Abstract
Low-cost measurement of the concentration of key volatile fatty acids (VFAs) would be very useful to improve the operation of a number of important bioprocesses, through monitoring and control. In situ microbial fuel cells (MFC)-based VFA sensors could replace the current generation of relatively complicated and expensive techniques for online VFA analysis. Cyclic voltammetry (CV) was used to determine the oxidation peak potentials for three VFA species (acetic, butyric and propionic). The dynamic behaviours and static sensitivities were recorded for 10-ml single chamber cubic MFC-based VFA sensors, separately acclimated on acetate, butyrate and propionate, while the anode was poised at a potential corresponding to the oxidation peak from CVs. The current responses at the fixed working electrode potentials were analysed for each VFA sensor in response to different concentration (0–250 mg/L) of the corresponding and other VFA species. When corresponding VFAs were supplied to the sensors, the range of the sensor was increased from 40 to 220 mg/L with no response to other VFA species in acetate- and propionate-enriched reactors.
Similar content being viewed by others
Keywords
1 Introduction
Microbial fuel cell (MFC) technology when first discovered was known almost exclusively for electricity generation, but later, its applications to biosensing have attracted the attention of many researchers, primarily for mainly toxicity analyses [1, 2] and biochemical oxygen demand (BOD; [3, 4] . A few reports have also been published in relation to analysing specific substrates such as lactate, for example, the work of Kim et al. [5] and volatile fatty acid (VFA) by Kaur et al. [6] . In previous studies, an MFC-based VFA sensor using cyclic voltammetry (CV) as one of the methods was introduced by Kaur et al. [6]. The methods were improved later by using immobilization of microbial community with natural and conductive polymers [7] . In CV-based sensor, the measured parameter is oxidation potential of an electrochemical reaction. The working principle relies on the fact that when a voltage is applied to an electrode in solution, a current flow occurs because of an electrochemical reaction. The voltage at which these reactions occur is indicative of a particular reaction and/or particular species [8] . However, for a sensor to meet the expected criteria of a robust deployable VFA sensing, the range and selectivity required improvement .
In an MFC, the biocatalyst present in the anodic chamber influences several aspects of the overall performance. Anode potential is one of the important factors controlling the synergistic interactions within and between microbial populations and current flow as it can influence the electron-liberating capacity of the biocatalyst [9, 10] . Setting the anode potential to a fixed voltage using a potentiostat in bioelectrochemical system (BES) can exert effects on the system performance, bacterial growth and/or composition of consortia [9, 11] . The positive effects of setting anode potential have already been presented in relation to pure cultures with ranges 0.095–0.595 V (vs. Standard Hydrogen Electrode (SHE), Shewanella putrefaciens) [12] , −0.16–0 V (vs. SHE, Geobacter sulfurreducens) [13] and 0.27–0.32 V (vs. SHE, Pseudomonas putida F1; [14] . While the use of set potentials for efficient performance at bioanodes has received significant attention in the context of improving start-up time [9, 15, 16 ] , and power generation and bioelectrochemical behaviour [14, 17] , only a limited number of reports have been published regarding the application of set potentials to improve the degradation of recalcitrant organics, and likewise, the importance of set potentials in measuring different substrates has not been published so far for MFCs-based sensors . This study presents results that indicate an improved range for the sensor, achieved by using specific poised potentials on the basis of oxidation peaks obtained from CVs, in relation to a set of specific VFA-enriched MFC sensors. Offline testing of samples collected from a scaled-up MFC running on sucrose and an electrodialysis (ED) cell with sucrose and grass separately as feed was also undertaken to investigate if the industrial implementation of the sensor could be plausible.
2 Material and Methods
2.1 MFC Sensor Set-Up
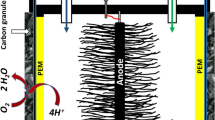
Three cubic MFCs were constructed from Perspex material with external dimensions (50 × 50 mm) to have a single cylindrical chamber with internal diameter of 25 mm. The thickness of the front and outer cover was 6 mm, and the middle chamber was 14.20-mm thick. The three individual parts were joined together using aluminium screws to hold the sandwiched assembly, including the membrane electrode assemble (MEA) of anode, cation exchange membrane (CMI-7000, Membrane International Inc. NJ, USA) and cathode. For anode material, a plain carbon cloth (BASF Fuel Cell Inc., NJ, USA) was used, with an exposed surface area of 4.9 cm2. The cathode was prepared on plain carbon cloth (CCP40, Fuel Cell Earth LLC, MA, USA) as described by Cheng et al. [18] . The system was sealed to make it watertight, giving a total liquid reactor volume in the anode chamber of 10 ml. These MFCs were enriched with 0.8 ml of sludge using 20 mM of acetate, propionate and butyrate as electron donor and 9.2 ml of media with composition as described previously by Kim et al. [19] .
2.2 Calculations
Current (I) and power (P = IV) were calculated as described in the previous report [20] . The current (Cs−1) was calculated according to I = V/R, where V is the measured voltage and R is the resistance. The percentage error was calculated for comparing the MFC-measured responses to the gas chromatograph (GC) responses obtained from various samples as follows:
where C approx is the concentration from MFC sensor array and C exact is the concentration from GC.
2.3 Electrochemical and Chemical Analysis
The poised potential and CV experiments were carried out to characterize the oxidation–reduction reaction of VFAs on the anode biofilm by using PC 4/750 potentiostat, Gamry. A conventional three-electrode system was employed with the anode as the working electrode, the cathode (2.5 cm2, 0.35 mg/cm2; 10 % Pt; E-Tek, NJ) as the counter electrode and an Ag/AgCl reference electrode as shown in Fig. 17.1.
The potentials were shifted from −750 to + 750 mV at a scan rate of 50 mV/s while monitoring the current response. The voltage and current across the load were monitored using a virtual instrument-based data logging system (National Instruments, LabVIEW™) connected to an analogue input on the input/output (I/O) card, respectively. Acetate, butyrate and propionate were measured using a Perkin Elmer headspace gas chromatograph (model number HS40XL, Perkins Elmer, Waltham, Massachusetts, USA) in conjunction with a flame ionization detector (FID) and a Nukol free fatty acid phase (FFAP) column (30 m × 0.32 mm; Supleco) running at 190 °C and 14 psi with nitrogen as a carrier gas according to the method by Cruwys et al. [21] .
3 Results and Discussion
3.1 Anode Posed Potential Investigation
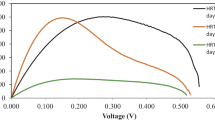
CV tests were conducted in previous study by Kaur et al. [6] to demonstrate that current generation was coupled to VFA oxidation and to examine if the concentration of the substrate can be detected from the current produced. It was concluded that CV can be used to measure and speciate VFAs in MFCs for a limited range of concentrations. The characteristic shapes of the cyclic voltammogram obtained were very distinctive for each VFA-enriched MFC. The peak potential for acetate- and propionate-enriched reactors (AMFC and PMFC, respectively) was consistent (i.e. −0.260 and −0.220 V) with ± 30 mV of peak shift. In contrast to AMFC and PMFC, butyrate-enriched reactor (BMFC) showed a shift of peak current potential ranging between ± 0.100 V. This potential shift is believed to be because of the degradation of butyrate to short-chain fatty acids as the BMFC sensor gives a current response to all three VFAs. The potential for BMFC was then selected on the basis of peak potential measured just before the calibration of the sensor , and the potential was −0.100 V. The selection of potentials was based on the experimental results, as theoretically, the redox potential for all the VFAs lies between −0.290 and −0.280 mV. Figure 17.2 shows the different oxidation peak potentials in AMFC, PMFC and BMFC, that is, −260, −220 and −100 mV, respectively.
3.2 Calibration with Standard and Mixed VFA
After enrichment and oxidation peak potential selection, the constant voltage/current was maintained, operating the MFCs in fed-batch mode with 5 mM substrate concentration. The calibration of individual VFA-enriched sensors was done by using standard concentrations of respective VFA ranging from 0 to 200 mg/L. Before calibration, reactors were shifted to the anaerobic chamber to keep the anode environment anaerobic and also to maintain the temperature at 30 °C. The anode chamber was rinsed with phosphate buffer and then refilled with media without any substrate in it. The current was stabilized for 1 h at preselected and respective poised potential for the individual VFAs . This stable current was taken as a baseline current. Then, concentration of the respective substrate was increased stepwise and measured for 10 min to achieve the stable current. Before the anolyte was refreshed in each measurement, the anode chamber was washed using deionized (DI) water to remove the organic matter that may have remained on the chamber wall. The current responses at the fixed working electrode potentials were analysed for each VFA sensor in response to different concentration (0–200 mg/L) of the corresponding and other VFA species at the appropriate poised potentials. When corresponding VFAs were supplied to the sensors, the range of the sensor was increased from 40 to 200 mg/L with no response to other VFA species in the case of AMFC and PMFC. This implies that the specific poise potentials also retained the selectivity as previously observed by Kaur et al. [6] and a further improved applicability of the sensor. The range achieved from individual VFA-enriched MFC and R 2 values is summarizedin Table 17.1 with the equation of linear fit and respective static sensitivities also included.
3.3 Measurement of Samples in MFC Sensor
A study of the application of biosensors to the real world is necessary before moving toward the ultimate ambition for any such device’s commercialization. Keeping in mind the application of the sensor presented in this study, we considered the samples from a tubular MFC and an ED cell running on complex substrates. A total of ten samples were collected from tubular MFC and ED cell and were analysed for their VFA content through GC. The maximum and minimum concentrations of mixed VFA in all samples are summarized in Table 17.2. In order to test the ability of the MFC sensor array to measure mixed VFAs contained in samples, the specific potential was poised for the individual MFC using miniaturized Ag/AgCl saturated in KCl as reference. The sensor responses were checked and compared against the GC results. The results obtained from individual MFC sensor device on addition of these samples are discussed in the following sections:
3.3.1 AMFC and PMFC Sensor Response
Most of the samples obtained from the sucrose-fed tubular MFC and ED cell were within the range of AMFC and PMFC and were not diluted further for measurement. All samples were measured twice, and the mean value of current was plotted on a calibration curve, analysed at the same time and under the same conditions, to derive a value of equivalent target compound concentrations. Table 17.2 summarizes the typical responses obtained from AMFC with respect to each sample. The maximum and minimum concentrations of acetate in the samples were 148 and 0 mg/L, respectively. For zero concentration, the response was < 20 mg/L on the calibration curve, which indicates a low amount of acetate in the sample, possibly due to the degradation of the butyric acid in the samples. Both concentrations gave very close correlations, with < 10 % error when compared with GC response, even in the presence of other VFAs . This shows that AMFC could be a promising candidate for measuring acetate concentrations in low VFA concentration samples. The propionate sensor was then tested for measuring propionate concentrations within the samples and also the effect of other VFAs in the samples. In contrast to AMFC, the concentration for propionate in all samples was very low, that is, < 70 mg/L with a maximum in the sample from ED S10, and the minimum was 0 mg/L. When measured in c-PMFC, these samples showed responses < 50 mg/L. Table 17.2 shows the responses obtained from MFC- and ED-derived samples in PMFC. Although the concentrations were not measured with high accuracy compared to the GC, no higher current was generated even in the presence of high butyric acid concentrations. This shows that the propionate sensor was specific to its corresponding VFA and might be inhibited by other unknown factors, resulting in a lower response. It is important to analyse the system performance with a larger number of samples with higher concentrations of propionate to assess how accurate the quantification of the target compound is.
3.3.2 BMFC Sensor Response
The samples were diluted in the ratio of (1:2) wherever required, with the same buffer solution that was used during the calibration. As BMFC responded to all VFAs , it was calibrated for both butyrate and total VFA (TVFA; Ac + Pr + Bu) concentrations with increasing and equal proportions of each VFA. Where AMFC and PMFC showed comparable results to GC measurements, it was found that in BMFC, it was difficult to determine the accurate concentration of the VFAs. The current responses obtained in BMFC were higher than expected in almost all samples. The current responses obtained indicate a low accuracy and repeatability of the measurement outputs from BMFC. In Table 17.2, the highlighted negative values of %error show the greater responses and high % error with respect to GC responses. As the butyric acid-enriched sensor always gives a higher response to acetate and propionate concentration as opposed to the butyric concentration, the higher current responses were in fact expected. The synergistic effect of combined VFAs in samples affected the BMFC more than AMFCs and PMFCs. The reliability of the sensor response decreases when butyric concentration in samples is ≥ 100 mg/L. In sample MFC S3 and ED S1, the concentration of the butyric acid was > 100 mg/L, which resulted in the randomly decreased current output with high %error.
This decrease might be due to the maximum detection limit of the BMFC for butyric acid, beyond which the concentration inhibits the microbial respiration. It is expected that anaerobic processes in, for example, still waters, sewers and primary waste waters, industrial wastewaters and other diluted environments may usefully be monitored for VFAs at low concentrations. Also, the application of such sensors at very low concentration in, for example, BES such as microbial electrolysis or desalination, or MFCs able to reduce concentrations to low levels of VFAs , could be important. The control of these devices or indeed aerobic or membrane effluent polishing systems could all benefit from the measurement of VFAs in or close to the ranges presented. In the present state of the sensor, it is recommended that the calibration should be done frequently to extract the more useful data. The calibration methods can be improved according to the implementation of the sensor system and the electrochemical method used for the analysis. Further detailed analysis of the bacterial communities involved should be considered for better understanding of the sensor signals. This would also help in understanding the detailed mechanisms behind the electron transfer between different bacterial communities and the electrode on the addition of individual VFA. Appropriate data processing in order to disaggregate coupled information should be considered in order to deliver VFA species-specific measurements from mixed samples. Given existing advances in biological sciences, and coupled with advances in various other scientific and engineering disciplines, it is plausible that existing VFA measurement methods can be replaced by MFC-based VFA sensor.
4 Conclusions
The results obtained showed a behaviour which might support interpretation from the developed array of VFA biosensors, when applied to environmental applications. The MFCs were able to generate significant responses, although complexity in analysing the samples may present interferences which defy simple analysis. The range of the sensor was improved from 40 to 200 mg/L. Where the acetate and propionate were poised 10 ml, MFCs sensors gave comparable results; the butyrate sensor needs further calibration, and deeper study is required to understand the complex chain of mechanisms. More analyses of the VFA biosensor are recommended, submitting it to different environmental samples. Convincing data have been produced which demonstrate a degree of robustness, selectively good measurement accuracy (in AMFC and PMFC) when compared with GC analysis. It is proposed with some confidence that the presented study on VFA sensing will contribute to understanding the place of MFC technology in the sensing of VFAs.
References
Patil S, Harnisch F, Schroder U (2010) Toxicity response of electroactive microbial biofilms—a decisive feature for potential biosensor and power source applications. ChemPhysChem 11(13):2834–2837
Stein NE, Hamelers HMV, van Straten G, Keesman KJ (2012) On-line detection of toxic components using a microbial fuel cell-based biosensor. J Process Control 22(9):1755–1761
Chang IS, Jang JK, Gil GC, Kim M, Kim HJ, Cho BW, Kim BH (2004) Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens Bioelectron 19(6):607–613
Di Lorenzo M, Curtis TP, Head IM, Velasquez-Orta SB, Scott K (2009) A single chamber packed bed microbial fuel cell biosensor for measuring organic content of wastewater. Water Sci Technol 60(11):2879–2887
Kim HJ, Moon Sik Hyun, In Seop Chang, Kim BH (1999) A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefacians. J Microb Bio 9(3):365–367
Kaur A, Kim JR, Michie I, Dinsdale RM, Guwy AJ, Premier GC (2013) Microbial fuel cell type biosensor for specific volatile fatty acids using acclimated bacterial communities. Biosens Bioelectron 47(0):50–55
Kaur A, Ibrahim S, Pickett CJ, Michie IS, Dinsdale RM, Guwy AJ, Premier GC (2014) Anode modification to improve the performance of a microbial fuel cell volatile fatty acid biosensor. Sens Actuators B Chem 201(0):266–273
D’Souza SF (2001) Microbial biosensors. Biosens Bioelectron 16(6):337–353
Aelterman P, Freguia S, Keller J, Verstraete W, Rabaey K (2008) The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol 78(3):409–418
Schroder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9(21):2619–2629
Kumar A, Siggins A, Katuri K, Mahony T, O’Flaherty V, Lens P, Leech D (2013) Catalytic response of microbial biofilms grown under fixed anode potentials depends on electrochemical cell configuration. Chem Eng J 230(0):532–536
Carmona-Martinez AA, Harnisch F, Kuhlicke U, Neu TR, Schroder U (2013) Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 93:23–29
Wei J, Liang P, Cao X, Huang X (2010) A new insight into potential regulation on growth and power generation of Geobacter sulfurreducens in microbial fuel cells based on energy viewpoint. Environ Sci Technol 44(8):3187–3191
Friman H, Schechter A, Nitzan Y, Cahan R (2012) Effect of external voltage on Pseudomonas putida F1 in a bio electrochemical cell using toluene as sole carbon and energy source. Microbiology 158(2):414–423
Chou T-Y, Whiteley CG, Lee D-J (2014) Anodic potential on dual-chambered microbial fuel cell with sulphate reducing bacteria biofilm. Int J Hydrogen Energy 39(33):19225–19231
Huang L, Chai X, Chen G, Logan BE (2011) Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ Sci Technol 45(11):5025–5031
Wagner RC, Call DF, Logan BE (2010) Optimal set anode potentials vary in bioelectrochemical systems. Environ Sci Technol 44(16):6036–6041
Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8:489–494
Kim JR, Premier GC, Hawkes FR, Rodriguez J, Dinsdale RM, Guwy AJ (2010) Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate. Bioresour Technol 101(4):1190–1198
Min B, Kim J, Oh S, Regan JM, Logan BE (2005) Electricity generation from swine wastewater using microbial fuel cells. Water Res 39(20):4961–4968
Cruwys JA, Dinsdale RM, Hawkes FR, Hawkes DL (2002) Development of a static headspace gas chromatographic procedure for the routine analysis of volatile fatty acids in wastewaters. J Chromatogr A 945(1–2):195–209
Acknowledgements
This research was funded by the RCUK Energy Programme, SUPERGEN Biological Fuel Cell Consortium project (EP/H019480/1). The Energy Programme is an RCUK cross-council initiative led by EPSRC and contributed by ESRC, NERC, BBSRC and STFC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kaur, A., Dinsdale, R., Guwy, A., Premier, G. (2015). Improved Dynamic Response and Range in Microbial Fuel Cell-Based Volatile Fatty Acid Sensor by Using Poised Potential. In: Sayigh, A. (eds) Renewable Energy in the Service of Mankind Vol I. Springer, Cham. https://doi.org/10.1007/978-3-319-17777-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-17777-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17776-2
Online ISBN: 978-3-319-17777-9
eBook Packages: EnergyEnergy (R0)