204. Springer, New York, pp 31–48. doi:10.1007/978-1-4615-6864-3_3
Ziff RM, McGrady ED (1985) The kinetics of cluster fragmentation and depolymerisation. J Phys A Math Gen 18(15):3027
McCoy BJ, Madras G (1997) Degradation kinetics of polymers in solution: dynamics of molecular weight distributions. AICHE J 43(3):802–810. doi:10.1002/aic.690430325
Rabek JB (1975) Oxidative degradation of polymers. In: Bamford CH, Tipper CFH (eds) Comprehensive chemical kinetics: degradation of polymers, vol 14. Elsevier Scientific Publishing Company, Amsterdam
Bartsev SI, Gitelson JI (2016) A mathematical model of the global processes of plastic degradation in the World Ocean with account for the surface temperature distribution. Dokl Earth Sci 466(2):153–156. doi:10.1134/s1028334x16020033
Leejarkpai T, Suwanmanee U, Rudeekit Y, Mungcharoen T (2011) Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag 31(6):1153–1161. doi:10.1016/j.wasman.2010.12.011
Beyler CL, Hirschler MM (2002) Thermal decomposition of polymers. In: SFPE handbook of fire protection engineering, vol 2, Sect. 1, Chap. 7. National Fire Protection Association, Quincy
Ehrenstein GW (2012) Polymeric materials: structure, properties, applications. Hanser Gardner Publications, Inc., München
Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Bjorn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Pham HV, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B Biol Sci 364(1526):2027–2045. doi:10.1098/rstb.2008.0284
Endo S, Takizawa R, Okuda K, Takada H, Chiba K, Kanehiro H, Ogi H, Yamashita R, Date T (2005) Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar Pollut Bull 50(10):1103–1114. doi:10.1016/j.marpolbul.2005.04.030
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35(2):318–324. doi:10.1021/es0010498
Ashton K, Holmes L, Turner A (2010) Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull 60(11):2050–2055. doi:10.1016/j.marpolbul.2010.07.014
Delle Site A (2001) Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J Phys Chem Ref Data 30(1):187–439. doi:10.1063/1.1347984
Bajracharya RM, Manalo AC, Karunasena W, Lau K-T (2014) An overview of mechanical properties and durability of glass-fibre reinforced recycled mixed plastic waste composites. Mater Des 62:98–112. doi:10.1016/j.matdes.2014.04.081
Hale SE, Meynet P, Davenport RJ, Martin Jones D, Werner D (2010) Changes in polycyclic aromatic hydrocarbon availability in River Tyne sediment following bioremediation treatments or activated carbon amendment. Water Res 44(15):4529–4536. doi:10.1016/j.watres.2010.06.027
Allan IJ, Harman C, Kringstad A, Bratsberg E (2010) Effect of sampler material on the uptake of PAHs into passive sampling devices. Chemosphere 79(4):470–475. doi:10.1016/j.chemosphere.2010.01.021
Fernandez LA, MacFarlane JK, Tcaciuc AP, Gschwend PM (2009) Measurement of freely dissolved PAH concentrations in sediment beds using passive sampling with low-density polyethylene strips. Environ Sci Technol 43(5):1430–1436. doi:10.1021/es802288w
Adams RG, Lohmann R, Fernandez LA, MacFarlane JK (2007) Polyethylene devices: passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ Sci Technol 41(4):1317–1323. doi:10.1021/es0621593
Booij K, Smedes F, van Weerlee EM (2002) Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers. Chemosphere 46(8):1157–1161. doi:10.1016/S0045-6535(01)00200-4
Fries E, Zarfl C (2012) Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE). Environ Sci Pollut Res 19(4):1296–1304. doi:10.1007/s11356-011-0655-5
Wanner U, Fuhr F, DeGraaf AA, Burauel P (2005) Characterization of non-extractable residues of the fungicide dithianon in soil using 13C/14C-labelling techniques. Environ Pollut 133(1):35–41. doi:10.1016/j.envpol.2004.04.017
Mordaunt CJ, Gevao B, Jones KC, Semple KT (2005) Formation of non-extractable pesticide residues: observations on compound differences, measurement and regulatory issues. Environ Pollut 133(1):25–34. doi:10.1016/j.envpol.2004.04.018
Bern L (1990) Size-related discrimination of nutritive and inert particles by freshwater zooplankton. J Plankton Res 12(5):1059–1067. doi:10.1093/plankt/12.5.1059
Rosenkranz P, Chaudhry Q, Stone V, Fernandes TF (2009) A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ Toxicol Chem 28(10):2142–2149. doi:10.1897/08-559.1
Besseling E, Wegner A, Foekema EM, van den Heuvel-Greve MJ, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the Lugworm Arenicola marina (L.) Environ Sci Technol 47(1):593–600. doi:10.1021/es302763x
Bilotta GS, Brazier RE (2008) Understanding the influence of suspended solids on water quality and aquatic biota. Water Res 42(12):2849–2861. doi:10.1016/j.watres.2008.03.018
Muncke J (2009) Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ 407(16):4549–4559. doi:10.1016/j.scitotenv.2009.05.006
Lithner D, Nordensvan I, Dave G (2012) Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile-butadiene-styrene, and epoxy to Daphnia magna. Environ Sci Pollut Res 19(5):1763–1772. doi:10.1007/s11356-011-0663-5
Lithner D, Damberg J, Dave G, Larsson A (2009) Leachates from plastic consumer products – screening for toxicity with Daphnia magna. Chemosphere 74(9):1195–1200. doi:10.1016/j.chemosphere.2008.11.022
Bejgarn S, MacLeod M, Bogdal C, Breitholtz M (2015) Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere 132:114–119. doi:10.1016/j.chemosphere.2015.03.010
Bradley LE, Driffield M, Guthrie J, Harmer N, Oldring TPK, Castle L (2009) Analytical approaches to identify potential migrants in polyester-polyurethane can coatings. Food Addit Contam Part A 26(12):1602–1610. doi:10.1080/19440040903252256
Bradley EL, Driffield M, Harmer N, Oldring PKT, Castle L (2008) Identification of potential migrants in epoxy phenolic can coatings. Int J Polym Anal Charact 13(3):200–223. doi:10.1080/10236660802070512
Mattsson K, Hansson LA, Cedervall T (2015) Nano-plastics in the aquatic environment. Environ Sci Process Impacts 17:1712–1721. doi:10.1039/c5em00227c
Syberg K, Khan FR, Selck H, Palmqvist A, Banta GT, Daley J, Sano L, Duhaime MB (2015) Microplastics: addressing ecological risk through lessons learned. Environ Toxicol Chem 34(5):945–953. doi:10.1002/etc.2914
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22. doi:10.1016/j.envpol.2007.06.006
Oberdörster E, Zhu S, Blickley TM, McClellan-Green P, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms. Carbon 44(6):1112–1120. doi:10.1016/j.carbon.2005.11.008
Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL (1997) The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res 14(11):1568–1573
Dhawan A, Pandey A, Sharma V (2011) Toxicity assessment of engineered nanomaterials: resolving the challenges. J Biomed Nanotechnol 7(1):6–7. doi:10.1166/jbn.2011.1173
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17(5):315–325. doi:10.1007/s10646-008-0206-0
Boxall AB, Chaudhry MQ, Sinclair CJ, Jones A, Aitken R, Jefferson B, Watts C (2007) Current and future predicted environmental exposure to engineered nanoparticles. Report by Central Science Laboratory for the Department of Environment Food and Rural Affairs
Musee N (2011) Simulated environmental risk estimation of engineered nanomaterials: a case of cosmetics in Johannesburg City. Hum Exp Toxicol 30(9):1181–1195. doi:10.1177/0960327110391387
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43(24):9216–9222. doi:10.1021/es9015553
Senjen R, Hansen SF (2011) Towards a nanorisk appraisal framework. C R Phys 12(7):637–647. doi:10.1016/j.crhy.2011.06.005
Foss Hansen S, Larsen BH, Olsen SI, Baun A (2007) Categorization framework to aid hazard identification of nanomaterials. Nanotoxicology 1(3):243–250. doi:10.1080/17435390701727509
Lambert S, Scherer C, Wagner M (2017) Ecotoxicity testing of microplastics: considering the heterogeneity of physico-chemical properties. Integr Environ Assess Manage 13(3):470–475. doi:10.1002/ieam.1901
Commission E (2002) Methyloxirane (propylene oxide): summary risk assessment report. Institute for Health and Consumer Protection-European Chemicals Bureau
Harrad S, Abdallah MA-E, Rose NL, Turner SD, Davidson TA (2009) Current-use brominated flame betardants in water, sediment, and fish from English lakes. Environ Sci Technol 43(24):9077–9083. doi:10.1021/es902185u
Alaee M, Arias P, Sjodin A, Bergman A (2003) An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int 29(6):683–689. doi:10.1016/S0160-4120(03)00121-1
Brennholt N, Heß M, Reifferscheid G (2017) Freshwater Microplastics: Challenges for Regulation and Management. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_12 (in this volume)
Arvidsson R (2012) Contributions to emissions, exposure and risk assessment of nanomaterials. Doctoral Thesis, Chalmers University of Technology
Hansen SF (2009) Regulation and risk assessment of nanomaterials – too little, too late? PhD Thesis, Technical University of Denmark
Nguyen Phuc T, Matsui Y, Fujiwara T (2011) Assessment of plastic waste generation and its potential recycling of household solid waste in Can Tho City, Vietnam. Environ Monit Assess 175(1–4):23–35. doi:10.1007/s10661-010-1490-8
Acknowledgements
Scott Lambert receives funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 660306. Martin Wagner receives funding by the German Federal Ministry for Transportation and Digital Infrastructure and the German Federal Ministry for Education and Research (02WRS1378 and 01UU1603).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Lambert, S., Wagner, M. (2018). Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview. In: Wagner, M., Lambert, S. (eds) Freshwater Microplastics . The Handbook of Environmental Chemistry, vol 58. Springer, Cham. https://doi.org/10.1007/978-3-319-61615-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-61615-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61614-8
Online ISBN: 978-3-319-61615-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)