You have full access to this open access chapter, Download chapter PDF
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Case-Based Glossary with Tips and Tricks
Clinical features | Marco Patelli | |
Radiology | Giorgia Dalpiaz Marta Fiscaletti Marco Piolanti | |
Pathology | Alessandra Cancellieri |
Air crescent sign | Page 252 | |
Air trapping | Page 255 | |
Angiogram sign | Page 257 | |
Beaded septum sign | Page 259 | |
Bubble-like lucencies | Page 261 | |
Butterfly sign | Page 263 | |
Cheerio sign | Page 265 | |
Comet tail sign | Page 267 | |
Crazy paving | Page 269 | |
Finger-in-glove sign | Page 271 | |
Galaxy sign | Page 273 | |
Halo sign | Page 275 | |
Head-cheese sign | Page 277 | |
Reversed batwing sign | Page 279 | |
Reversed halo sign | Page 281 | |
Snowflake sign | Page 283 | |
Tree in bud, bronchiolar | Page 285 | |
Tree in bud, vascular | Page 287 |
Air Crescent Sign
Meniscus or cap sign

Clinical History
(Patient A) Male in his 70s with fever, cough, and hemoptysis: immunocompromised patient with neutropenia.
(Patient B) Male in his 70s, he underwent coronary artery bypass due to ischemic heart disease. No dyspnea, immunocompetent, BPCO, and persistent productive cough.
HRCT
(Patient A) In the left upper lobe, axial HRCT image shows rounded area of consolidation surrounded by a very thin black area (air crescent sign) (►). An area of ground-glass opacity (GGO) also coexists in the same lobe ( ).
).
(Patient B) In the left upper lobe, coronal HRCT image shows cystic bronchiectases with intracavitary material surrounded by a crescent-shaped thin black area (air crescent sign) ( ).
).

Causes of Air Crescent Sign
Common
-
Angioinvasive aspergillosis
-
Mycetoma (also defined aspergilloma)
Rare
-
Abscess
-
Cavitary neoplasm
-
Granulomatosis with polyangiitis (Wegener granulomatosis)
-
Hematoma
-
Hydatid cyst
-
Pneumocystis jiroveci pneumonia
-
Tuberculosis (TB)
Tips and Tricks
-
Please pay attention to the state of the patient immunocompetence and if there was a preexisting cystic or cavitary lung disease in a previous CT. As a matter of fact, invasive aspergillosis should be suspected in any patient with neutropenia who develops a fever and presents the air crescent sign inside a consolidation or mass (Patient A). On the contrary, the air crescent sign of mycetoma, also referred to as the Monad sign, is seen in an immunocompetent host with preexisting cystic or cavitary lung disease, usually from tuberculosis or sarcoidosis (Patient B).
-
Mycetoma is usually located in the upper lobes. Lower lobes and multifocal distribution should increase the suspicion of a different diagnosis.
Management and Diagnosis
Both patients were positive for serum biomarker galactomannan together with the presence of Aspergillus in the bronchoalveolar lavage (BAL) (septate hyphae branching at 45° on a necrotic background; see the images below).
Final diagnosis of Patient A: angioinvasive aspergillosis
Final diagnosis of Patient B: mycetoma

Pearls
-
Air crescent sign is recognized as a crescent-shaped or circumferential area of radiolucency within a parenchymal consolidation or nodular opacity. This sign is often seen in two types of Aspergillus infection: angioinvasive and mycetoma.
-
Pathogenesis of air crescent sign in angioinvasive Aspergillus infection. It is caused by parenchymal cavitation, which typically occurs 2 weeks after the detection of the initial radiographic abnormality. The nodules are composed of infected hemorrhagic and infarcted lung tissue. As the neutrophil count recovers and the patient mounts an immune response, peripheral reabsorption of necrotic tissue causes the retraction of the infarcted center, and air fills the space in between. This creates an air crescent within the nodules and is a good prognostic finding because it marks the recovery phase of the infection. This sign is seen in approximately 50 % of patients. Angioinvasive Aspergillus infection is often fatal.
-
Pathogenesis of air crescent sign in mycetoma (aspergilloma). It is caused by the presence of a fungus ball inside a cavity separated from the wall of the cavity by an airspace of variable size. Air crescent sign in mycetoma, also referred to as the Monad sign, was first described in 1954 by Pesle and Monod. It is seen in an immunocompetent host with preexisting cystic or cavitary lung disease, usually from tuberculosis or sarcoidosis. The radiographic appearance is often that of a gravity-dependent mass within a preexisting cavity.
Hansell DM (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697
Nitschke A (2013) Monod sign. J Thorac Imaging 28:W120
Franquet T (2001) Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 21(4):825
Air Trapping
Gas trapping

Clinical History
Female in her 60s, ex-smoker. Bronchial asthma from 15 years, on continuous therapy during the last 3 years. Clinical reevaluation for worsening cough shows bronchial obstruction, not reversible.
HRCT
Coronal inspiratory (Insp.) and expiratory (Exp.) CT images with minimum intensity projection (MinIP) algorithm show patchy areas of black and white aspect due to air trapping. Coronal CT image with maximum intensity projection (MIP) shows small solid nodules with random distribution.

Causes of Air Trapping
With Bronchiectasis
-
Constrictive bronchiolitis (CB)
-
Congenital conditions (cystic fibrosis, primary ciliary dyskinesia, Swyer–James Syndrome – SJS,
Williams–Campbell syndrome)
-
Infection (atypical mycobacteria, tuberculosis, ABPA)
With Interstitial Lung Disease
-
Constrictive bronchiolitis (CB), DIPNECH, sarcoidosis, hypersensitivity pneumonitis (HP), andcollagen vascular disease (CVD)
Tips and Tricks
-
In our patient air trapping is not associated with bronchiectasis but with interstitial lung disease (nodules) displaying solid density and random distribution. The presence of random nodules rules out the diagnosis of sarcoidosis or hypersensitivity pneumonitis.
-
The coexistence of patchy areas of air trapping with solid random nodules supports the diagnosis of constrictive bronchiolitis in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH).
Management and Diagnosis
The patient underwent a surgical lung biopsy. Nodules (A) are composed of a proliferation of neuroendocrine cells, as demonstrated by the positivity with anti-chromogranin antiserum (B). The neuroendocrine proliferation can also consist in a linear growth within the airway wall (C). As a result, fibrosis of the bronchioles can ensue, both in the form of stenosis and complete obliteration of the lumen (D).
Final diagnosis: diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH)

Pearls
-
Air trapping is the retention of air in the lung distal to an obstruction (usually partial). Air trapping is seen on expiration CT scans as parenchymal areas with less than normal increase in attenuation and lack of volume reduction. Comparison between inspiratory and expiratory CT scans can be helpful when air trapping is subtle or diffuse.
-
Pathogenesis. Small-airway disease results from different causes (please see the table above).
-
DIPNECH, when associated with bronchiolar fibrosis, is also known as Aguayo–Miller syndrome after the name of the authors who first published a clinical series of six cases in 1992. The majority of patients presenting with DIPNECH are middle-aged females with symptoms of cough and dyspnea, obstructive abnormalities on pulmonary function testing, and radiographic imaging showing pulmonary nodules, air trapping, and mild bronchiectases. In general, the clinical course remains stable; however, progression to respiratory failure does occur. Long-term follow-up and treatment remain unclear. Transbronchial biopsy in search of a specific etiology is often a first step. If this remains negative, then a video-assisted thoracoscopic surgical biopsy may be necessary. Please also refer to DIPNECH in the Dark Lung Diseases chapter.
Hansell DM (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697
Kligerman SJ (2015) Mosaic attenuation: etiology, methods of differentiation, and pitfalls. Radiographics 35(5):1360
Nassar AA (2011) Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview. Am J Respir Crit Care Med 184(1):8
Angiogram Sign
Lightning sign (n.d.e.)

Clinical History
Woman in her 70s, previous smoker with dry cough, asthenia, and chest right pain for 6 months. Recent diagnosis of autoimmune disease. The chest radiography shows consolidation not responsive to medical treatment (“non-resolving pneumonia”).
CT
In the lower right lobe, CT shows consolidation with visibility of patent white vessels crossing the lesion (angiogram sign). In the upper right lobe, a parenchymal nodule is also visible ( ).
).

Causes of Angiogram Sign
Common Disease
-
Adenocarcinoma
-
Pneumonia
Rare Disease
-
Lipoid pneumonia
-
Lymphoma
-
Metastasis of gastrointestinal adenocarcinomas
-
Obstructive pneumonitis due to central lung tumors
Tips and Tricks
-
Please note that the consolidation presents a mass-like aspect without bronchogram sign. The vessels inside the lesion (angiogram sign) appear thinned and stretched. This feature suggests the presence of material that occupies the alveoli with mass effect.
-
In our case the mass-like consolidation with angiogram sign is associated with a nodule. These imaging features are nonspecific and may resemble a variety of benign and malignant chest disorders including adenocarcinoma. However, the diagnosis of pulmonary MALT lymphoma should be considered in patient with the imaging features described above and with a history of autoimmune disorder.
Management and Diagnosis
The bronchoscopy showed endobronchial vegetation into the proximal tract of the lower right bronchial pyramid. Pathologic analysis showed fragments of a diffuse proliferation composed of small centrocyte-like lymphocytes (Figure A). The proliferating lymphocytes are CD20 positive, a feature consistent with B-cell derivation (Figure B) (Courtesy of S. Damiani, Bellaria Hospital, University of Bologna).
Diagnosis: low-grade B-cell pulmonary lymphoma MALT

Pearls
-
Angiogram sign refers to the visualization of pulmonary vessels within an consolidation, on contrast-enhanced CT scanning. The vessels are prominently seen against a background of a relatively low-attenuation lesion. It has been initially described in 1990 by Im as a specific sign of lobar bronchoiolo-alveolar carcinoma. In the following years, this sign has been reported to be present in several different benign and malignant diseases (please see the table).
-
Pathogenesis. The vessels are prominently seen due to the variable low-attenuation density of the consolidation. CT low-attenuating lung consolidations with angiogram sign after intravenous contrast material administration may be due to the presence in the airspace of fat (lipoid pneumonia), mucus (obstructive pneumonia with abundant accumulation of secretions), necrosis (necrotizing pneumonia), or mucin (primary or metastatic mucinous adenocarcinoma) (please also refer to the Cheerio sign in this chapter).
-
Pulmonary MALToma does not present pathognomonic imaging features. The commonest radiological manifestations are pulmonary mass, or mass-like area of consolidation and multiple pulmonary nodules. Common associated features include air bronchograms, positive angiogram sign on contrast-enhanced CT, and halo of ground-glass opacity (halo sign). Please also refer to MALToma in the Alveolar Diseases chapter.
Im JG (1990) Lobar bronchioloalveolar carcinoma: “angiogram sign” on CT scans. Radiology 176(3):749
Beaded Septum Sign
Beaded appearance, nodular septal thickening

Clinical History
Man in his 20s with cough, dyspnea, and restrictive ventilatory defect at pulmonary function tests. The chest radiography shows multiple micronodules predominant in the upper and middle zone.
HRCT
HRCT at the level of the right upper lobe shows diffuse nodular thickening of the interlobular septa (beaded septum sign ►) and subpleural micronodules (➨). Axial unenhanced CT (mediastinal window) shows bilateral hilar and subcarinal calcified lymph node enlargement.

Causes of Beaded Septum Sign
Common
-
Lymphangitic carcinomatosis (LC)
Rare
-
Amyloidosis
-
Lymphoproliferative disease (lymphoma, leukemia)
-
Sarcoidosis
Tips and Tricks
-
Small and dense nodules beaded some septa (see above, left) but also the subpleural space with the so-called perilymphatic distribution (“avid of pleura”). This pattern is most typical of sarcoidosis, lymphangitic spread of carcinoma or other neoplasms, and lymphoproliferative disease.
-
Note that a grouping of small subpleural nodules adjacent to the costal margins coexists. They are due to agglomerates of nodules and arranged linearly along the pleural surfaces, thus mimicking focal thickening (pseudoplaques ➨). Pseudoplaques are thought to be most commonly seen in association with granulomatous disease and in particular with sarcoidosis.
-
The distribution of the enlarged lymph nodes is a key sign for diagnosis of some diseases. Bilateral hilar lymph node enlargement may be a feature of infection (particularly fungal or mycobacterial infection) or malignancy (e.g., lymphoma), but sarcoidosis is the most common cause of bilateral lymph node enlargement.
-
Calcifications are often present in chronic sarcoidosis.
-
In our case the beaded septum sign, together with perilymphatic micronodules, pseudoplaques, and the symmetric lymphadenopathy with punctate calcifications are highly suggestive of sarcoidosis.
Management and Diagnosis
Transbronchial biopsy (TBB) can easily show non-necrotizing, well-formed granulomas, due to their centrilobular distribution (Figure A  ).
).
Transbronchial needle aspiration (TBNA) from mediastinal lymph nodes can also provide granulomas with similar features (please also refer to TBB and TBNA in the chapter Clinical Approach to DLD).
Final diagnosis: sarcoidosis

Pearls
-
Beaded septum sign consists of nodular thickening of interlobular septa reminiscent of a row of beads (Please see Figure B and Septal Pattern, subset Nodular). The beaded septum sign was initially described as a sign of lymphangitic spread of cancer, although thoracic sarcoidosis in the literature has been known as a “great mimicker” and can manifest with various pattern on HRCT, like nodular septal thickening simulating lymphangitic carcinomatosis.
-
Sarcoidosis, septal. Beaded septum sign often is an ancillary sign. It may be a predominant radiologic feature in only 15–20 % of patients with sarcoidosis. Other atypical manifestations of sarcoidosis, such as mass-like or alveolar opacities, honeycomb-like cysts, miliary opacities, mosaic attenuation, tracheobronchial involvement, and pleural disease, and complications such as aspergillomas may also be seen. The most common pattern in sarcoidosis, which helps to make a diagnosis, is the presence of micronodules with a perilymphatic distribution (avid of pleura) and bilateral, symmetric hilar lymph node enlargement.
Andreu J (2004) Septal thickening: HRCT findings and differential diagnosis. Curr Probl Diagn Radiol 33:226
Criado E (2010) Pulmonary sarcoidosis: typical and atypical manifestation at high-resolution CT with pathologic correlation. Radiographics 30:1567–1586
Bubble-Like Lucencies
Bubbly consolidation, pseudocavitations

Clinical History
Female in her 60s with asthenia, chronic dyspnea, arthralgia, and low-grade fever for about 2 months. Chest X-ray shows bilateral patchy consolidations. The picture remains unchanged after antibiotic therapy (“non-resolving pneumonia”).
HRCT
Patchy bilateral lung disease with alveolar pattern in the form of peripheral consolidations. All lesions show multiple bubble-like hyperlucencies (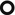 ) and surrounding ground-glass opacity (halo sign
) and surrounding ground-glass opacity (halo sign  ).
).

Causes of Bubble-Like Lucencies
Neoplastic
-
Adenocarcinoma, primary or metastatic
-
BALT lymphoma
Nonneoplastic
-
Infection (e.g., TB), organizing pneumonia (OP), and pulmonary infarction
Tips and Tricks
-
The presence of hyperlucencies within a consolidation or within a subsolid nodule should not always be considered as a synonym of necrosis or cavitation. Small, rounded, or oval hyperlucencies, similar to bubbles (bubble-like lucencies), may be the expression of ectatic bronchioles (
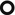 ); in this last case, therefore, they are pseudocavitations.
); in this last case, therefore, they are pseudocavitations. -
The association between persistent opacities (“non-resolving pneumonia”) with bubble-like hyperlucencies and extended halo sign (
 ) is suggestive of primary pulmonary adenocarcinoma, more frequently of the mucinous type.
) is suggestive of primary pulmonary adenocarcinoma, more frequently of the mucinous type. -
Infectious disease (mainly, secondary tuberculosis), pulmonary infarction, organizing pneumonia (OP), and BALT lymphoma are other possible etiologies, more rarely encountered.
Management and Diagnosis
The patient underwent bronchoscopy. Bronchoalveolar lavage (BAL) showed increased lymphocyte and neutrophil count, absence of malignancy, and negative bacteriological examination and culture for mycobacteria. Histologic sampling by transbronchial biopsy (TBB, images below) revealed the presence of a small focus of adenocarcinoma; neoplastic cells grow along the alveolar septa (lepidic growth ►). They show a fence-like arrangement in a fence and show abundant intracytoplasmic mucin, with positive TTF-1 expression.
Final diagnosis: primary mucinous adenocarcinoma of the lung

Pearls
-
Bubble-like lucencies are small lucencies inside both nodules and consolidations. The origin of these pseudocavitations has been clarified by histopathological studies in patients with adenocarcinoma, in particular with the in situ mucinous adenocarcinoma (previously known as mucinous bronchoalveolar carcinoma or BAC).
-
Pathogenesis. Bubble-like lucencies (pseudocavitation) can be formed by a valve mechanism by bronchiolar obstruction or by desmoplastic bronchiolar traction or paracicatricial emphysema. Also, lucency can result from spared pulmonary lobules.
-
Lepidic growth is defined as cancer cells which proliferate respecting the microscopic structure of pulmonary alveoli and papering them like butterflies (Lepidoptera) on a fence.
-
Mucinous adenocarcinomas of the lung often manifest as diffuse lung involvement (multilobar and bilateral), showing patchy and extensive consolidations (pneumonia-like), often with bubble-like lucencies and possible halo sign. It also may display air bronchograms and the CT-angiogram sign, or it may be also associated with nodules, prevalently subsolid. It carries a worse prognosis than non-mucinous variants (survival by about 30 % at 5 years versus 70 %). Please also refer to adenocarcinoma in the Alveolar Diseases chapter.
Gaeta M (1999) Radiolucencies in bronchioloalveolar carcinoma: CT-pathologic correlation. Eur Radiol 9:55
Butterfly Sign
Bat’s wing/angel wing opacities

Clinical History
Woman in her 50s turned to the emergency room for sudden onset of hemoptysis and acute respiratory distress. The patient had also been suffering from low-grade fever and diffuse arthralgia for several months. At admission a chest radiography was performed, followed by a pulmonary HRCT study. A sinonasal CT was later performed due to upper airway symptoms.
CT
HRCT shows bilateral pulmonary involvement in the form of extensive ground-glass opacities. Distribution is central: note the presence of “subpleural sparing” (butterfly pulmonary opacities). CT scan of the paranasal sinuses shows bilateral maxillary sinus mucosal thickening ( ).
).

Causes of Butterfly Pulmonary Opacities
Acute
-
Hydrostatic pulmonary edema, diffuse alveolar hemorrhage, pneumocystis pneumonia, viralpneumonia, and aspiration or inhalation pneumonia
Chronic
-
Pulmonary alveolar proteinosis (PAP), adenocarcinoma, lipoid pneumonia (LP), “alveolar”sarcoidosis, and lymphoma/leukemia
Tips and Tricks
-
Diffuse parenchymal opacification, either with GGO or consolidations, constitute the alveolar pattern, which encompasses a wide range of diagnostic hypotheses. Consequently, it is very useful to consider the “time” factor: distinguishing acute from chronic opacification is very helpful in reducing the differential diagnoses (please see the tables at the end of Alveolar Pattern).
-
Bat’s wing distribution with acute symptoms primarily refers to a hydrostatic pulmonary edema. However, other acute lung diseases may show this distribution, such as diffuse alveolar hemorrhage (DAH), pneumonia, or inhalation/aspiration pneumonia.
-
The presence of Bat’s wing or butterfly pulmonary opacities without pleural effusion, and of other signs of pulmonary venous hypertension, makes the hypothesis of hydrostatic pulmonary edema unlikely.
-
Bat’s wing opacities in a patient with hemoptysis, acute respiratory distress, and signs of sinus inflammation support the final diagnosis of granulomatosis with polyangiitis (Wegener’s granulomatosis).
Management and Diagnosis
Laboratory tests showed iron deficiency anemia and hematuria (pulmonary renal syndrome).
Bronchoscopy ruled out the presence of bronchial lesions, and the bronchoalveolar lavage (BAL) showed hemosiderin-laden macrophages on a background of red blood cells (please see the image below). Laboratory tests were positive for antibodies c-ANCA and negative for other antibodies.
Final diagnosis: granulomatosis with polyangiitis (Wegener’s granulomatosis)
Follow-up: complete resolution of the opacities (see the coronal HRCT image below).

Pearls
-
Butterfly or Bat’s wing sign refers to the presence of bilateral parenchymal opacities, with perihilar distribution and sparing of the periphery of the lungs. It is classically described in the chest X-ray but is best appreciated on CT.
-
Pathogenesis. It is not clearly established and there are various hypotheses. The most shared is that of a propulsive effect of the respiratory cycle: the effect is more pronounced at the periphery determining a flow of fluids toward the hilum. Otherwise the contractile properties of the alveolar septa may boost edema toward the hilum. One other hypothesis is that of an increase in hydraulic conductivity centrally with the over hydration of the tissues.
-
Granulomatosis with polyangiitis (Wegener’s granulomatosis) is a systemic granulomatous necrotizing vasculitis. It is characterized by diffuse systemic involvement of the microcirculation: precapillary arterioles, capillaries, and capillary venules. DAH occurs in approximately 10 % of patients. According to the Chapel Hill classification of 2012, Wegener’s granulomatosis belongs to the systemic “small vessels vasculitis,” along with microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (Churg–Strauss).
Jennette JC (2013) Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 17:603
Cheerio Sign
Tarallucci sign (n.d.e.)

Clinical History
Man in his 50s with weight loss, chronic dyspnea on exertion, no fever. The chest X-ray shows multiple nodules throughout both lungs and consolidations in the lower lobes, not responsive to medical treatment.
HRCT
Axial HRCT shows multiple nodules containing central lucencies (Cheerio sign) (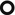 ). Some nodules are feeding vessels (►). Coronal contrast-enhanced CT image shows normally enhancing pulmonary vessels within basal consolidations (➨).
). Some nodules are feeding vessels (►). Coronal contrast-enhanced CT image shows normally enhancing pulmonary vessels within basal consolidations (➨).

Causes of Cheerio Sign
Neoplastic
-
Adenocarcinoma, primary or metastatic
-
Langerhans cell histiocytosis (LCH)
-
Lymphoma
-
Sarcoma
-
Squamous cell carcinoma
Nonneoplastic
-
Infection (fungal or mycobacterial)
-
Rheumatoid arthritis (RA) and granulomatosis with polyangiitis (former Wegener’s granulomatosis, often macronodules)
Tips and Tricks
-
In case of a diffuse nodular pattern, it is useful to identify the fissure and the pleural surface to define the distribution of the nodules (random, perilymphatic, or centrilobular). If only few nodules touch the pleura (indifferent to the pleura), as in this case, their distribution is random. Random nodules are expression of hematogenous spread of disease, supported by the “feeding vessel” site of some of them (►).
-
The second step involves the evaluation of the morphological characteristics of the nodules. They are solid and have different size due to poussées of hematogenous spread. Some of them have a central lucency (like a Tarallucci or Cheerios) due to possible necrosis or bronchiolar ectasia (
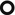 ); some of them and also some non-Cheerio nodules have halo sign that can be an expression of perinodular bleeding, neoplastic infiltration, or inflammation.
); some of them and also some non-Cheerio nodules have halo sign that can be an expression of perinodular bleeding, neoplastic infiltration, or inflammation. -
The CT scans after intravenous contrast material administration show normally enhancing pulmonary vessels within lung parenchyma consolidation (angiogram sign) (➨). The vessels appear normal in the right lower lobe and are thinned and stretched in the left lower lobe (compare their caliber!). This latter feature suggests the presence of material that occupies the alveoli with mass effect. The consolidations are low-attenuating in comparison with the chest wall musculature. The low density cannot be secondary to ischemia since the vessels are patent, but it is due to the presence of hypodense material which occupies the alveoli (necrosis, mucin, and fat) (please also refer to the Angiogram sign in this chapter).
Management and Diagnosis
A total-body CT, performed to define the general clinical condition, demonstrates a partially cystic solid mass in the pancreatic tail ( ) with a adrenal metastasis (►). The lung biopsy showed (see Figure below, right), a small nodule of neoplastic glands with prominent mucinous differentiation TTF1 negative. The fine needle aspiration (FNA) of lateral cervical lymph detects metastasis from pancreatic mucinous adenocarcinoma.
) with a adrenal metastasis (►). The lung biopsy showed (see Figure below, right), a small nodule of neoplastic glands with prominent mucinous differentiation TTF1 negative. The fine needle aspiration (FNA) of lateral cervical lymph detects metastasis from pancreatic mucinous adenocarcinoma.
Final diagnosis: metastatic pancreatic mucinous adenocarcinoma

Pearls
-
Cheerio sign is defined by a nodule with a central lucency seen on CT, similar to the ring-shaped “Cheerios breakfast cereal” and to Italian “Tarallucci”. The sign was first described in 1993 by Sandra Reed in low-grade adenocarcinoma of the lung. Many subsequent reports have shown that other diseases may be responsible for the “Cheerio sign” such as the Langerhans cell histiocytosis (LCH), metastatic gastrointestinal adenocarcinoma, mycobacterial/fungal infections, and necrobiotic rheumatoid nodules.
-
Pathogenesis. The Cheerio appearance is determined by the proliferation of either neoplastic cells or nonmalignant cells around a patent airway. Any cell proliferation surrounding other types of central radiolucencies, such as pseudocavitation, cavitation, alveoli, or multiple thin-walled cysts, can also produce appearances similar to Cheerios.
Chou SH (2013) Cheerio sign. J Thorac Imaging 28(1):W4
Comet Tail Sign
Crab nippers sign, parachute sign

Clinical History
Male patient in his 70s, asymptomatic, with past exposure to asbestos. Chest X-ray showed multiple bilateral pleural plaques and a right basal nodule. The patient underwent a chest CT with contrast medium.
CT
MPR sagittal images show in the right lower lobe a subpleural oval parenchymal lobulated opacity connected to the ipsilateral hilum by curvilinear bands (►). It is also adherent to a pleural plaque with some linear calcifications, the latter well visible in the image with mediastinal window ( ).
).

Causes of Comet Tail Sign
Common
-
Asbestosis
Rare
-
Congestive heart failure, pulmonary infarct, Dressler syndrome, parapneumonic effusion, tuberculous effusion, nonspecific pleurisy, uremic pleurisy, trauma, malignancies, and sarcoidosis
Tips and Tricks
-
The peripheral parenchymal lesion has oval lobulated morphology and is strictly adhering to a pleural plaque, which shows in its context some minute hyperdense calcifications. The contrast enhancement of the nodule is homogenous.
-
In the sagittal reconstruction, the pulmonary vessels and bronchial branches afferent to the lesion appear as curved strands extending from the lesion to the hilum. They appear like the tail of a comet, while the nucleus is represented by the lesion, hence the term comet tail sign.
-
Note the presence of volume loss of the involved lobe (that is revealed by the downward displacement of the fissure ➨).
-
The past history of exposure to asbestos together with CT pictures suggests the diagnosis of asbestosis with rounded atelectasis.
Management and Diagnosis
In the light of the CT findings and to avoid the use of invasive procedures, a PET/CT scan has been performed, which showed no areas of tracer uptake, nor at the level of the pulmonary lesion (►) or in the pleural lesions or mediastinal lymph nodes.
The patient was sent to radiological follow-up, which showed a stationary picture, even 5 years later.
Final diagnosis: rounded atelectasis in asbestos-related disease

Pearls
-
Comet tail sign is a finding that can be seen on CT scans of the chest. It consists of a curvilinear opacity that extends from a subpleural “mass” toward the ipsilateral hilum. The comet tail sign is produced by the distortion of vessels and bronchi leading to an adjacent area of round atelectasis, which is the mass. The bronchovascular bundles resemble a comet tail. It has also been defined in several ways in literature: parachute sign and crab nippers sign. This sign has a high specificity (90 %) for rounded atelectasis, which is more frequent in men. Also, rounded atelectasis has been variously defined: Blesovsky syndrome, “folded” lung, atelectatic pseudotumor, and shrinking pleuritis with atelectasis.
-
At PET/CT, rounded atelectasis is not avid, since it is metabolically non-active. PET/CT may be useful to confirm the diagnosis, without resorting to invasive biopsy investigations. However, some malignancies, like pulmonary adenocarcinoma in situ (former BAC), carcinoid, and metastatic renal cell carcinoma, may show no uptake. Furthermore, there are reports of mild positivity at PET, possibly due to associated inflammation.
-
Curiosity. The comet tail of rounded atelectasis is not the only comet tail in radiology. There is the comet tail sign in sonography. It is a reverberation artifact generated by calcific, crystalline, or other highly reflective interfaces.
Sobocińska M (2014) Rounded atelectasis of the lung: a pictorial review. Pol J Radiol 79:203
Crazy Paving
Palladian sign

Clinical History
Man in his 40s, smoker (20 pack/year), no environmental or occupational exposures. Chronic clinical history (exertional dyspnea for several months with productive cough). The chest radiograph showed bilateral parenchymal consolidations. The chest X-ray picture was unchanged after medical treatment (“non-resolving pneumonia”). HRCT was then performed.
HRCT
In the left lower lobe, extended areas of ground-glass opacity with superimposed interlobular septal thickening and intralobular lines (crazy paving) are visible (Figure A). Cranially in the same lobe, diffuse ground-glass opacities coexisting with multiple small cysts are present (Figure B).

Causes of Crazy Paving
Acute
-
Pulmonary edema, infection, diffuse alveolar hemorrhage (DAH), acute interstitial pneumonia (AIP), acute respiratory distress syndrome (ARDS), drug-induced pneumonitis, and DAD superimposed on UIP
Subacute/Chronic
-
Pulmonary alveolar proteinosis (PAP, the most frequent), lipoid pneumonia (LP), chronic eosinophilic pneumonia (CEP), organizing pneumonia (OP), sarcoidosis (alveolar), tuberculosis, primitive pulmonary neoplasms (adenocarcinoma, MALT lymphoma), nonspecific interstitial pneumonia (NSIP), and radiation pneumonitis
Tips and Tricks
-
Crazy paving may be present in many diseases. To narrow the differential diagnosis, first of all, is necessary to evaluate whether the process is acute or subacute/chronic (please see the table above).
-
Our patient presents chronic symptoms together with signs of fibrosis consisting of a little volume loss, distortion of the architecture, and also a festooned course of the fissure. Small cysts coexist, which probably have mixed origin (honeycombing and traction bronchiolectasis, Figure B).
-
Crazy-paving sign in association with a fibrotic pattern considerably narrows the range of diagnostic possibilities, as well as the subacute course. The HRCT findings may be attributable to an interstitial pneumonia (NSIP-type) or to an exogenous lipoid pneumonia.
Management and Diagnosis
Pulmonary function tests (restrictive) and arterial blood gas analysis (mild hypoxemia) result nonspecifically. Transbronchial biopsy (TBB) was performed, revealing chronic interstitial infiltrate, including lipid-laden macrophages; alveolar septa result thickened, with hyperplasia of type II pneumocytes (please see the images below). Only after obtaining a new medical history, the patient referred the long-term use of nasal lubricating oils, used to relieve the symptoms induced by the chronic use of cocaine.
Final diagnosis: exogenous lipoid pneumonia

Pearls
-
Crazy paving refers to the appearance of ground-glass opacity with superimposed interlobular septal thickening and intralobular reticular thickening, seen on HRCT.
-
Pathogenesis. Ground-glass opacity (GGO) is created by different materials (inflammatory, proteinaceous, neoplastic, etc.) partially filling the alveoli. Also it may be the expression of the thickening of the alveolar walls and of the interstitium. The “linear” component may be due to the smooth interlobular septal thickening and/or to thickening of the intralobular interstitium and/or also to deposition of material along the peripheral walls of the lobules. Therefore, the pathogenesis of the crazy paving is variable: it may be due to alveolar filling processes (airspace disease), to an interstitial disease, or to a combination of the two.
-
Exogenous lipoid pneumonia is an inflammatory disease caused by the inhalation or aspiration of fats or oils, especially mineral oils. Patients at risk are those with swallowing dysfunction and those who use lubricating oils for endotracheal and nasogastric tubes or nasal sprays to contrast the dryness of mucous membranes. Also at risk are patients with chronic constipation with prolonged use of laxatives.
Rossi SE (2003) “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview.Radiographics. 23(6):1509
Laurent F (1999) Exogenous lipoid pneumonia: HRCT, MR, and pathologic findings. Eur Radiol 9:1190
Finger-in-Glove Sign
Rabbit ear appearance, mickey mouse appearance, toothpaste-shaped opacities, Y-shaped opacities, V-shaped opacities, and hand-in-glove sign

Clinical History
Woman in her 30s with recurrent allergic bronchial asthma exacerbations, bilateral bronchiectases diagnosed when she was 22 year-old, expectoration of dark mucous plugs and hemoptysis.
HRCT
Bilateral extensive central bronchiectases filled with mucus and fluid.

Causes of Finger-in-Glove Sign
Nonobstructive
-
Allergic bronchopulmonary aspergillosis (ABPA)
-
Cystic fibrosis (CF)
Obstructive
-
Broncholithiasis
-
Congenital segmental bronchial atresia
-
Foreign body
-
Neoplasms, benign: hamartoma and lipoma
-
Neoplasms, malignant: carcinoma and carcinoid
-
TB
Tips and Tricks
-
The bilateral and extensive distribution of the finger-in-glove sign is suggestive for nonobstructive nature. Both in allergic bronchopulmonary aspergillosis (ABPA) and in cystic fibrosis (CF), bronchiectases involve central airways with upper-mid-lung predominance.
-
The presence of bilateral and extensive finger-in-glove sign in a patient with history of asthma is suggestive of ABPA.
-
Caveat! At the periphery of the lung, bronchiectases with mucoid impaction may assume a nodular-like appearance.
Management
The patient underwent fiberoptic bronchoscopy and bronchoalveolar lavage (BAL) which showed mucoid impaction (Figures A) and saprophytic proliferation of Aspergillus organisms (Figure B, inset).
Final diagnosis: allergic bronchopulmonary aspergillosis (ABPA)

Pearls
-
Finger-in-glove sign can be seen on either chest radiograph or chest CT and refers to branching tubular or fingerlike opacities which often originate from the hila and are directed peripherally.
-
Pathogenesis. The fingerlike opacities extending out from the hila represent dilated bronchi filled with mucus (mucoid impaction) (please see Figure B).
-
Pulmonary aspergillosis can be subdivided into five categories: 1. aspergilloma, 2. hypersensitivity reaction (ABPA), 3. semi-invasive (chronic necrotizing) aspergillosis, 4. airway-invasive aspergillosis, and 5. angioinvasive aspergillosis.
-
ABPA, first described in 1952 by Hinson et al., is most often seen in patients with asthma. The mucoid impaction is caused by saprophytic proliferation of Aspergillus organisms within the dilated and thickened bronchi (Figure B).
-
The HRCT scan may be useful in the diagnosis of ABPA in asthmatic patients because the combination of centrilobular nodules often seen as branching opacities (tree-in-bud pattern), central bronchiectases in three or more lobes, and mucoid impaction is highly suggestive of ABPA. In approximately 25 % of patients with mucoid impactions, HRCT scan with mediastinal window may show high-attenuation mucus secondary to the deposition of calcium salts.
-
CT may be helpful, moreover, for differentiating mucoid impactions from other causes of branching opacities (e.g., arteriovenous malformations) as well as for indicating a particular disease process or processes.
Nguyen ET (2003) The gloved finger sign. Radiology 227:453
Martinez S (2008) Mucoid impactions: finger-in-glove sign and other CT and radiographic features. Radiographics 28(5):1369
Galaxy Sign
Sarcoid galaxy sign

Clinical History
Man in his 30s underwent chest X-ray for low-grade fever which showed a nodule in the right lung. The patient received a broad-spectrum antibiotic therapy, but the lesion appeared unchanged at control X-ray performed 1 month later. HRCT and contrast-enhanced CT were then performed.
CT
HRCT shows a macronodule in the right lower lobe with peripheral nodular margination consistent with the galaxy sign ( ). Numerous confluent nodules along the costal pleura and the fissure also coexist (►). Contrast-enhanced CT shows lymphadenopathy at hilar and subcarinal level.
). Numerous confluent nodules along the costal pleura and the fissure also coexist (►). Contrast-enhanced CT shows lymphadenopathy at hilar and subcarinal level.

Causes of Galaxy Sign
-
Sarcoidosis
-
Tuberculosis (TB)
Tips and Tricks
-
The solid macronodule has irregularly micronodular margins and satellite micronodules. These features are defined as galaxy sign, described in sarcoidosis and active tuberculosis. For the differential diagnosis, it is mandatory to find out the associated lesions. Please carefully look at the periphery of the lung and note the presence of numerous nodules along the costal pleura and the fissure (►) (“avid of pleura”). This perilymphatic nodules are typically present in sarcoidosis and not in TB.
-
Please note that coalescent costal small nodules mimic the appearance of a pleural plaque (►). This feature is defined “pseudoplaques” and is associated with sarcoidosis, silicosis, and coal-worker’s pneumoconiosis (CWP).
-
Contrast-enhanced CT with mediastinal window shows slightly enlarged lymph nodes in the hilar and subcarinal stations.
-
The presence of the galaxy sign with micronodules “avid of pleura”, pseudoplaques, and coexistence of bilateral nodal enlargement suggests the diagnosis of sarcoidosis.
Management and Diagnosis
The patient underwent transbronchial biopsy (TBB): bronchiolar wall with a non-necrotizing granuloma with well-defined margins (Figure A ➨); minimal inflammation coexists. Microorganisms were not found.
Final diagnosis: sarcoidosis

Pearls
-
Galaxy sign consists of confluent nodules with multiple small peripheral nodules emanating from the margins of the central nodule (see Figure B
 ). It was initially described as the “sarcoid galaxy”, by Nakatsu et al. in 2002. In 2005, Heo et al. described the presence of the same sign in a series of patients with active tuberculosis, and they named it “clusters of small nodules”. Please also refer to Galaxy sign in the Nodular Pattern.
). It was initially described as the “sarcoid galaxy”, by Nakatsu et al. in 2002. In 2005, Heo et al. described the presence of the same sign in a series of patients with active tuberculosis, and they named it “clusters of small nodules”. Please also refer to Galaxy sign in the Nodular Pattern. -
Pathogenesis. The galaxy sign results from the coalescence of granulomas, creating the appearance of a nodule. Granulomas become less concentrated at the periphery of the lesion, justifying the irregularity and micronodularity of the margins and the satellite micronodules.
-
Sarcoidosis. Bilateral hilar lymph node enlargement is the most common finding, followed by interstitial lung disease. At HRCT, the most typical findings of pulmonary involvement are micronodules with a perilymphatic distribution and bilateral hilar lymph nodes. Multifocality of the galaxy sign supports the diagnosis of sarcoidosis.
-
TB can also present with a galaxy sign in the upper lobes and the superior segments of the lower lobes. A single isolated focus of the galaxy sign supports the diagnosis of TB. Associated findings can be very helpful as well: necrotic nodules or consolidation and tree-in-bud opacities.
-
Mimicker: neoplasm. The irregular margins should not be mistaken for the spiculated contour of lung carcinoma. The presence of symmetric mediastinal lymphadenopathies suggests sarcoidosis.
-
Mimicker: silicosis and coall workers pneumoconiosis (CWP). The fibrotic stage of these pneumoconioses may resemble the galaxy sign. Signs of fibrosis such as architectural distortion, bronchiectases, and paracicatricial emphysematous destruction are crucial for the differential diagnosis.
Nakatsu M (2002) Large coalescent parenchymal nodules in pulmonary sarcoidosis: “sarcoid galaxy” sign. AJR Am J Roentgenol 178:1389
Criado E (2010) Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 30(6):1567
Aikins A (2012) Galaxy sign. J Thorac Imaging 27(6):W164
Halo Sign

Clinical History
An immunocompetent man in his 70s underwent chest X-ray for weight loss and low-grade fever. Chest X-ray showed a small nodule in the right lung.
HRCT
CT showed a nodule with halo sign, together with a concomitant parahilar soft density tissue with extended halo of ground-glass opacity. A total-body contrast-enhanced CT after 1 month of medical therapy showed a thoracic unchanged picture (“non-resolving lesions”) but also a pancreatic lesion (please see the contrast enhancement abdominal CT image below  ).
).

Causes of Halo Sign
Infection
-
Fungi, viruses, bacteria, mycobacteria, and parasites
Noninfectious diseases
-
Granulomatosis with Polyangiitis (GPA), formerly defined Wegener Granulomatosis (WG), organizing pneumonia (OP), eosinophilic diseases, amyloidosis, amiodarone-induced toxicity, and endometriosis
Neoplasms
-
Adenocarcinoma; lymphoproliferative diseases; hemorrhagic metastasis of angiosarcoma, choriocarcinoma, melanoma, osteosarcoma, and renal cell carcinoma; nonhemorrhagic metastases of adenocarcinoma of the digestive tract, pancreas, and lung; Kaposi’s sarcoma; and primary angiosarcoma
Tips and Tricks
-
Although nonspecific, the halo sign is important because the clinical setting and associated radiological features may give a clue to the differential diagnosis.
-
In an immunocompromised patient, the halo sign suggests infection, Kaposi’s sarcoma, or a lymphoproliferative disorder. If the patient is neutropenic, it strongly suggests angioinvasive aspergillosis.
-
In immunocompetent patients, primary lung adenocarcinoma is the most frequent cause of halo sign. On the contrary, metastatic adenocarcinoma from other size rarely presents with halo sign. In both neoplastic conditions, the halo reflects histopathologically a lepidic growth pattern.
-
In our immunocompetent patient, the neoplastic nature of the lung lesions is suggested by a concomitant, likely malignant, focal pancreatic lesion (please see the abdominal CT below).
Management and Diagnosis
Abdominal contrast-enhanced CT showed a focal hypodense pancreatic lesion suspect for cystic neoplasm ( ). Pancreasectomy showed a mucinous adenocarcinoma.
). Pancreasectomy showed a mucinous adenocarcinoma.
Transbronchial biopsy (TBB): mucinous adenocarcinoma, showing positivity with ck7 and ck20 and negativity with TTF1, consisting in metastasis from pancreatic adenocarcinoma.
Final diagnosis: pulmonary metastatic pancreatic mucinous cystadenocarcinoma

Pearls
-
Halo sign is defined as a ground-glass opacity which circumferentially surrounds a pulmonary nodule or mass. The sign was originally described by Kuhlman in association with invasive pulmonary aspergillosis, but, actually, many infective, inflammatory, and neoplastic diseases may present with this pattern (see the table).
-
Pathogenesis. The halo sign is more frequently associated with hemorrhagic nodules, although it may be the expression of neoplastic or inflammatory infiltration. In the so-called hemorrhagic nodules, the halo is the expression of perinodular hemorrhage, which is produced by various mechanisms: infarction, broncho-arterial fistula, spillage from neovascularization, or vasculitis.
-
Metastases from adenocarcinoma. It is known from histopathologic studies that metastases from adenocarcinoma may spread into the lung along intact alveolar walls (lepidic growth), in a fashion similar to primary adenocarcinoma (former BAC). In another pattern of growth, tumor cells fill the alveolar spaces in a manner analogous to that of exudative pneumonia (airspace pattern). Four CT features were used to classify lesions as airspace metastases: (a) airspace nodules, (b) parenchymal consolidation containing air bronchogram and/or showing angiogram sign, (c) focal or extensive ground-glass opacities, and (d) nodule(s) with a “halo” sign.
Parrón M (2008) The halo sign in computed tomography images: differential diagnosis and correlation with pathology findings. Arch Bronconeumol 44(7):386
Head-Cheese Sign
Hog’s head-cheese sign, mixed (infiltrative and obstructive) disease

Clinical History
Male patient in his 40s. Minimal exertional dyspnea from a few months with occasional flaring up. Thoracic chest X-ray negative.
HRCT
The selected CT images through the mid-lung zones reveal a patchy pattern of a mixed density appearing as juxtaposition of regions of low, normal, and high attenuation (ground-glass opacity). It is defined “head-cheese sign”. Low-density lobular areas due to air trapping are more visible on expiratory CT ( ).
).

Causes of Head-Cheese Sign
Common
-
Hypersensitivity pneumonia (HP)
Rare
-
Atypical infection with bronchiolitis (e.g., Mycoplasma pneumoniae)
-
Smoking-related interstitial lung disease (RB-ILD, DIP)
-
Sarcoidosis
Tips and Tricks
-
In the presence of mixed density, it is mandatory to perform an expiratory chest CT to find out the coexistence of air trapping, which appears as darker areas on expiratory CT. In our patient, note that some dark areas present a polygonal morphology with lobular size suggesting small-airway obstruction as a component of the disease.
-
Look at the images carefully and note that there are also, although difficult to recognize, some diffuse centrilobular subsolid nodules (snowflake nodules). This associated sign suggests primarily a diagnosis of subacute hypersensitivity pneumonitis (HP). CT differential diagnosis with RB-ILD may be challenging due to a similar pattern; however, the latter is a smoking-related disease and, as a consequence, wall thickening and centrilobular emphysema coexist.
-
Integrating clinical findings and laboratory tests may indicate the most likely diagnosis in the setting of the head-cheese sign.
Management and Diagnosis
Further anamnesis revealed that the patient was a pigeon fancier. Laboratory findings revealed serum-precipitating antibodies against pigeons.
A second HRTC after removal of the birds and steroid therapy was performed, which showed a clear clinical and radiologic improvement.
Final diagnosis: hypersensitivity pneumonitis (HP)

Pearls
-
Head-cheese sign is characterized by the juxtaposition of lobular regions of low, normal, and high attenuation. Head cheese, believe it or not, is not a cheese and is often not made of head. It is actually a type of terrine, with bits of meat scavenged from various parts of various animals (including the head) usually from calves or pigs. It has a heterogeneous mosaic pattern, ranging from light to dark.
-
Pathogenesis. The head-cheese sign is indicative of a mixed infiltrative and obstructive process. The ground-glass opacity and consolidation component represent the infiltrative portion of the underlying disease (figure above

 ). Low attenuation lobules reflect obstructive small-airway disease with resultant air trapping and vasoconstriction from localized hypoxia (
). Low attenuation lobules reflect obstructive small-airway disease with resultant air trapping and vasoconstriction from localized hypoxia ( ). Please also refer to head-cheese sign in the Alveolar Pattern.
). Please also refer to head-cheese sign in the Alveolar Pattern. -
Hypersensitivity pneumonitis (HP) is the prototype disease showing the head-cheese sign. It is classified into acute, subacute, and chronic; however, there are actually many overlaps between these phases. In particular, HRCT does not allow to discern an acute from a subacute form. Findings are centrilobular GG nodules, patchy or diffuse GGO, mosaic pattern, and the head-cheese sign. Rarely, acute HP may present as ARDS with DAD. From a histopathological point of view, HPs are heterogeneous and can be characterized by various alterations such as cellular bronchiolitis, organizing pneumonia, or nonspecific interstitial pneumonia with ill-defined granulomas.
-
Chung et al. showed that well-defined bronchovascular nodules and nodules along the pleural surface helped distinguish sarcoidosis from HP.
Chong BJ (2014) Headcheese sign. J Thorac Imaging 29(1):W13
Chung MH (2001) Mixed infiltrative and obstructive disease on high-resolution CT: differential diagnosis and functional correlates in a consecutive series. J Thorac Imaging 16(2):69
Reversed Bat Wing Sign
Reversed butterfly sign, reversed pulmonary edema, and photographic negative of pulmonary edema

Clinical History
Male in his 30s with mild fever and cough. Chest X-ray shows consolidations in the upper lobes and bilateral, symmetrical hilar enlargement.
HRCT
Coronal HRCT image confirms the presence of bilateral airspace disease with predilection for the peripheral upper lobes of the lungs. Close-up of axial image shows two rounded areas of clustered nodules ( ).
).

Causes of Reversed Batwing Sign
Common
-
Chronic eosinophilic pneumonia (CEP) and organizing pneumonia (OP)
Rare
-
Sarcoidosis, alveolar
-
Contusions
-
Infarcts
Tips and Tricks
-
The disorders presenting with peripheral pulmonary consolidations can be remembered by the easy mnemonic AEIOU (Alveolar sarcoidosis, Eosinophilic pneumonia, Infarcts, OP, contUsions). This acronym was firstly suggested by Jannette Collins (please see the reference in the next page).
-
In our patient the lesions mainly involve the upper lung zones. This location is more frequent in CEP and in “alveolar” sarcoidosis. It is possible in OP but not typical for infarcts or contusions.
-
In the close-up axial image, please note the presence of two rounded clusters of multiple tiny nodules (the so-called sarcoid cluster sign
 ); the latter may be associated both with sarcoidosis and tuberculosis.
); the latter may be associated both with sarcoidosis and tuberculosis. -
A careful visualization of coronal CT image reveals a convex aspect of the hila suggesting a pathological involvement. Enlarged lymph nodes are also visible in the subcarinal region.
-
Mediastinal and symmetrical hilar lymphadenopathy together with “sarcoid cluster sign” and peripheral (Reversed bat wing sign) nonexcavated consolidations in the upper lobes provide clues to the diagnosis of sarcoidosis.
Management and Diagnosis
Surgical biopsy of carinal mediastinal lymph nodes shows non-necrotizing microgranulomatous inflammation (please see the images below). Special stain for micobacteria (Ziehl-Neelsen) was negative.
Final diagnosis: sarcoidosis

Pearls
-
Reversed bat wing sign is a radiographic sign characterized by bilateral peripheral opacities sparing the perihilar region.
-
Sarcoid cluster sign corresponds to rounded or long clusters of multiple small nodules in the pulmonary parenchyma which are close to each other but not confluent. It may be seen in both pulmonary sarcoidosis and pulmonary tuberculosis.
-
Alveolar sarcoidosis. The so-called “acinar” or “alveolar” form of sarcoidosis is a definition derived from conventional radiology and refers to sarcoidosis mimicking an alveolar pattern. “Alveolar” sarcoidosis is seen in 10–20 % of patients with sarcoidosis. HRCT shows bilateral patchy alveolar consolidations, which may show air bronchograms or ground-glass opacities. It is usually bilateral and symmetric and involves the middle and upper zones of the lungs. Associated CT findings of nodules in a perilymphatic distribution and mediastinal and hilar lymphadenopathy provide clues to the correct diagnosis.
-
CEP and OP. The classic radiographic and chest CT scan finding is peripheral, nonsegmental, homogeneous alveolar opacities, often with air bronchograms. Areas of ground-glass attenuation are common. In CEP the lesions involve mainly the middle and upper lung zones. On the contrary in OP, the lesions may be prevalent in the lower zones.
-
Infarcts. Only 15 % or less of thromboemboli cause pulmonary infarction. It is unknown why some emboli cause infarction and others do not, but it is likely due to compromise of both the pulmonary and bronchial arterial circulation. Pulmonary infarction results in airspace opacities that may be multifocal and predominantly peripheral in the lower lung zones.
-
Contusions. Pulmonary contusions result in the leakage of blood and edema fluid into the interstitial and alveolar spaces. On CT, contusions present as areas of consolidation, ground-glass opacification, or both which tend to be peripheral, nonsegmental, and geographic in distribution.
Collins J, Stern E (2015) Chest Radiology: The Essentials. 3rd edition, Wolters Kluwer
Reversed Halo Sign
Atoll sign

Clinical History
Male in his 50s presents with cough and low-grade fever for about a month. Chest X-ray showed small parenchymal opacities, basal and bilateral. The lesions did not regress after a cycle of broad-spectrum antibiotics (“non-resolving pneumonia”).
HRCT
In the right lower lobe, multiple GGOs, rounded, one of them conformed to “semicircle,” with a denser peripheral ring (atoll sign) are present ( ). In the left basal lobes, there are peripheral consolidations and thin arcade-like opacities (the so-called perilobular sign) (►).
). In the left basal lobes, there are peripheral consolidations and thin arcade-like opacities (the so-called perilobular sign) (►).

Causes of Atoll Sign
Infection
-
Fungal pneumonia (pneumocystosis, paracoccidioidomycosis, histoplasmosis, mucormycosis, angioinvasive aspergillosis), bacterial infection (TB, bacterial pneumonia), and virus infection (H1N1)
Noninfectious and Nonneoplastic
-
Cryptogenic organizing pneumonia (COP, the more frequent) and secondary OP, chronic eosinophilic pneumonia (CEP), nonspecific interstitial pneumonia (NSIP), sarcoidosis, granulomatosis with polyangiitis (Wegener), lymphoid interstitial pneumonia (LIP), acute fibrinous organizing pneumonia (AFOP), hypersensitivity pneumonia (HP), exogenous lipoid pneumonia, post-embolic infarction, radiotherapy, and percutaneous RF ablation
Neoplastic
-
Lymphomatoid granulomatosis, lung adenocarcinoma, and metastases
Tips and Tricks
-
The reversed halo sign/atoll sign is a nonspecific sign. To narrow the differential diagnosis, the evaluation must include clinical data and other radiological findings: first of all, it is necessary to rule out clinical signs and symptoms of infection. In severely immunocompromised patients, it should be considered expression of opportunistic invasive fungal infection, until proven otherwise. In AIDS patients it may be caused by Pneumocystis jirovecii infection.
-
In neoplastic patients it may be the expression of secondary lesions, but also of an OP following chemotherapy or radiotherapy.
-
In our patient, the concomitant presence of “chronic” peripheral ground-glass opacities and consolidations, mostly of thin arcade-like opacities (
 ), suggests the diagnosis of COP, while other diagnoses result less likely.
), suggests the diagnosis of COP, while other diagnoses result less likely.
Management and Diagnosis
Serological testing for a connective tissue disorder and infection was negative. A drug history was ruled out. Bronchoalveolar lavage (BAL) excluded the possibility of organisms or neoplastic cells. Transbronchial biopsy (TBB) showed plugs of fibroblastic tissue within bronchioles and surrounding alveoli (please see Figure A ➨).
Final diagnosis: cryptogenic organizing pneumonia (COP)

Pearls
-
Reversed halo sign. According to the glossary of the Fleischer society, reversed halo sign is defined as a focal rounded area of ground-glass opacity surrounded by a more or less complete ring of consolidation. This sign was at first described in 1996 by Voloudaki et al. who reported this finding in two cases of bronchiolitis obliterans with organizing pneumonia (BOOP – now defined OP). The definition of atoll sign was at first coined by Zompatori et al. in 1999, in a case report of a patient with BOOP. Still in 1999, Marlow et al. used the term “fairy ring” to describe the atoll sign in a case of sarcoidosis. In organizing pneumonia (OP, Figure B above), the central ground-glass opacity corresponded histopathologically to the area of alveolar septal inflammation and cellular debris (
 ) and the ring-shaped or crescentic peripheral airspace consolidation, to the area of organizing pneumonia (►). Reversed halo sign was initially considered highly specific for OP, where it is present in about 20 % of the cases. Later, the presence of this sign has been described in several different diseases (please see table above).
) and the ring-shaped or crescentic peripheral airspace consolidation, to the area of organizing pneumonia (►). Reversed halo sign was initially considered highly specific for OP, where it is present in about 20 % of the cases. Later, the presence of this sign has been described in several different diseases (please see table above).
Maturu VN (2014) Reversed halo sign: a systematic review. Respir Care 59(9):1440.
Snowflake Sign
Snowflake nodules, fluffy nodules, nodular GGO, ill-defined nodules, and airspace nodules

Clinical History
Male in his 50s, never smoker, not allergy sufferer. Kidney transplant at age of 40 years after a 7 year dialysis for postinfectious severe renal failure. Slowly progressive dyspnea and functional limitation exercise. Lung function was normal except for a reduced mild DLCO and mild hypocapnia.
HRCT
CT images show bilateral fluffy snowflake nodules. Some nodules are confluent involving whole lobules. (Images courtesy of Gaetano Rea, Naples, Italy)

Causes of Snowflake Sign
Common
-
Hypersensitivity pneumonitis (HP), subacute
-
Respiratory bronchiolitis–interstitial lung disease (RB-ILD)
Rare
-
Follicular bronchiolitis (FB)
-
Hemorrhage
-
Hot tub lung (HTL)
-
“Metastatic” pulmonary calcification (MPC)
-
Pulmonary capillary hemangiomatosis (PCH)
Tips and Tricks
-
Note as the nodules stop at a certain distance from the pleural surfaces (“pavid of the pleura”). So a centrilobular distribution can be assumed.
-
To reduce the number of differential diagnoses look at the cranio-caudal distribution of the nodules. The prevalent distribution in the upper-medium lobes, as present in our patient, is often present in the RB-ILD, in the “metastatic” pulmonary calcification and in the subacute HP.
-
The size of the nodules is another helping parameter. Tiny centrilobular nodules may be visible in RB-ILD. Tiny nodules are randomly distributed in HTL.
-
The presence or absence of associated signs may be crucial. Lobular air trapping is often associated with subacute HP. The association with moderate centrilobular emphysema is suggestive of RB-ILD. Calcification in the vessels of the chest wall is suggestive for “metastatic” pulmonary calcification. Main pulmonary arterial enlargement due to pulmonary arterial hypertension turns to pulmonary capillary hemangiomatosis (PCH).
-
Last but not least, think about the anamnesis: allergy in HP, heavy smoker (RB-ILD), hemoptysis (hemorrhage), and conditions that directly or indirectly result in hypercalcemia, e.g., chronic renal failure (“metastatic” pulmonary calcification).
Management and Diagnosis
Neck ultrasonography showed a left solid nodule due to possible parathyroid origin. Parathyroid hormone (PTH) blood test: 220 pg/ml (normal values 15–69),
Skeletal scintigraphy showed a widespread increased activity at the third and top of both lung fields ( ). The total-body PET/CT shows intense metabolic activity area in correspondence of the left paratracheal region (SUV max 37
). The total-body PET/CT shows intense metabolic activity area in correspondence of the left paratracheal region (SUV max 37  ). No evidence of further metabolic hyperactivity areas at the level of other body segments was present. At surgery, a poorly differentiated parathyroid carcinoma, infiltrating the outer capsule, was observed.
). No evidence of further metabolic hyperactivity areas at the level of other body segments was present. At surgery, a poorly differentiated parathyroid carcinoma, infiltrating the outer capsule, was observed.
Final diagnosis: “metastatic” pulmonary calcification due to parathyroid carcinoma

Pearls
-
The snowflakes sign refers to nodules appearing with subsolid density (nodular ground-glass opacities) like snowflakes.
-
Pathogenesis. The appearance of low-density CT nodules is due to the partial alveolar filling or minimum interstitial peribronchiolar thickening. Both the pathogenic mechanisms are inferior to CT spatial resolution, and then the final common effect is low-density lesions.
-
Metastatic pulmonary calcification (MPC) is a metabolic lung condition, rare cause of centrilobular snowflake nodules, sometimes calcified. This entity is secondary to the deposition of calcium in the normal lung parenchyma. The word metastatic is put in brackets because it is a misnomer (nde).
-
Causes of MPC. It occurs in association with conditions that directly or indirectly result in hypercalcemia, e.g., chronic renal insufficiency, primary or secondary hyperparathyroidism, vitamin D toxicity, intravenous therapy of calcium, multiple myeloma, and massive osteolytic metastasis.
Belém LC (2014) Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 108(5):668
Tree-in-Bud Sign, Bronchiolar
Centrilobular branching opacities, budding tree, V- or Y-shaped branching pattern, and jacks

Clinical History
A 65-year-old retired man, nonsmoker. Two-day history of acute shortness of breath without fever.
HRCT
Bilateral widespread tree-in-bud sign with ill-defined margins and uniform distribution in all lobes together with thickening of the bronchial wall.

Causes of Bronchiolar Tree in Bud
Infections
-
Bacterial (Mycobacterium TB, non-TB Mycobacterium and Staphylococcus aureus, Haemophilus influenzae)
-
Fungal (Aspergillus)
-
Viral (Respiratory syncytial virus, Cytomegalovirus)
Congenital
-
Cystic fibrosis (CF)
Immunologic Disorders
-
Allergic bronchopulmonary aspergillosis (ABPA)
Connective Tissue Disorders
-
Rheumatoid arthritis (RA) and Sjögren syndrome
Neoplasms
-
Endobronchial spread of adenocarcinoma
Other Causes
-
Aspiration, inhalation (toxic fumes and gases), and diffuse panbronchiolitis
Tips and Tricks
-
The identification of the tree-in-bud sign should urge you to determine its location together with clinical history. Gravity-dependent distribution and esophageal abnormality or hiatal hernia with tree-in-bud opacities were associated with aspiration. Upper lung predominance is commonly encountered in patients with cystic fibrosis.
-
Please look for further imaging findings (e.g., cavitated consolidation or nodules and necrotic lymphadenopathy support the diagnosis of infection). In our patient the thickening of the bronchial wall is not specific but supports the airway involvement.
-
Widespread tree-in-bud sign with ill-defined margins, together with acute shortness of breath, supports the diagnosis of inhalation of toxic fumes and gases or infection.
-
Scrutinize patient history, including appropriate exposure history, as this may aid in determining the most likely diagnosis. In our patient the acute and rapid onset of shortness of breath without fever supports the diagnosis of inhalation of toxic fumes and gases. Only after a second anamnesis, the patient admits that he had weeded in the garden some days before the onset of symptoms using a high dose of forbidden herbicide.
Management and Diagnosis
Transbronchial biopsy (TBB) performed after a week shows organizing DAD (please see Figure A).
Final diagnosis: acute inhalation of toxic fumes

Pearls
-
Tree in bud (also referred to as branching opacities, budding tree or children’s toy jacks, see above, Figure B) is referred to a centrilobular branching opacity whose appearance resembles a budding tree. The branching opacities end with small nodular opacities usually well recognizable at the periphery of the lungs. It is not visible on chest X-ray.
Rossi SE (2005) Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 25:789
Tree in Bud: Vascular
TIB, centrilobular branching opacities, budding tree, V- or Y-shaped branching pattern, and jacks

Clinical History
Female in her 50s, nonsmoker, with cough for about 1 month, mild dyspnea, fatigue, and weight loss. For the sudden worsening of dyspnea, associated with hypoxemia and hypercapnia, a CT angiography was performed to rule out a pulmonary thromboembolism.
CT
Image with lung window shows in the right middle lobe an area of clustered peripheral vascular branching opacities: the small vessels are ectatic with small nodules at their ends, similar to gems ( ). This finding corresponds to the so-called vascular tree in bud. Contrast-enhanced CT shows no intraluminal arterial filling defects related to pulmonary thromboembolism. However, it reveals mediastinal and hilar enlarged lymph nodes (
). This finding corresponds to the so-called vascular tree in bud. Contrast-enhanced CT shows no intraluminal arterial filling defects related to pulmonary thromboembolism. However, it reveals mediastinal and hilar enlarged lymph nodes ( ). Ectasia of the common arterial trunk is also visible (
). Ectasia of the common arterial trunk is also visible ( ).
).

Causes of Vascular Tree in Bud
Neoplastic
-
Extrapulmonary primary malignancies (breast, liver, renal, stomach, prostate, and ovarian cancers)
Nonneoplastic
-
Cellulose and talc granulomatosis
Tips and Tricks
-
The common pulmonary artery is considered enlarged when it is larger than the adjacent ascending aorta. This comparison, however, can be misleading in the presence of a concomitant aortic ectasia. This occurrence is not uncommon in the elderly. Quantitatively, the common arterial trunk should be considered as dilated when its size is > 2.9 cm. This value has a sensitivity of 87 % and a specificity of 89 % for the diagnosis of pulmonary hypertension.
-
The association of vascular tree-in-bud pattern with lymphadenopathies and signs of pulmonary hypertension should raise the suspicion of neoplastic thrombotic microangiopathy.
Management and Diagnosis
Echocardiogram confirmed pulmonary arterial hypertension. The patient was transferred to the ICU for the worsening of dyspnea and hypoxemia. PET/CT revealed the presence of an ovarian hypermetabolic mass and enlarged abdominal and thoracic lymph nodes. About a month later, the patient died from cardiorespiratory arrest. Postmortem examination revealed thrombosis of a pulmonary arteriole with fociof neoplastic cells resulting positive for cytokeratin (CK). Arteriolar eccentric intimal hyperplasia (►) associated with neoplastic lymphangitis was present ( ). These findings confirm the radiological suspicion of neoplastic thrombotic microangiopathy. The ovarian mass turns out to be a carcinoma with positivity for cytokeratin (CK). Images courtesy of Pathology Department of S. Orsola Hospital (Bologna) and Pneumology Unit of Arco (Trento) – Italy.
). These findings confirm the radiological suspicion of neoplastic thrombotic microangiopathy. The ovarian mass turns out to be a carcinoma with positivity for cytokeratin (CK). Images courtesy of Pathology Department of S. Orsola Hospital (Bologna) and Pneumology Unit of Arco (Trento) – Italy.
Final diagnosis: pulmonary tumor thrombotic microangiopathy

Pearls
-
Tree in bud is referred to a centrilobular branching opacity whose appearance resembles a budding tree. The branching opacities end with small nodular opacities usually well recognizable at the periphery of the lungs. Tree in bud is often due to bronchiolar disease (please see also bronchiolar Tree-in-bud sign in this chapter), rarely due to peripheral pulmonary vascular disease responsible of the so-called vascular tree in bud.
-
Vascular tree in bud is rare and often due to neoplastic conditions such as adenocarcinoma.
-
Pathogenesis. The neoplastic vascular TIB is secondary to neoplastic thrombosis with or without microangiopathy.
-
Pulmonary tumor thrombotic microangiopathy is a distinct and rare variant of neoplastic pulmonary thrombosis, found in 3.3 % of the autopsies of patients with extra-thoracic malignancy, especially adenocarcinomas. It is characterized by neoplastic thrombosis of the centrilobular arterioles and intimal fibrocellular hyperplasia induced by the tumor. These changes increase vascular resistance resulting in severe pulmonary arterial hypertension. Patients present with cough and progressive dyspnea and may develop a fatal acute right heart failure.
-
Another kind of vascular TIB is the cellulose and talc granulomatosis, secondary to i.v. injection of drugs prepared for oral administration.
Rossi SE (2005) Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 25:789
Franquet T (2002) Thrombotic microangiopathy of pulmonary tumors: a vascular cause of tree-in-bud pattern on CT. AJR Am J Roentgenol 179:897
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dalpiaz, G., Cancellieri, A. (2017). Case-Based Glossary with Tips and Tricks. In: Dalpiaz, G., Cancellieri, A. (eds) Atlas of Diffuse Lung Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-42752-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-42752-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42750-8
Online ISBN: 978-3-319-42752-2
eBook Packages: MedicineMedicine (R0)

