Abstract
Duchenne muscular dystrophy (DMD) is a devastating, degenerative muscle disease that affects ~1 in every 3500 male births. DMD arises from mutations in the DMD gene that prevent expression of its encoded protein, Dystrophin (Burghes et al. Nature 328:434–437, 1987). Interestingly, patients with Dmd mutations that delete certain segments of the Dystrophin coding region, but maintain protein reading frame, have a much milder form of the disease, known as Becker Muscular Dystrophy (BMD). This observation has spurred interest in developing “exon skipping” strategies in which certain mutation-containing or mutation-adjacent Dmd exons are intentionally removed in order to restore protein reading frame, and thereby Dystrophin expression, in DMD patients (Beroud et al. Hum Mutat 28:196–202, 2007; Yokota et al. Expert Opin Biol Ther 7:831–842, 2007).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Recently our lab (Tabebordbar et al. 2015) and others (Long et al. 2015; Nelson et al. 2015) reported a novel strategy to accomplish permanent sequence-specific modification of the Dmd gene in vivo in the mdx mouse model of DMD. This strategy utilizes the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9 RNA guided gene editing system, delivered using adeno-associated virus (AAV) vectors. We showed that, when administered systemically, AAV-Dmd-CRISPR enables sequence-targeted genome modification in each of the key affected cell types and organs of DMD model mdx mice, including cardiomyocytes, skeletal muscle fibers and endogenous muscle stem cells (Tabebordbar et al. 2015). Gene editing in these cells restores Dystrophin protein reading frame and expression, recovers muscle contractile function, enhances muscle resilience in the face of controlled muscle damage, and establishes a pool of therapeutically modified progenitors that can participate in subsequent muscle regenerative events.
These studies provided a critical advance in allowing programmable genome editing that can irreversibly modify disease-causing mutations in the affected tissues of dystrophic individuals. Moreover, the results represent critical proof-of-concept evidence demonstrating the feasibility of systemic gene editing in vivo, which has the potential to recover Dystrophin expression in up to 80% of patients with DMD (Beroud et al. 2007; Yokota et al. 2007). Yet, important challenges remain for the future therapeutic application of Dmd-CRISPR gene editing, including enhancing the efficiencies with which gene editing may be accomplished in muscle fibers and satellite cells and circumventing the possible emergence of a host immune response to the bacterial Cas9 endonuclease, which could interfere with gene editing and/or lead to elimination of gene-edited cells. Overcoming these challenges will be crucial for developing clinically relevant strategies to accomplish safe, efficient and durable in vivo gene editing for DMD.
Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is one of the most common X-linked genetic disorders in humans; it arises from point mutations, deletions or duplications in the DMD gene that prevent expression of its encoded protein, Dystrophin (Burghes et al. 1987; Koenig et al. 1987). Dystrophin is an essential structural protein in skeletal and cardiac muscle (Ervasti and Campbell 1991). Its primary function is to link the cytoskeleton of muscle fibers to the extracellular matrix and thereby stabilize the muscle fiber membrane (Straub et al. 1992). Absence of functional Dystrophin protein increases the susceptibility of dystrophic muscle fibers to contraction-induced injury (Campbell and Kahl 1989). Increased cytosolic calcium following mechanical stress, activation of proteases (particularly calpains), destruction of membrane constituents and ultimately muscle fiber necrosis occur frequently in dystrophic muscles (reviewed in Tabebordbar et al. 2013). Due to continual myofiber destruction in dystrophic muscle, the resident pool of regenerative muscle stem cells (known as “satellite cells”) must support repeated rounds of activation and regeneration in an attempt to compensate for ongoing damage. As the disease advances, satellite cells show reduced capacity to regenerate muscle, possibly due to cell-intrinsic defects (Dumont et al. 2015) or proliferation-induced reductions in telomere length (Sacco et al. 2010). Absent an adequate regenerative response, fat and fibrotic tissue replace muscle fibers, leading to further weakening and wasting (Wallace and McNally 2009).
Current Gene-Targeted Therapeutic Strategies for DMD
Current treatment options for DMD are disappointingly limited and focus mainly on managing symptoms and suppressing the immune and inflammatory response (Muir and Chamberlain 2009; Partridge 2011). Patients are typically diagnosed at 3–5 years of age, they are wheelchair-bound in their second decade, and they have an average life expectancy of only about 30 years. In contrast, a related group of patients with mutations that impact this same gene but maintain its open reading frame produce an internally deleted but still partially functional Dystrophin protein that results in a markedly less severe disease known as Becker Muscular Dystrophy (BMD; England et al. 1990; Nakamura et al. 2008; Taglia et al. 2015). Many BMD patients are not diagnosed until adolescence or even adulthood and some enjoy a normal life span. These observations have provided motivation for the generation of rationally modified, truncated versions of Dystrophin for therapeutic application in DMD, including engineered “microdystrophins” and endogenous exon “skipped” DMD mRNAs.
The extremely large size of the DMD gene (2.4 Mb) and its encoded mRNA (14 kb) makes it very difficult, if not impossible, to package full-length dystrophin expression cassettes into clinically relevant viral vectors such as Adeno-associated viruses (AAVs), which have a packaging capacity of <5 kb. This limitation has propelled the generation of truncated mini- (6–8 kb) and microdystrophin (<4 kb) genes (Harper et al. 2002), which reduce the Dystrophin protein to its most essential functional elements. These rationally designed microgenes delete large regions of the internal Rod domain of Dystrophin, which contains 24 spectrin-like repeats and comprises 80% of the overall protein (Chamberlain 2002), while maintaining much of its functional integrity. Microdystrophin genes can be packaged into viral vectors for exogenous delivery and ectopic expression from ubiquitous or muscle-specific promoters (Fabb et al. 2002; Gregorevic et al. 2004), and delivery by AAVs results in effective expression of protein products that correctly localize to the sarcolemma and recruit other dystrophin glycoprotein complex (DGC) proteins. Importantly, while microdystrophins are not equivalent in function to full-length Dystrophin, they have been shown to ameliorate DMD pathologies in mdx mice (Harper et al. 2002; Wang et al. 2000) and dystrophin-deficient canine models (Shin et al. 2013). A related approach—exon skipping—similarly generates a modified Dystrophin protein product, but in this case the endogenous Dmd pre-mRNA transcript is targeted to remove mutation-carrying and/or mutation-adjacent exons from the mRNA. By choosing specific exons for removal, exon skipping approaches are able to generate Dmd mRNAs with restored reading frame.
In both gene complementation by microdystrophin and exon skipping approaches, the overall goal is to convert a severe DMD mutation, lacking Dystrophin protein expression entirely, into a milder BMD-like one, via expression of a truncated but still partially functional protein. It has been estimated that exon skipping strategies for Dmd could ultimately provide significant therapeutic benefit to the majority (~80%) of existing DMD patients (Beroud et al. 2007; Yokota et al. 2007), while complementation by ectopic expression of microdystrophin could in theory be useful for any mutation that abrogates Dystrophin protein production.
Challenges for Therapeutic Exon Skipping and Microdystrophin Delivery Strategies
Clinical application of exon skipping approaches to date has relied on antisense oligonucleotides (AONs) designed to mask splice donor and acceptor sequences in mutation-affected or mutation-adjacent exons. However, for many of the therapeutically relevant Dmd exons, exon-skipping AONs have not yet been developed or have not progressed to clinical trials (https://www.sarepta.com/our-pipeline). In addition, in a recent clinical trial, AON-mediated skipping of Dmd exon 51 failed to achieve sufficient rescue of Dystrophin protein to meet predetermined clinical endpoints (Lu et al. 2014). Importantly, even with relatively stable chemistries (Goyenvalle et al. 2015), AONs have a defined half-life (Goyenvalle et al. 2015; Vila et al. 2015) and require repeated (weekly) administration. This need for recurrent treatment increases the cost and potential side effects of AON therapy. Also, delivery of AONs to cardiac muscle has been more challenging than delivery to skeletal muscle, and delivery to resident muscle stem cells, if it occurs, is unlikely to be effective due to the dilution of AONs that occurs during cell proliferation. Thus, any benefit from AON therapy in satellite cells would be lost during muscle regenerative responses, which require proliferation of satellite cells and their progeny. Strategies in which AONs are delivered virally, by embedding within small nuclear RNAs, appear to suffer from similar progressive loss of the viral genome and its encoded AONs from dystrophic muscles (Vulin et al. 2012; Le Hir et al. 2013).
Relatedly, exogenous gene supplementation therapies using partially functional engineered microdystrophin constructs have encountered some challenges in clinical application. An initial Phase I clinical trial of microdystrophin gene therapies in human DMD patients yielded suboptimal transgene expression despite continued presence of vector genomes, possibly due to pre-existing or acquired T cell-mediated immune responses to dystrophin epitopes or AAV capsid proteins (see below), disease-associated inflammatory responses, CMV promoter silencing, or low AAV tropism (Bowles et al. 2012; Mendell et al. 2010). Additional Phase I trials of microdystrophin therapies utilizing different gene regulatory elements and AAV serotypes are currently underway (ClinicalTrials.gov Identifier: NCT02376816), and may mitigate these concerns; however, similar to AON delivery, delivery of AAV-microdystrophin to muscle satellite cells is unlikely to result in sustained transgene production, as the episomal AAV genome will be diluted with successive cell divisions. These challenges that have been encountered in the development of effective AON and microdystrophin therapies highlight the need for further evaluation of alternative strategies that could provide an efficient, permanent, one-time, systemic treatment to restore expression of Dystrophin in skeletal and cardiac muscles, as well as muscle satellite cells, of DMD patients.
Gene-Editing Approaches to Restore Dystrophin Function in DMD
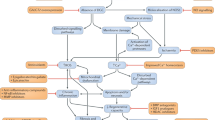
In a recent report (Tabebordbar et al. 2015), we described a novel genome-targeted editing approach (Fig. 1), based on Dmd exon skipping approaches, that was designed to accomplish irreversible removal of a mutated segment of the Dmd gene in the affected tissues of mdx mice, an animal model of human DMD (Sicinski et al. 1989). We further showed that this approach resulted in production of functional Dystrophin protein and improved muscle stability and contractility (Tabebordbar et al. 2015). Our approach made use of the CRISPR-Cas9 gene editing system, which allows the introduction of user-defined “cuts” in the genome. Each CRISPR-Cas9 gene editing complex consists of a Cas9 endonuclease and a programmable guide RNA (gRNA) that probes the genome for protospacer-adjacent motifs (PAM) [e.g., –NGG (Ran et al. 2013a) or –NNGRR(T) (Ran et al. 2015)]. Upon PAM recognition and base-pairing of the gRNA with an adjacent complementary DNA sequence, Cas9 creates a double-strand break (DSB) in the genomic DNA. Introduction of DSBs at two sites in the same linear stretch of DNA favors excision of the intervening sequence, and repair of this lesion by non-homologous end joining (NHEJ) juxtaposes the remaining 5′ and 3′ sequences (Canver et al. 2014; Tabebordbar et al. 2015). Alternatively, inclusion of a homologous donor template enables repair by homology directed recombination (HDR), leading to incorporation of precise nucleotide changes, encoded in the donor template, at the site of the DSB. Changes introduced by HDR can range from a single base pair to insertions of entire genes or even large cassettes of multiple genes (Urnov et al. 2005; Ding et al. 2013; Voit et al. 2014). Significantly, the relative activity of NHEJ and HDR repair mechanisms can vary with cell type, cell cycle and developmental stage, which can have important ramifications for the efficacy and outcome of therapeutic genome modification (Yang et al. 2016).
Gene-editing strategy for recovery of Dystrophin expression in DMD model mice. Mdx mice with a mutation in the Dmd gene were injected with AAV particles carrying clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 endonucleases and paired guide RNAs targeting the mutated Dmd exon23. This procedure led to excision of the targeted DNA and restored Dmd gene reading frame and Dystrophin expression in gene-edited skeletal muscle fibers, cardiomyocytes and muscle stem cells following local delivery or delivery via the bloodstream, in dystrophic mice. Gene-edited nuclei are shown in green and non-edited nuclei are shown in blue. The mutated Dmd mRNA is degraded and Dystrophin expression is lacking in the dystrophic tissues of untreated mice (graphical summary describes data reported in Tabebordbar et al. 2015. See text for details)
CRISPR-Cas9 RNA guided endonucleases (RGENs) have been used to target both expressed and non-expressed genes in multiple cell types from multiple organisms both in vitro (Cho et al. 2013; Cong et al. 2013; DiCarlo et al. 2013; Ding et al. 2013; Friedland et al. 2013; Hwang et al. 2013; Mali et al. 2013; Wu et al. 2016) and in vivo (Ding et al. 2014; Xue et al. 2014; Yin et al. 2014; Ran et al. 2015; Yang et al. 2016). Published data demonstrate the utility of this system for multi-organ gene targeting of many distinct cell lineages, including hepatocytes, muscle fibers, cardiomyocytes, and muscle regenerative stem cells (Long et al. 2015; Nelson et al. 2015; Ran et al. 2015; Tabebordbar et al. 2015; Yang et al. 2016). We adapted the CRISPR-Cas9 system for Dmd editing in cardiac and skeletal muscle in vivo by utilizing a smaller Cas9 ortholog from Staphyloccocus aureus (SaCas9), which could be packaged into recombinant AAV particles using the muscle-tropic serotype 9 (Zincarelli et al. 2008). Our strategy (Fig. 1) employed a dual AAV system (termed “AAV-Dmd-CRISPR”), which, due to AAV packaging limitations, was superior to single vector systems in terms of gene editing efficiency (Tabebordbar et al. 2015). In the dual system (Tabebordbar et al. 2015), the first AAV delivers SaCas9, driven by a strong CMV promoter, whereas the second AAV carries two gRNAs that target sequences in the introns flanking mouse Dmd exon 23 (“Dmd23 gRNAs”), each driven by a U6 promoter. This targeting of intronic sequences is important because it allows for tolerance of small insertions and deletions that are common with NHEJ-mediated repair of DNA DSBs (Symington and Gautier 2011). When injected intramuscularly or systemically into adult (P42) or early postnatal (P3) recipient mice, which carry a nonsense mutation (mdx) in Dmd exon 23, AAV-Dmd-CRISPR caused excision of exon 23 in heart cells (cardiomyocytes), skeletal muscle fibers and muscle stem cells [satellite cells, marked by transgenic expression of the fluorescent zsGreen protein from the Pax7 promoter (Bosnakovski et al. 2008)], producing an exon 23-deleted Dystrophin mRNA that, when translated, generated a truncated but functional Dystrophin protein (Tabebordbar et al. 2015; Fig. 1). Dystrophin protein restoration in AAV-Dmd-CRISPR treated mdx mice improved structural and functional aspects of the muscle, increased muscle strength and improved resistance to eccentric contraction-induced damage. Importantly, AAV-Dmd-CRISPR gene editing complexes could be disseminated systemically and were functional in both neonatal and adult mice. Exon-deleted transcripts represented almost 50% of total Dmd mRNA in muscle after intramuscular delivery in adults and 5–15% in skeletal and cardiac muscles after systemic delivery in neonates (Tabebordbar et al. 2015).
Importantly, and emphasizing the robustness and reproducibility of these results, similar outcomes were reported simultaneously by two other groups (Long et al. 2015; Nelson et al. 2015) using different Cas9 proteins and regulatory elements (Long et al. 2015), different AAV serotypes (Nelson et al. 2015), different routes of systemic administration (Long et al. 2015), and different gRNAs (Long et al. 2015; Nelson et al. 2015). All three groups reported gene editing in skeletal muscle fibers and cardiomyocytes, with efficiencies in skeletal muscle reported by Long et al. and Nelson et al. to vary from 1 to 67% Dystrophin + fibers, depending on the delivery approach used (local vs. systemic), dose of virus and age of the recipient animals. Long et al. also documented Dmd modification in vascular smooth muscle cells but not in brain, and our group, as discussed above, demonstrated detectable editing in endogenous muscle satellite cells (Tabebordbar et al. 2015). Finally, by analyzing treated muscle tissues at 4, 8, and 12 weeks after AAV injection, Long et al. ascertained that the percentage of dystrophin-positive myofibers might increase over time, and Nelson et al. observed that dystrophin restoration could be maintained for at least 6 months after treatment, indicating the potential long-term efficacy of AAV-Dmd-CRISPR therapies. Promisingly, differences in experimental design among the three studies and the varying efficiencies obtained suggest that multiple parameters may be adjusted and optimized to enhance genomic editing and increase dystrophin protein expression levels for more effective treatment of disease phenotypes by Dmd-CRISPR.
In summary, published work from our lab and others provides strong evidence supporting the efficacy of in vivo genome editing to correct disruptive mutations in DMD in a relevant dystrophic mouse model (Long et al. 2015; Nelson et al. 2015; Tabebordbar et al. 2015). These data indicate that programmable CRISPR complexes can be delivered locally and systemically to terminally differentiated skeletal muscle fibers, cardiomyocytes and smooth muscle cells, as well as regenerative muscle satellite cells, in neonatal and adult mice, where they mediate targeted gene modification, restore Dystrophin expression and partially recover functional deficiencies of dystrophic muscle. As prior studies in mice and humans indicate that Dystrophin levels as low as 3–15% of wild type are sufficient to ameliorate pathologic symptoms in the heart and skeletal muscle (van Putten et al. 2012, 2013, 2014; Long et al. 2014), and levels as low as 30% can completely suppress the dystrophic phenotype (Neri et al. 2007), the level of Dystrophin expression that is potentially achievable by one-time administration of AAV-Dmd-CRISPR urges further development of this system, which could be used independently or together with other therapies, including AON-mediated exon skipping (Aartsma-Rus et al. 2009) and AAV-mediated delivery of engineered “microdystrophins” (Harper et al. 2002; Ramos and Chamberlain 2015), as discussed above.
Remaining Challenges for Therapeutic Development of DMD-CRISPR
Taken together, the rodent studies described above provide strong pre-clinical proof-of-concept data that should inspire further evaluation and optimization of AAV-CRISPR as a new therapeutic option for DMD patients, either as a stand-alone intervention or in conjunction with other existing DMD therapies. Below, we discuss a number of challenges that remain to be overcome before realizing the potential of this approach in human patients.
Challenges of DMD-CRISPR Delivery
Engineered recombinant AAVs are particularly attractive vectors for both local and systemic delivery of gene editing complexes due to their general non-pathogenicity in human populations, their relatively low immunogenicity, and their inability to integrate efficiently into the genome (Gao et al. 2004; Boutin et al. 2010). Because of these traits, AAVs are currently in use in several human clinical trials (Mingozzi and High 2011; Kotterman and Schaffer 2014), and the immune response to AAV vectors has been extensively studied in both animal models and humans. Because engineered AAV vectors do not replicate and do not encode viral proteins, immune responses to AAVs are directed solely at the viral capsid and exhibit a relatively low pro-inflammatory profile (Mingozzi and High 2011). While pre-existing and acquired immunity to AAV remains a challenge for systemic, and repeated, administration of AAV vectors in human populations, these issues have been investigated for several decades and promising pharmacologic and physical strategies have emerged (Mingozzi and High 2011). In addition, clinical responses to AAV administration have been monitored in hundreds of human subjects, with little evidence as yet of acute adverse events (Mingozzi and High 2011). Thus, the successful application of AAV-mediated therapy in multiple human trials suggests that the immune response to AAV itself is unlikely to preclude gene editing therapies based on AAV delivery.
Still, a clear limitation of current AAV systems is that levels of gene targeting achieved in mouse models by AAV-mediated delivery of CRISPR-Cas9 to muscle satellite cells are rather low (<5% of satellite cells targeted; Tabebordbar et al. 2015), suggesting a need to investigate additional AAV serotypes to identify those with optimal tropism for satellite cells. Directed evolution and in vivo selection have been used recently to engineer novel AAV capsids with high tropism for tissues that are difficult to transduce with naturally occurring AAVs, such as human hepatocytes in a xenograft liver model (Lisowski et al. 2014) and the outer retina after injection into the eye’s vitreous humor (Dalkara et al. 2013). In addition, transduction rates of blood-forming hematopoietic stem cells have been improved through incorporation of novel amino acid substitutions in capsids (Song et al. 2013a, b). Thus, the application of directed evolution and in vivo selection strategies for generating novel AAV serotypes with high tropism for satellite cells represents an exciting future direction for increasing gene-editing efficiencies in these cells in vivo.
On the other hand, the development of alternative delivery strategies that enable transient expression of DMD-CRISPR may hold some advantages, particularly since the therapeutic effect of gene-editing approaches does not depend on persistent expression of Cas9 and gRNAs. Transient expression of CRISPR components could mitigate several of the possible adverse effects associated with prolonged Cas9 exposure, including potential genomic toxicity and immunogenicity (Wang et al. 2015). Indeed, in vitro experiments indicate that transient expression of Cas9 does produce lower off-target effects (Kim et al. 2014; Zuris et al. 2015).
Recent advances in lipid nanoparticle-mediated delivery of Cas9:gRNA complexes in vitro (Kim et al. 2014; Woo et al. 2015; Zuris et al. 2015) and Cas9 mRNA in vivo (Yin et al. 2016) provide additional promising avenues that may circumvent the challenges of AAV immunity. Delivering Cas9 and gRNAs conjugated with cell penetrating peptides (CPPs) has also been useful in targeting gene-editing complexes to human cell lines in culture (Ramakrishna et al. 2014), and combining this approach with incorporation of novel muscle-homing peptides (Gao et al. 2014) could potentially be effective for in vivo delivery of DMD-CRISPR.
Potential Immune Response to Restored Dystrophin Protein
A possible immune response to the repaired DMD protein is also of potential concern for clinical application of DMD-CRISPR-mediated gene editing; however, due to large variations in the types of DMD mutations seen in patients (Aartsma-Rus et al. 2009), it is likely that the nature of individual immune responses to Dystrophin protein will vary as well and will depend at least in part on the nature of the mutation and the frequency with which “natural” exon skipping, which gives rise to revertant fibers in both DMD patients and mdx mice (Hoffman et al. 1990; Burrow et al. 1991; Klein et al. 1992; Nicholson et al. 1993; Fanin et al. 1995; Uchino et al. 1995; Lu et al. 2000), may allow for endogenous exposure and tolerance to near-full length Dystrophin. Interestingly, in gene therapy trials for hemophilia B, in which AAV vectors were used to deliver Factor IX (F. IX), no subjects developed immune responses against the F.IX transgene, even though some carried null mutations in the F.IX gene (Manno et al. 2006; Nathwani et al. 2011). Similarly, promising results from studies using “microdystrophin” in mice and primates suggest that this protein is effectively expressed for up to 5 months without overt T cell or cytokine responses (Rodino-Klapac et al. 2010). These data argue that acquired immunity against the therapeutic protein also may not be therapy limiting. On the other hand, results from a clinical trial using intramuscular AAV-mediated delivery of microdystrophin, expressed under the control of a ubiquitous CMV promoter, revealed the presence in some patients of T cells recognizing self and non-self Dystrophin epitopes (Mendell et al. 2010). Interestingly, these T cells were present both before and after vector injection in two of the six patients, raising the possibility that screening for pre-existing immunity to Dystrophin protein in larger cohorts of DMD patients could provide useful information relevant to patient inclusion and exclusion criteria in future trials. Anti-Dystrophin antibodies were not detected in any of the treated patients; however, detection of Dystrophin-specific T cells and a lack of transgene expression in muscles of patients injected with AAV-microdystrophin (with the exception of two patients analyzed 6 weeks after injection) may suggest a cytotoxic response against fibers expressing microdystrophin. Thus, currently available data point to a compelling need for further studies to investigate more deeply the potential immune response to restored Dystrophin expression in dystrophic muscle.
Pre-existing and Acquired Immunity to Cas9
Potential immunity to the Cas9 endonuclease is also a significant consideration for therapeutics development in humans. An essential component of the CRISPR-based gene-editing machinery, Cas9 is a bacterially derived protein whose expression in transduced cells can evoke both humoral and cellular responses (Wang et al. 2015; and see below). Additionally, about 20% of individuals in the human population are persistent carriers of Staphylococcus aureus and another 60% have been periodic carriers at some point in their lives (Kluytmans et al. 1997). Thus, a significant fraction of potential patients is likely to have been exposed to the Cas9 protein from this species, raising the possibility that a pre-existing anti-Cas9 immune response could modulate the efficacy of CRISPR-mediated gene editing for recovery of Dystrophin expression in dystrophic muscles. Moreover, as emerging data suggest that the immune system and its products can modulate the expression of AAV-encoded transgenes (Mingozzi and High 2011), as well as components of cellular DNA damage response pathways (Jackson and Bartek 2009; Calvo et al. 2012), Cas9-induced immune responses could potentially alter both the degree of on-target DMD editing and the frequency and types of off-target modifications induced. Thus, while studies in our lab and others (Long et al. 2015; Nelson et al. 2015; Tabebordbar et al. 2015) clearly demonstrate that anti-Cas9 immunity does not preclude gene editing in vivo, Cas9 immune responses could, nevertheless, have profound implications for the persistence of therapeutic benefit in the muscle and other tissues. We therefore believe that it is particularly important at this juncture to begin to assess the nature and consequences of the immune response to the foreign Cas9 protein itself and to determine whether preventing or ameliorating this response might improve the efficiency, durability, repeatability and/or safety of Cas9-mediated therapeutic gene editing.
Assessing Mutagenic Events at On-Target and Off-Target Sites
Off-target modifications pose a potential threat for gene editing approaches because the unintended activity of CRISPR-Cas9 at these locations can cause pathogenic modifications that impair cellular function or promote tumorigenesis. Furthermore, because in general Cas9-induced DNA DSBs can be repaired by either HDR or NHEJ, editing can result in different outcomes, depending on the number of alleles affected and the type of modification introduced. Thus, it is critical to develop tools that enable facile assessment of mutagenic potential in an un-biased genome-wide manner, since such evaluations are likely to show patient- and gRNA-specific variation.
Recent advances have developed several different strategies to reduce genome-wide off-target mutations of Streptococcus pyogenes Cas9 (SpCas9). These strategies include use of paired SpCas9 nickases (Ran et al. 2013b), gRNAs with reduced length of the guide sequence (Fu et al. 2014) and the engineering of SpCas9 variants with amino acid substitutions in the DNA binding domain that reduce off-target rates (Kleinstiver et al. 2016; Slaymaker et al. 2016). Yet, there is still need for improving the specificity of SpCas9 and its smaller orthologs (e.g., SaCas9), and this issue is particularly important for the targeting of muscle stem cells, which have substantial proliferative capacity. The risk of generating undesired and deleterious mutations at proto-oncogene loci or at loci critical to stem cell function by CRISPR-Cas9 transduction of these cells must be rigorously analyzed before proceeding further with clinical translation of gene editing for DMD.
Enabling HR for Precise Repair of Dmd
Prior work in mice demonstrates that DMD pathology in skeletal muscle can be reversed by transplantation of sorted muscle stem cells isolated from wild-type animals (carrying a normal copy of the Dmd gene; Cerletti et al. 2008; Sacco et al. 2010). However, muscle stem cells are extremely rare, cannot be expanded effectively ex vivo, must be delivered by intramuscular injection (as they fail to migrate to muscle tissue when injected intravenously), and do not engraft cardiac muscle, which also is affected by Dmd mutation. These significant complications have limited the application of stem cell transplantation therapy to DMD, despite promising results in individually injected muscle groups.
Likewise, as discussed above, recent reports document the feasibility of AAV-based delivery of gene-editing complexes into cardiac and skeletal muscle in vivo and demonstrate that this system could be used to specifically excise a mutated segment of the Dmd gene in mdx mice to restore Dmd reading frame and allow production of a partially functional Dystrophin protein that improves muscle stability and contractility (Long et al. 2015; Nelson et al. 2015; Tabebordbar et al. 2015). However, it is important to note that the “first-generation” gene-editing strategies applied in these studies do not produce a full length Dystrophin. Instead, these approaches generate an internally truncated protein analogous to that seen in patients with BMD. While BMD is a markedly less severe disease compared to DMD, BMD patients still experience muscle pathology, and so, while clearly providing a potential clinical benefit, this approach is not fully curative for DMD. For this reason, future studies should be aimed at achieving full restitution of Dystrophin protein expression through precise gene editing to restore the normal Dmd gene sequence. Importantly, as conventional wisdom holds that HDR is limited to proliferative cells and DSBs introduced into post-mitotic cells (e.g., muscle fibers) will be repaired instead by NHEJ, it is likely that achieving precise repair of the Dmd gene will require efficient co-delivery into muscle satellite cells of CRISPR/Cas9, Dmd-targeting guide RNAs, and donor DNA template to direct HDR. Such a feat will, in turn, likely necessitate the identification of novel or optimized delivery vehicles that exhibit high satellite cell tropism (see above). While certainly challenging, success in such an approach would represent a very promising treatment strategy for DMD.
Gene-Editing Therapy in Combination with AONs or Microdystrophin
AAV-mediated delivery of expression constructs encoding for AONs has been shown to enable widespread exon skipping, restoration of Dystrophin protein production and improvement of muscle function in short-term animal studies (Goyenvalle et al. 2004, 2012; Denti et al. 2006; Le Guiner et al. 2014); however, long-term studies in a more severe mouse model (Le Hir et al. 2013) and also in the Golden retriever model of DMD (Vulin et al. 2012) revealed that vector genomes are lost from dystrophic muscle upon muscle damage and also over time. This observation can be explained by injury-induced loss or degeneration of muscle fibers that previously were transduced by the AAV vector and subsequent incorporation of new satellite cell-derived nuclei to the muscle. Importantly, as noted above, the low rate of satellite cell transduction with the AAV serotypes tested thus far, together with the likelihood that these cells and their progeny proliferate prior to incorporation into muscle fibers, makes it doubtful that additional vector genomes are delivered to muscle via fusion of satellite cell progeny in this system. Consistent with this, acute muscle damage by cardiotoxin injury of AAV injected mouse dystrophic muscle results in rapid loss of vector genome from the muscle (Le Hir et al. 2013). Irreversible gene correction of regenerating satellite cells and their progeny, achieved by gene editing, has the potential to overcome this challenge. Moreover, to avoid immune response complications related to re-administration of AAV, non-viral delivery of DMD-CRISPR to dystrophic muscle could potentially be used to complement viral delivery of AONs or microdystrophin to achieve long-term and persistent Dystrophin restoration.
Possible Application of CRISPR-mediated gene editing Strategies in Other Diseases
The reprogrammable targeting of the Cas9 endonuclease via easily constructed gRNAs presents the exciting possibility of utilizing this system to treat a wide range of genetic diseases. Results from Dmd targeting by AAV-CRISPR in mdx mice are most immediately pertinent to other muscle disorders that are likewise amenable to mRNA splicing modulation, i.e., exon skipping or exon retention strategies, conventionally achieved by AONs. These disorders include primary dysferlinopathies, such as limb-girdle muscular dystrophy type 2B, resulting from mutations in the large dysferlin protein coding region that may be skipped inconsequentially if contained in redundant C2 domains (Wein et al. 2010). AONs also have been used in spinal muscular atrophy (SMA) to interrupt the function of an intronic splicing silencer that would otherwise result in the omission of exon 7 of the survival motor neuron 2 (SMN2) protein product, thereby allowing compensation for the loss-of-function of its paralog, survival motor neuron 1 (SMN1) in SMA patients (Burghes and McGovern 2010). Furthermore, the use of AAV-CRISPR as an AON alternative is suitable for non-muscle specific diseases like Leber congenital amaurosis (Maeder and Gersbach 2016).
Aside from complementing and potentially superseding the use of AONs in exon exclusion strategies, NHEJ-mediated DNA excision is applicable more generally for the targeted removal of specific genomic elements associated with disease. For example, chemokine receptor 5 (CCR5) is a critical human immunodeficiency virus type 1 (HIV-1) co-receptor that is necessary for the fusion to and infection of cells by CCR5-tropic virions (Broder and Collman 1997). Mutations in the CCR5 gene can confer immunity to HIV-1 infection, and transplantation of hematopoietic stem cells carrying the same mutated gene has been aggressively pursued as a possible curative treatment (Allers et al. 2011). By using Cas9 and paired gRNAs, researchers have been able recently to selectively mutate the CCR5 gene and thereby provide resistance of immune cells to HIV-1 infection (Kang et al. 2015; Mandal et al. 2014). Moreover, CRISPR-Cas9 can be used to directly target and disrupt integrated proviral genomes (Vulin et al. 2012; Ebina et al. 2013; Kennedy and Cullen 2015; Wang et al. 2015). Other uses may include the removal of excess nucleotides in trinucleotide repeat disorders (Park et al. 2015) and the knock-out of proprotein convertase subtilisin/kexin type 9 (PCSK9) involved in hypercholesterolemia (Ding et al. 2014; Ran et al. 2015; Wang et al. 2016). Finally, approaches utilizing co-delivery of CRISPR components with a donor DNA template to correct mutations via activation of the HDR pathway are also currently under development to treat cystic fibrosis (Schwank et al. 2013), hemophilia A (Park et al. 2015), hereditary tyrosinemia (Yin et al. 2014), sickle cell disease (Orkin 2016), severe combined immunodeficiency (Booth et al. 2016), and other, predominantly loss-of-function genetic diseases.
Conclusions and Perspective
Three independent studies have provided evidence for AAV-mediated delivery of CRISPR components targeting Dmd and restoring Dystrophin expression in dystrophic cardiac and skeletal muscle (Long et al. 2015; Nelson et al. 2015; Tabebordbar et al. 2015). One study (Tabebordbar et al. 2015) also showed Dmd gene targeting in dystrophic muscle stem cells. Correction of Dmd in dystrophic satellite cells provides a critical reservoir of myogenic progenitors capable of producing Dystrophin-expressing muscle fibers and represents a potential advantage compared to conventional transgene-mediated gene therapy. Transgenes delivered by AAV are generally maintained as non-replicating episomes and thus are diluted during expansion of satellite cells and their myoblast progeny. In contrast, CRISPR-mediated gene editing allows for irreversible modification of Dmd in satellite cells and their progeny, a result that is even more advantageous if the gene-corrected cells are selected for, or enriched, in dystrophic tissue. Expansion of clusters of naturally occurring Dystrophin-expressing revertant fibers in mdx muscle, which depends on muscle regeneration, suggests that such a selective advantage may exist for Dystrophin-expressing satellite cells in dystrophic muscle (Yokota et al. 2006). It would be interesting to test if gene-corrected satellite cells are selectively enriched in dystrophic muscles after induced muscle degeneration and regeneration. Furthermore, it would be informative to examine whether permanent gene correction of dystrophic satellite cells (and their progeny) prevents the loss of Dystrophin-expressing nuclei in muscle fibers, which is typically seen with traditional gene therapy approaches (Vulin et al. 2012; Le Hir et al. 2013). Minimizing off-target activity of Cas9 nuclease, analyzing potential immune responses against CRISPR components and therapeutic gene products and developing non-viral delivery approaches for transient expression of DMD-CRISPR in dystrophic muscle will also be important to help to move gene editing technology towards clinical application for DMD. In addition, it is important to keep in mind that the efficacy and safety of this approach in non-rodent dystrophy models is yet to be studied. Canine models of DMD, including the golden retriever muscular dystrophy (GRMD) model, exhibit more severe dystrophic phenotypes that show greater similarity to human DMD phenotypes than the mdx mouse model (Kornegay et al. 2012). Therefore, preclinical studies in dog models might better indicate the therapeutic potential of in vivo gene editing for DMD. The recently developed human muscle xenograft model also provides a unique and informative opportunity for studying the efficacy of DMD-CRISPR in correcting mutations in human dystrophic muscle fibers and satellite cells in vivo (Zhang et al. 2014). Finally, to assess the likelihood of vertical transfer of gene-editing events to the next generation after systemic gene editing, germline and also transplacental transmission of AAV-CRISPR should be rigorously analyzed. AAV9 has been shown to penetrate the placenta (Picconi et al. 2014) in mice, a finding that should be taken into consideration for planning clinical application of this technology. Still, the possibility to directly modify the human genome to correct deleterious mutations that lead to devastating human diseases, such as DMD, presents unprecedented promise for the future of regenerative medicine.
References
Aartsma-Rus A, Fokkema I, Verschuuren J, Ginjaar I, van Deutekom J, van Ommen GJ, den Dunnen JT (2009) Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat 30:293–299
Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T (2011) Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 117:2791–2799
Beroud C, Tuffery-Giraud S, Matsuo M, Hamroun D, Humbertclaude V, Monnier N, Moizard MP, Voelckel MA, Calemard LM, Boisseau P, Blayau M, Philippe C, Cossee M, Pages M, Rivier F, Danos O, Garcia L, Claustres M (2007) Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat 28:196–202
Booth C, Gaspar HB, Thrasher AJ (2016) Treating immunodeficiency through HSC gene therapy. Trends Mol Med 22:317–327
Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M (2008) Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26:3194–3204
Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C (2010) Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 21:704–712
Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, Grieger JC, Govindasamy L, Agbandje-McKenna M, Xiao X, Samulski RJ (2012) Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Therapy J Am Soc Gene Therapy 20:443–455
Broder CC, Collman RG (1997) Chemokine receptors and HIV. J Leukoc Biol 62:20–29
Burghes AH, Logan C, Hu X, Belfall B, Worton RG, Ray PN (1987) A cDNA clone from the Duchenne/Becker muscular dystrophy gene. Nature 328:434–437
Burghes AH, McGovern VL (2010) Antisense oligonucleotides and spinal muscular atrophy: skipping along. Genes Dev 24:1574–1579
Burrow KL, Coovert DD, Klein CJ, Bulman DE, Kissel JT, Rammohan KW, Burghes AH, Mendell JR (1991) Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. CIDD Study Group. Neurology 41:661–666
Calvo JA, Meira LB, Lee CY, Moroski-Erkul CA, Abolhassani N, Taghizadeh K, Eichinger LW, Muthupalani S, Nordstrand LM, Klungland A, Samson LD (2012) DNA repair is indispensable for survival after acute inflammation. J Clin Invest 122:2680–2689
Campbell KP, Kahl SD (1989) Association of dystrophin and an integral membrane glycoprotein. Nature 338:259–262
Canver MC, Bauer DE, Dass A, Yien YY, Chung J, Masuda T, Maeda T, Paw BH, Orkin SH (2014) Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J Biol Chem 289:21312–21324
Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ (2008) Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134:37–47
Chamberlain JS (2002) Gene therapy of muscular dystrophy. Hum Mol Genet 11:2355–2362
Cho SW, Kim S, Kim JM, Kim JS (2013) Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol 31:230–232
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV (2013) In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5:189ra176
Denti MA, Rosa A, D’Antona G, Sthandier O, De Angelis FG, Nicoletti C, Allocca M, Pansarasa O, Parente V, Musaro A, Auricchio A, Bottinelli R, Bozzoni I (2006) Body-wide gene therapy of Duchenne muscular dystrophy in the mdx mouse model. Proc Natl Acad Sci USA 103:3758–3763
DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM (2013) Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res 41:4336–4343
Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan CA, Musunuru K (2013) Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12:393–394
Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, Rader DJ, Musunuru K (2014) Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res 115:488–492
Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA (2015) Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21:1455–1463
Ebina H, Misawa N, Kanemura Y, Koyanagi Y (2013) Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 3:2510
England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, Bulman DE, Harris JB, Davies KE (1990) Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343:180–182
Ervasti JM, Campbell KP (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121–1131
Fabb SA, Wells DJ, Serpente P, Dickson G (2002) Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet 11:733–741
Fanin M, Danieli GA, Cadaldini M, Miorin M, Vitiello L, Angelini C (1995) Dystrophin-positive fibers in Duchenne dystrophy: origin and correlation to clinical course. Muscle Nerve 18:1115–1120
Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA (2013) Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods 10:741–743
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM (2004) Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 78:6381–6388
Gao X, Zhao J, Han G, Zhang Y, Dong X, Cao L, Wang Q, Moulton HM, Yin H (2014) Effective dystrophin restoration by a novel muscle-homing peptide-morpholino conjugate in dystrophin-deficient mdx mice. Mol Ther 22:1333–1341
Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, Danos O (2004) Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306:1796–1799
Goyenvalle A, Babbs A, Wright J, Wilkins V, Powell D, Garcia L, Davies KE (2012) Rescue of severely affected dystrophin/utrophin-deficient mice through scAAV-U7snRNA-mediated exon skipping. Hum Mol Genet 21:2559–2571
Goyenvalle A, Griffith G, Babbs A, El Andaloussi S, Ezzat K, Avril A, Dugovic B, Chaussenot R, Ferry A, Voit T, Amthor H, Buhr C, Schurch S, Wood MJ, Davies KE, Vaillend C, Leumann C, Garcia L (2015) Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med 21:270–275
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10:828–834
Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS (2002) Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8:253–261
Hoffman EP, Morgan JE, Watkins SC, Partridge TA (1990) Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci 99:9–25
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461:1071–1078
Kang H, Minder P, Park MA, Mesquitta WT, Torbett BE, Slukvin II (2015) CCR5 disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 virus. Mol Therapy Nucleic acids 4:e268
Kennedy EM, Cullen BR (2015) Bacterial CRISPR/Cas DNA endonucleases: a revolutionary technology that could dramatically impact viral research and treatment. Virology 479–480:213–220
Kim S, Kim D, Cho SW, Kim J, Kim JS (2014) Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 24:1012–1019
Klein CJ, Coovert DD, Bulman DE, Ray PN, Mendell JR, Burghes AH (1992) Somatic reversion/suppression in Duchenne muscular dystrophy (DMD): evidence supporting a frame-restoring mechanism in rare dystrophin-positive fibers. Am J Hum Genet 50:950–959
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK (2016) High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529:490–495
Kluytmans J, van Belkum A, Verbrugh H (1997) Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520
Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM (1987) Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50:509–517
Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Li J, Nghiem P, Detwiler DA, Larsen CA, Grange RW, Bhavaraju-Sanka RK, Tou S, Keene BP, Howard JF Jr, Wang J, Fan Z, Schatzberg SJ, Styner MA, Flanigan KM, Xiao X, Hoffman EP (2012) Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome 23:85–108
Kotterman MA, Schaffer DV (2014) Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 15:445–451
Le Guiner C, Montus M, Servais L, Cherel Y, Francois V, Thibaud JL, Wary C, Matot B, Larcher T, Guigand L, Dutilleul M, Domenger C, Allais M, Beuvin M, Moraux A, Le Duff J, Devaux M, Jaulin N, Guilbaud M, Latournerie V, Veron P, Boutin S, Leborgne C, Desgue D, Deschamps JY, Moullec S, Fromes Y, Vulin A, Smith RH, Laroudie N, Barnay-Toutain F, Rivière C, Bucher S, Le TH, Delaunay N, Gasmi M, Kotin RM, Bonne G, Adjali O, Masurier C, Hogrel JY, Carlier P, Moullier P, Voit T (2014) Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther 22:1923–1935
Le Hir M, Goyenvalle A, Peccate C, Precigout G, Davies KE, Voit T, Garcia L, Lorain S (2013) AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol Ther 21:1551–1558
Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, Nygaard S, Grompe M, Alexander IE, Kay MA (2014) Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506:382–386
Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN (2014) Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345:1184–1188
Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, Olson EN (2015) Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351:400–403
Lu QL, Morris GE, Wilton SD, Ly T, Artem’yeva OV, Strong P, Partridge TA (2000) Massive idiosyncratic exon skipping corrects the nonsense mutation in dystrophic mouse muscle and produces functional revertant fibers by clonal expansion. J Cell Biol 148:985–996
Lu QL, Cirak S, Partridge T (2014) What can we learn from clinical trials of exon skipping for DMD? Mol Ther Nucl Acids 3:e152
Maeder ML, Gersbach CA (2016) Genome-editing technologies for gene and cell therapy. Mol Therapy J Am Soc Gene Therapy 24:430–446
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Mandal PK, Ferreira LM, Collins R, Meissner TB, Boutwell CL, Friesen M, Vrbanac V, Garrison BS, Stortchevoi A, Bryder D, Musunuru K, Brand H, Tager AM, Allen TM, Talkowski ME, Rossi DJ, Cowan CA (2014) Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15:643–652
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12:342–347
Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM (2010) Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med 363:1429–1437
Mingozzi F, High KA (2011) Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 12:341–355
Muir LA, Chamberlain JS (2009) Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med 11:e18
Nakamura A, Yoshida K, Fukushima K, Ueda H, Urasawa N, Koyama J, Yazaki Y, Yazaki M, Sakai T, Haruta S, Takeda S, Ikeda S (2008) Follow-up of three patients with a large in-frame deletion of exons 45-55 in the Duchenne muscular dystrophy (DMD) gene. J Clin Neurosci 15:757–763
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365:2357–2365
Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, Asokan A, Zhang F, Duan D, Gersbach CA (2015) In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351:403–407
Neri M, Torelli S, Brown S, Ugo I, Sabatelli P, Merlini L, Spitali P, Rimessi P, Gualandi F, Sewry C, Ferlini A, Muntoni F (2007) Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord 17:913–918
Nicholson LV, Johnson MA, Bushby KM, Gardner-Medwin D (1993) Functional significance of dystrophin positive fibres in Duchenne muscular dystrophy. Arch Dis Child 68:632–636
Orkin SH (2016) Recent advances in globin research using genome-wide association studies and gene editing. Ann N Y Acad Sci 1368:5–10
Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S, Kim JH, Kim DW, Kim JS (2015) Functional correction of large factor VIII gene chromosomal inversions in hemophilia a patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell 17:213–220
Partridge TA (2011) Impending therapies for Duchenne muscular dystrophy. Curr Opin Neurol 24:415–422
Picconi JL, Muff-Luett MA, Wu D, Bunchman E, Schaefer F, Brophy PD (2014) Kidney-specific expression of GFP by in-utero delivery of pseudotyped adeno-associated virus 9. Mol Ther Methods Clin Dev 1:14014
Ramakrishna S, Kwaku Dad AB, Beloor J, Gopalappa R, Lee SK, Kim H (2014) Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res 24:1020–1027
Ramos J, Chamberlain JS (2015) Gene therapy for Duchenne muscular dystrophy. Expert Opin Orphan Drugs 3:1255–1266
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013a) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F (2013b) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520:186–191
Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N, Sprinkle S, Campbell KJ, Sahenk Z, Clark KR, Walker CM, Mendell JR, Chicoine LG (2010) Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther 18:109–117
Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM (2010) Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143:1059–1071
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13:653–658
Shin JH, Pan X, Hakim CH, Yang HT, Yue Y, Zhang K, Terjung RL, Duan D (2013) Microdystrophin ameliorates muscular dystrophy in the canine model of duchenne muscular dystrophy. Mol Therapy J Am Soc Gene Therapy 21:750–757
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244:1578–1580
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F (2016) Rationally engineered Cas9 nucleases with improved specificity. Science 351:84–88
Song L, Li X, Jayandharan GR, Wang Y, Aslanidi GV, Ling C, Zhong L, Gao G, Yoder MC, Ling C, Tan M, Srivastava A (2013a) High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS One 8:e58757
Song L, Kauss MA, Kopin E, Chandra M, Ul-Hasan T, Miller E, Jayandharan GR, Rivers AE, Aslanidi GV, Ling C, Li B, Ma W, Li X, Andino LM, Zhong L, Tarantal AF, Yoder MC, Wong KK Jr, Tan M, Chatterjee S, Srivastava A (2013b) Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy 15:986–998
Straub V, Bittner RE, Leger JJ, Voit T (1992) Direct visualization of the dystrophin network on skeletal muscle fiber membrane. J Cell Biol 119:1183–1191
Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45:247–271
Tabebordbar M, Wang ET, Wagers AJ (2013) Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu Rev Pathol 8:441–475
Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, EY W, Xiao R, Ran FA, Cong L, Zhang F, Vandenberghe LH, Church GM, Wagers AJ (2015) In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351:407–411
Taglia A, Petillo R, D’Ambrosio P, Picillo E, Torella A, Orsini C, Ergoli M, Scutifero M, Passamano L, Palladino A, Nigro G, Politano L (2015) Clinical features of patients with dystrophinopathy sharing the 45-55 exon deletion of DMD gene. Acta Myol 34:9–13
Uchino M, Tokunaga M, Mita S, Uyama E, Ando Y, Teramoto H, Miike T, Ando M (1995) PCR and immunocytochemical analyses of dystrophin-positive fibers in Duchenne muscular dystrophy. J Neurol Sci 129:44–50
Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC (2005) Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435:646–651
van Putten M, Hulsker M, Nadarajah VD, van Heiningen SH, van Huizen E, van Iterson M, Admiraal P, Messemaker T, den Dunnen JT, t Hoen PA, Aartsma-Rus A (2012) The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS One 7:e31937
van Putten M, Hulsker M, Young C, Nadarajah VD, Heemskerk H, van der Weerd L, t Hoen PA, van Ommen GJ, Aartsma-Rus AM (2013) Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J 27:2484–2495
van Putten M, van der Pijl EM, Hulsker M, Verhaart IE, Nadarajah VD, van der Weerd L, Aartsma-Rus A (2014) Low dystrophin levels in heart can delay heart failure in mdx mice. J Mol Cell Cardiol 69:17–23
Vila MC, Klimek MB, Novak JS, Rayavarapu S, Uaesoontrachoon K, Boehler JF, Fiorillo AA, Hogarth MW, Zhang A, Shaughnessy C, Gordish-Dressman H, Burki U, Straub V, Lu QL, Partridge TA, Brown KJ, Hathout Y, van den Anker J, Hoffman EP, Nagaraju K (2015) Elusive sources of variability of dystrophin rescue by exon skipping. Skelet Muscle 5:1–12
Voit RA, Hendel A, Pruett-Miller SM, Porteus MH (2014) Nuclease-mediated gene editing by homologous recombination of the human globin locus. Nucleic Acids Res 42:1365–1378
Vulin A, Barthelemy I, Goyenvalle A, Thibaud JL, Beley C, Griffith G, Benchaouir R, le Hir M, Unterfinger Y, Lorain S, Dreyfus P, Voit T, Carlier P, Blot S, Garcia L (2012) Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther 20:2120–2133
Wallace GQ, McNally EM (2009) Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71:37–57
Wang B, Li J, Xiao X (2000) Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA 97:13714–13719
Wang D, Mou H, Li S, Li Y, Hough S, Tran K, Li J, Yin H, Anderson DG, Sontheimer EJ, Weng Z, Gao G, Xue W (2015) Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther 26:432–442
Wang X, Raghavan A, Chen T, Qiao L, Zhang Y, Ding Q, Musunuru K (2016) CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo. Arterioscler Thromb Vasc Biol 36:783–786
Wein N, Avril A, Bartoli M, Beley C, Chaouch S, Laforet P, Behin A, Butler-Browne G, Mouly V, Krahn M, Garcia L, Levy N (2010) Efficient bypass of mutations in dysferlin deficient patient cells by antisenseinduced exon skipping. Hum Mutat 31:136–142
Woo JW, Kim J, Kwon SI, Corvalan C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33:1162–1164
Wu J, Hunt SD, Xue H, Liu Y, Darabi R (2016) Generation and characterization of a MYF5 reporter human iPS cell line using CRISPR/Cas9 mediated homologous recombination. Sci Rep 6:18759
Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, Cai W, Yang G, Bronson R, Crowley DG, Zhang F, Anderson DG, Sharp PA, Jacks T (2014) CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514:380–384
Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, Yu H, Xu C, Morizono H, Musunuru K, Batshaw ML, Wilson JM (2016) A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol 34:334–338
Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG (2014) Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 32:551–553
Yin H, Song CQ, Dorkin JR, Zhu LJ, Y L, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG (2016) Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol 34:328–333
Yokota T, QL L, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA (2006) Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J Cell Sci 119:2679–2687
Yokota T, Pistilli E, Duddy W, Nagaraju K (2007) Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin Biol Ther 7:831–842
Zhang Y, King OD, Rahimov F, Jones TI, Ward CW, Kerr JP, Liu N, Emerson CP Jr, Kunkel LM, Partridge TA, Wagner KR (2014) Human skeletal muscle xenograft as a new preclinical model for muscle disorders. Hum Mol Genet 23:3180–3188
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE (2008) Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16:1073–1080
Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, JH H, Maeder ML, Joung JK, Chen ZY, Liu DR (2015) Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 33:73–80
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Tabebordbar, M., Cheng, J., Wagers, A.J. (2017). Therapeutic Gene Editing in Muscles and Muscle Stem Cells. In: Jaenisch, R., Zhang, F., Gage, F. (eds) Genome Editing in Neurosciences. Research and Perspectives in Neurosciences. Springer, Cham. https://doi.org/10.1007/978-3-319-60192-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-60192-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60191-5
Online ISBN: 978-3-319-60192-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)