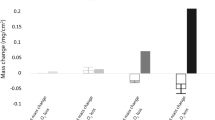
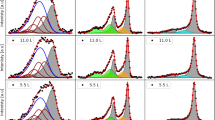
Abstract
The corrosion products produced on polycrystalline Ni metal and Ni-Cr (20%) (NiCr) alloy surfaces exposed to aqueous environments chosen to emulate possible solution conditions in the steam generator (SG) tubing of pressurized water reactors (PWR) were studied using XPS. Additional measurements modelling the distribution of oxidized Ni and Cr species on select alloy specimens were carried out using ToF SIMS. Exposure of Ni metal and NiCr alloy samples to mildly oxidizing potentials in basic solutions resulted in the preferential growth of a β-Ni(OH)2 phase; driven by the dissolution of metallic Ni at both 25°C and 150°C. The presence of β-Ni(OH)2, Cr(OH)3 and small amounts of a Cr6+-containing oxide on NiCr specimens oxidized under mildly oxidizing conditions at 150°C in neutral solutions suggested that the dissolution of both metallic Ni and Cr followed by the back deposition of the corresponding corrosion products was responsible for oxide growth under these conditions. In acidic media oxide nucleation at 150°C under mildly oxidizing potentials was determined to occur via the dissolution of both Ni and Cr species on NiCr specimens as well. The increased stability of Ni2+ in acidic solution led to a limited precipitation of β-Ni(OH)2 resulting in the formation of very thin oxides containing higher levels of Cr(OH)3. Reactions on metallic Ni and NiCr surfaces under highly oxidizing potentials resulted in an increase in the NiO content of these films compared to similar exposures carried out at milder oxidation conditions attributed to accelerated dehydration of the β-Ni(OH)2 phase. In addition, an increase in the Cr(OH)3 contribution on the alloy surface oxidized at a more oxidative potential suggested a more rapid dissolution of Cr under these conditions; overall, uneven films were formed from these conditions. The composition of the corrosion product formed after an exposure to a highly oxidizing potential was found to be unchanged following a subsequent reaction of equivalent length at a much lower oxidizing potential in basic solution.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
L. Liu, Y. Li, F. Wang, Electrochim. Acta 52 (2007) 2392.
R.S. Dutta, A. Lobo, R. Purandare, S.K. Kulkarni, G.K. Dey, Metall. Mater. Trans. A 33A (2002) 1437.
Y.Y. Andreev, E.A. Skryleva, I.A. Safonov, Prot. Met. Phys. Chem. S. 45 (2009) 181.
M. Lampimäki, K. Lahtonen, P. Jussila, M. Hirsimäki, M. Valden, J. Electron Spectrosc. Relat. Phenom. 154 (2007) 69.
S.E. Ziemniak, M. Hanson, Corros. Sci. 48 (2006) 498.
R.S. Dutta, Jagannath, G.K. Dey, P.K. De, Corros. Sci. 48 (2006) 2711.
B.A. Kobe, S. Ramamurthy, M.C. Biesinger, N.S. McIntyre, A.M. Brennenstühl, Surf. Interface Anal. 37 (2005) 478.
A. Machet, A. Galtayries, S. Zanna, L. Klein, V. Maurice, P. Jolivet, M. Foucault, P. Combrade, P. Scott, P. Marcus, Electrochim. Acta 49 (2004) 3957.
A. Machet, A. Galtayries, P. Marcus, P. Combrade, P. Jolivet, P. Scott, Surf. Interface Anal. 34 (2002) 197.
J.T. Francis, N.S. McIntyre, R.D. Davidson, S. Ramamurthy, A.M. Brennenstühl, A. McBride, A. Roberts, Surf. Interface Anal. 33 (2002) 29.
A. Rossi, B. Elsener, G. Hähner, M. Textor, N.D. Spencer, Surf. Interface Anal. 29 (2000) 460.
N.S. McIntyre, R.D. Davidson, T.L. Walzak, A.M. Brennenstühl, F. Gonzalez, S. Corazza, Corros. Sci. 37 (1995) 1059.
N.S. McIntyre, D.G. Zetaruk, D. Owen, J. Electrochem. Soc. 126 (1979) 750.
M.C. Biesinger, C. Brown, J.R. Mycroft, R.D Davidson, N.S. McIntyre, Surf. Interface Anal. 36 (2004) 1550.
B.P. Payne, A.P. Grosvenor, M.C. Biesinger, B.A. Kobe, N.S. McIntyre, Surf. Interface Anal. 39 (2007) 582.
B.P. Payne, M.C. Biesinger, N.S. McIntyre, J. Electron Spectrosc. Relat. Phenom. 175 (2009) 55.
M.C. Biesinger, B.P. Payne, L.W.M. Lau, A.R. Gerson, R.St.C. Smart, Surf. Interface Anal. 41 (2009) 324.
M.C. Biesinger, B.P. Payne, A.P. Grosvenor, L.W.M. Lau, A.R. Gerson, R.St.C. Smart, Appl. Sur. Sci. 257 (2011) 2717.
B.P. Payne, M.C. Biesinger, N.S. McIntyre, J. Electron Spectrosc. Relat. Phenom. 184 (2011) 29.
B.P. Payne, “X-ray Photoelectron Spectroscopy Studies on the Oxidation Processes of Nickel, Chromium and their Alloys” (Ph.D. thesis, University of Western Ontario, 2011), 110–127.
B. Beverskog, I. Puigdomenech, Corros. Sci. 39 (1997) 43.
B. Beverskog, I. Puigdomenech, Corros. Sci. 39 (1997) 969.
B. Beverskog, I. Puigdomenech, Corros. Sci. 55 (1999) 1077.
P. Jakupi, J.J. Noël, D.W. Shoesmith, Electrochem. Solid S. 13 (2010) C1.
P. Jakupi, F. Wang, J.J. Noël, D.W. Shoesmith, Corros. Sci. (2011) doi: 10.1016/j.corsci.2011.01.028.
D. Johnson, CoreWare for Windows, Version 3.2, Scribner Associates Inc. 1990–2010.
N. Fairley, CasaXPS Version 2.2.15, 1999–2009.
T.A. Carlson, G.E. McGuire, J. Electron Spectrosc. Relat. Phenom. 1 (1972/1973) 161.
B.R. Strohmeier, Surf. Interface Anal. 15 (1990) 51.
NIST Electron Inelastic Mean Free Path Database (Version 1.1) ©, U.S. Secretary of Commerce, 2000.
Ion-ToF (GmbH), IONSPEC, Version 3.14.9, Ion-ToF, Münster, Germany, 1995–2000.
J. Newman, J. Electrochem. Soc. 117 (1960) 198.
W.C. Ehrhardt, in “The Measurement and correction of electrolyte resistance in electrochemical tests” eds. S.R Taylor, L.L Scribner, ASTM STP 1056, American Society for Testing and Materials, Philadelphia, 1990, 27–59.
H. Corti, R. Crovetto, R. Fernandez-Prini, J. Solution Chem. 8 (1979) 897.
P.C. Ho, D.A. Plamer, R.H. Wood, J. Phys. Chem. B 104 (2000) 12084.
S.V. Kagwade, C.R. Clayton, G.P. Halada, Surf. Interface Anal. 31 (2001) 442.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 TMS (The Minerals, Metals & Materials Society)
About this paper
Cite this paper
Payne, B.P., Keech, P.G., McIntyre, N.S. (2011). X-Ray Photoelectron Study of the Oxides Formed on Nickel Metal and Nickel-Chromium 20% Alloy Surfaces Under Reducing and Oxidizing Potentials in Basic, Neutral and Acidic Solutions. In: Busby, J.T., Ilevbare, G., Andresen, P.L. (eds) Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems — Water Reactors. Springer, Cham. https://doi.org/10.1007/978-3-319-48760-1_65
Download citation
DOI: https://doi.org/10.1007/978-3-319-48760-1_65
Publisher Name: Springer, Cham
Online ISBN: 978-3-319-48760-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)