Abstract
Groundwater is the primary source of water for domestic, agricultural and industrial uses in many countries. Water resources of good quality are becoming an important issue being vital to health, safety and socio-economic development of man. The rising demands for hygienic water often cannot be met by surface water supplies. This has led to increased dependence on groundwater resources in many parts of the world. Hence suitable quantity and quality of groundwater become a more crucial alternative resource to meet the drastic increase in social and agricultural development and to avoid the expected deterioration of groundwater quality due to heavy abstraction for miscellaneous uses. Water quality and its management have received added attention in developing countries. Further intensive use for irrigation makes groundwater a critical resource for human activities. Thus water remains the principal driver for development in South Asia.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Groundwater is the primary source of water for domestic, agricultural and industrial uses in many countries. Water resources of good quality are becoming an important issue being vital to health, safety and socio-economic development of man. The rising demands for hygienic water often cannot be met by surface water supplies. This has led to increased dependence on groundwater resources in many parts of the world. Hence suitable quantity and quality of groundwater become a more crucial alternative resource to meet the drastic increase in social and agricultural development and to avoid the expected deterioration of groundwater quality due to heavy abstraction for miscellaneous uses. Water quality and its management have received added attention in developing countries. Further intensive use for irrigation makes groundwater a critical resource for human activities. Thus water remains the principal driver for development in South Asia.
The shallow aquifers (~50 m depth) in the Gangetic alluvial deposits encounter intensive groundwater draft. The middle Ganga plain (MGP) covering about 89 % geographical area of Bihar (~94,000 km2) holds potential alluvial aquifers. The tract is known for surplus food production and intensive groundwater extraction for drinking, irrigation, and industrial uses. Chemical quality plays a significant role in groundwater resource management in Bihar as the entire drinking and a major part of irrigation consumption is extracted from Quaternary aquifers (Saha 1999). Chemical composition of groundwater is controlled by many factors that include composition of precipitation, geological structure and mineralogy of the watersheds and aquifers, and geological processes within the aquifer (Andre et al. 2005). However, in a groundwater system of alluvial nature, anthropogenic influences may mask the normal chemical alteration trends. The impact of agricultural practices and domestic waste disposal pose a major threat to quality of shallow groundwater.
Groundwater arsenic contamination in fluvial plains of India and Bangladesh and its consequences to the human health have been reported as one of the world’s biggest natural groundwater calamities to mankind. Arsenic (As) occurrence in natural waters is the result of natural or anthropogenic sources, natural sources being the major cause of environmental As problems (Smedley and Kinniburgh 2002). Natural As in groundwater at concentrations above the drinking water standard of 10 μg/L is not uncommon. Extensive spread of As-contaminated areas in the Bengal basin and parts of the Ganga plain, which are virtually free of industrial, mining, or thermal water activities, and reports of similar problems in other deltas and floodplains suggest that As-contamination in groundwater in flood-delta region is geogenic. Recent studies have linked the occurrence of arsenic in aquifers with regional geology and hydrogeology, the most serious contamination being in alluvial/fluvial or deltaic plains (Mandal et al. 1996; Bhattacharya et al. 1997, 2004, 2009; Nickson et al. 2000; Acharyya 2002; Ahmed et al. 2004; Nath et al. 2008; Chauhan et al. 2009; Kumar et al. 2010; Bhowmick et al. 2013). The most common process that explains the As mobilization is the reductive dissolution of iron and oxides/hydroxide minerals and subsequent release of As into the groundwater, which occurs under anaerobic conditions (Bhattacharya et al. 1997; Nickson et al. 2000; Zheng et al. 2004; Mukherjee et al. 2008). Holocene alluvial sediment with slow flushing rate is one of the significant contributors to As enrichment in groundwater (Nordstrom 2002). The As-contaminated areas, in the Ganga plain, are exclusively confined to the Newer Alluvium (Holocene).
The investigated area is located in the middle Ganga plain, amid the flood-prone belt of Sone-Ganga interfluve region. The Quaternary deposits within 300 m below ground in As affected areas in Sone–Ganga interfluve is two-tier aquifer system as revealed by hydrostratigraphic analysis. The upper part of the shallow aquifer (within ~50 m bgl) is affected by groundwater As contamination (Saha 2009). Tracts of As-polluted areas are reported from adjoining parts of eastern Uttar Pradesh (U.P.), and northern parts of Jharkhand states, which are located in the middle section of the Ganga alluvial plain and of the deltaic Bengal basin located in lower Gangetic plain.
The paper intends to evaluate the natural and human induced chemical processes in the shallow alluvial aquifers and characterize the groundwater in parts of Bhojpur district in Bihar. A detailed periodic investigation was carried out to appraise the groundwater chemistry of As-contaminated aquifers. This study also investigates the mechanisms of As mobilization from Quaternary sedimentary aquifers in parts of MGP and its enrichment in the local groundwater.
2 Materials and Methods
2.1 Site Description
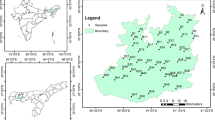
The study was carried out in two rural residential areas namely Semaria Ojha Patti and Kastulia in northern Bhojpur district of Bihar, India (Fig. 10.1). Bhojpur district lies between 25°10′ to 25°40′N and from 83°45′ to 84°45′E inhabiting population of 22,33,415 with population density 903 km−2. The study area is located in the middle-Gangetic alluvial plain amid the floodprone belt of Sone-Ganga interfluve region at about 8 km south of the Ganga river. Sone river, a right-hand perennial tributary of the Ganga, flows along the eastern boundary of the study area whereas the northern, southern and western boundaries are marked by the Ganga course. This region has some of the most fertile soil found in the country as new silt is continually laid down by the rivers every year. Annual normal rainfall in this part of MGP is 1,061 mm. The period of general rains (southwest monsoon) usually starts in third week of June with a sudden rise of relative humidity to over 70 % and a fall in temperature between about 5 °C and 8 °C from about 35 °C. The rainfall continues with intermissions till the end of September or the early part of October providing over 85 % of the annual rainfall.
Semaria Ojha Patti (~4 km2 in area) and Kastulia (~3 km2 in area), with population of 3,500 inhabitants and 1,500 inhabitants, respectively, are remote agricultural regions located at nearly 30 km from Ara, the district’s headquarter lying between two major important cities Patna and Buxar in the MGP in Bihar. Important land uses for economic activities to the local people in the study area are subsistence agriculture and pastoral production. Numerous mechanical bore-wells were found in the area drilled by farmers for irrigation. The depth of these wells ranges from 30 to 60 m yielding few litres to 30 L/s of water. The main cultivation for livelihood sustenance is focused on paddy, wheat, maize, pulses, and some cash crops (e.g. sugarcane) which are water intensive. The irrigation demand is largely met by the extraction from shallow aquifers in the area. There are no potable water supply networks in these rural localities; hence people rely mostly on groundwater extracted through hand-pumps and dug-wells.
2.2 Hydrogeological Settings
The MGP is predominantly alluvial deposits of Quaternary age. Most parts of the MGP are characterized by a rapidly filling and aggradational regime. The channels are not deeply incised in this area, and exposed bank sediments are those of the recent, aggrading floodplain system and the alluvial sequences in this area range in age from Middle Pleistocene to Holocene (Sinha et al. 2005). The sedimentation in the narrow and entrenched Sone-Ganga interfluve region of the active middle-Gangetic flood plain was influenced by sea level fluctuation during the Holocene, causing increased aggradations and formation of large fluvial lakes and swamps (Singh 2001). A higher sediment supply in the MGP may be a function of, apart from its greater proximity to the Himalayan front in the north, a higher crustal shortening rate and a higher average Holocene uplift rate (Lave and Avouac 2000). The newer alluvium, as distinct from the older unit, is characteristically unoxidised and consists of sand and silt-clay, which were mainly deposited in a fluvial and fluvio-lacustrine setting (Acharya 2005). The upper succession of the newer alluvium is predominantly argillaceous (silty clay or sandy clay), followed by fine to medium sand with occasional clay lenses forming the shallow aquifer (Saha et al. 2010). The As is generally associated with many types of mineral deposits and in particular those containing sulphide minerals such as arsenopyrite. However, elevated concentrations are sometimes found in fine grained argillaceous sediments and phosphorites (Thornton 1996).
X-ray Diffractrometry results of As-safe older alluvial and As-contaminated newer alluvial soil samples from the MGP reveals presence of minerals such as quartz, muscovite, chlorite, kaolinite, feldspar, amphibole and goethite (Shah 2008). The study area is confined within newer alluvium entrenched channel and floodplain of the Ganga river (Fig. 10.1). The area is underlain by a multi-layer sequence of sand (aquifer) alternating with aquitards like sandy-clay and clay, down to depth of 300 m. In the Sone-Ganga interfluves region, the Quaternary deposits within 300 m below ground exhibit two-tier aquifer system. The shallow aquifer system is confined within 120–130 m depth, followed by a laterally continuous 20–30 m thick clay/sandy-clay zone forming the aquitard. The deeper aquifer system exists below this aquitard, which continues down to 240–260 m below ground (Saha 2009). The shallow aquifer system is referred as active channel deposit and marked as newer alluvium. The groundwater in deep aquifer remains under confined to semi-confined condition in the affected Bhojpur district as revealed by the pumping test carried out by Saha (2009). The deep aquifer can, therefore, be developed for safe community based drinking water supply in the area.
2.3 Sampling, Field Measurements and Laboratory Analyses
A total of 20 groundwater samples were collected from 20 shallow wells (≤55 m depth) located in residential houses within the study areas based on general survey of water quality from the occupants of the respective houses during August 2006–May 2009. Sampling locations were chosen on the basis of availability of wells and in a manner such that the whole study area is uniformly covered. The wells’ locations are shown in Fig. 10.1. Some wells were selected from the houses where residents reported symptoms of arsenic poisoning. This was with a view of evaluating the quality and safety of the water being consumed, from elemental composition and possible health implication perspectives. The samples were collected from hand-pumps and few active dug-wells. First, the water was left to run from the sampling source (hand-pump) for 4–6 min to pump out the volume of water standing in the casing before taking the final sample and then water samples were collected in pre-cleaned, acid sterilized high density 250 ml polypropylene bottles. The physical parameters namely pH, Eh, EC and TDS were measured in-situ by portable corresponding digital instruments. Further, water samples were filtered through 0.45 μm Millipore membrane filter papers and immediately preserved in portable ice-box to minimize chemical alteration until they were brought at the earliest possible to the laboratory where the samples were kept at 4 °C in a thermostatic cold chamber until further processing and analyses. About 100 ml of sample was acidified with ultrapure HNO3 (2 % v/v) to pH ~2 to stabilize the dissolved metals and rest of the portion was kept unacidified. Additional samples were collected in chromic-acid-washed brown glass bottles and filtered with glass-fibre (GF/C) filter papers for the analysis of dissolved organic carbon (DOC).
Cations were analyzed in the acidified fraction, whereas the unacidified fraction was used for the analysis of anions. Bicarbonate content was determined following the potentiometric titration method (Clescerl et al. 1998). Anions and cation (NH4 +) were analyzed by Dionex ICS-90 Ion Chromatograph using the specific analytical columns “IonPac AS12A (4 × 200 mm)” for anions and “IonPac CS12A (4 × 250 mm)” for cations. Dissolved silica (H4SiO4) and DOC were determined from the unacidified water samples by the molybdosilicate method (Clescerl et al. 1998) and the vial digestion method using COD reactor, respectively; finally, absorbance measurements were carried out using a Cecil Spectrophotometer (model no. 594) for both the parameters. Metals such as Na, K, Ca, Mg, Fe and Mn were measured by Shimadzu AA-6800 Atomic Absorption Spectrophotometer (AAS) in Flame mode of analysis, whereas total dissolved As were quantified using AAS coupled with on-line hydride generator (HG). Samples were added with 20 % (w/v) KI (2 mL in 20 mL sample) and kept for 15 min reaction period for the reduction of As(V) to As(III) prior to the analysis. Further, As(III) was reduced to arsine (AsH3) by on-line HG using 1 % (w/v) NaBH4 in 0.1 % (w/v) NaOH and 5 M HCl as buffer solution. The inclusion of on-line HG generally increases the sensitivity of detection and reduces the possible interferences from the sample matrix. Merck-certified standard solutions and AnalR grade chemicals were used for quality control during analyses. Milli-Q water was used during processing and analyses of the samples. Standards for major and trace elements were analyzed as unknown intermittently to check precision as well as accuracy of the analysis and the precision was ≤5 %. The AAS was programmed to perform analyses in triplicates and RSD was found within ±3 % in most of the cases reflecting the precision of the analysis. The computed ionic balance errors for the analytical database were observed within a standard limit of ±5 % attesting good quality of the data.
3 Results and Discussion
3.1 Groundwater Chemistry
Chemical characteristics of groundwater as observed during investigation period in the study area are statistically presented in Table 10.1. Groundwaters are characterized as nearly neutral to moderately alkaline with pH ranging 7.5–8.3 (mean 7.9). The lower redox potential (Eh) recorded in groundwater wells (−41 mV to −15 mV; mean ~ −23 mV) suggest that groundwater in the studied area of MGP was under moderately reducing condition perhaps induced by frequent flooding in the region. The moderately reducing aquifer condition in the area is further confirmed by considerable presence of other redox-sensitive elements such as Fe and Mn discussed in next section. Significant variations in chemical composition indicate high salinity in many of the groundwaters, with total dissolved solids (TDS) ranging 375–1,087 mg/L (mean 602 mg/L) and conductivity (EC) in the range 581–1,643 μS/cm (mean 943 μS/cm). Higher EC implies intensive mineralization in groundwater. Irrigation increases the salinity of irrigation-return-flow from three to ten times that of the applied water (Todd 1980). Since irrigation is the primary user of water, irrigation-return-flow may be the major cause of groundwater pollution in the study area. Further recycling of saline groundwater for irrigation may result in a progressive increase in soil and groundwater salinity.
Major ion composition showing a wide range of variations in the parts of middle-Gangetic alluvial aquifers is dominated by HCO3 − (253.49–519.60 mg/L, mean 386.63 mg/L) and in lower proportion by Cl− (8.48–155.23 mg/L, mean 44.64 mg/L). Concentrations of NO3 − (3.03–133.80 mg/L, mean ~34.5 mg/L) and SO4 2− (3.99–94.56 mg/L, mean 27.56 mg/L) were relatively low in the samples, except for some locally high occurrences, contributing 0.75–13.61 % (mean 5.64 %) and 1.27–23.33 % (mean 6.33 %), respectively to the total anions. The dominance of HCO3 − accounting for 50.23–93.33 % (mean ~75 %) of the total anions, classifies the area as a recharge zone (Subba Rao 2007). The elevated HCO3 − reflects an apparent lithological control of major ion composition in groundwaters. Hence, this infers a dominance of mineral dissolution in the aquifers (Stumm and Morgan 1996). High HCO3 −concentrations are typical features of many of these groundwaters that can be attributed to the dissolution of carbonate via biodegradation of organic matter under local reducing condition and reaction of silicates with carbonic acid. The origin of Cl− derives mainly from the non-lithological sources and can also be contributed, especially, from the surface sources through the domestic wastewaters, septic tanks, irrigation-return flows and chemical fertilizers (Hem 1991). The Cl− contribution of 3.88–28.14 % (mean ~13 %) to the total anions in groundwater of the study area may be attributed to the influences of irrigation return-flows and chemical fertilizers. The agricultural activity is intensive and long-term, and no other sources are evident in the area. The NO3 − can be a direct indicator of anthropogenic contaminants from agricultural land uses and Cl− also indicates the same source because salinization due to seawater intrusion or brines from deep aquifers would not be expected in the newer alluvial regime.
Natural NO3 − sources are minor which include low concentration soil NO3 − from decay of sparse, natural vegetation and nitrogen-bearing rock units. Indeed, 25 % of the groundwater samples exceeds drinking water limit of 45 mg/L NO3 − (BIS 2003). In natural conditions, mainly from the non-lithological sources, the concentration of NO3 − does not exceed 10 mg/L in the water (Ritzi et al. 1993). The observed higher concentrations exceeding 10 mg/L, in 80 % of the monitored locations, reflect the man-made pollution (Hem 1991), apparently due to application of fertilizers targeted for higher crop yields in the study area. The shallow aquifers in Ganga Alluvial Plain at places are known for high NO3 − load (Handa 1983), often exceeding the limit for human consumption (45 mg/L) prescribed by BIS (2003). The SO4 2− is likely to be contributed by multiple sources, such as atmospheric deposition, agricultural activities and pyrite oxidation (N’egrel and Pauwels 2004). In general, high SO4 2− concentrations may be derived either by sulphide or SO4 2− weathering (Stallard and Edmond 1987). Because the shallow aquifers in the area were recharged from the irrigated agricultural field and were not affected by seawater, it might also possibly be inferred that SO4 2− dissolved in groundwaters were derived from agricultural activities and decomposition of organic matter. Higher SO4 2− concentrations in some cases may partly be attributed to pyrite oxidation. High levels of Cl− and SO4 2− generally observed together with high NO3 − levels in shallow groundwaters of agricultural areas are reported to be derived from fertilizers (Chae et al. 2004). Thus the observed higher concentrations of Cl−, SO4 2− and NO3 − are an indication of man-made source, as also reported by Hem (1991).
The PO4 3− concentrations, on the other hand, are fairly low (0.27–1.36 mg/L, mean 0.65 mg/L) accounting for 0.09–0.63 % (mean 0.25 %) to the total anions. Under natural conditions, the dissolved phosphate (PO4 3−) concentration should not exceed 0.5 mg/L, as the solubility of phosphate mineral is limiting (Subramanian 1984). High concentrations in some wells are apparently due to anthropogenic influences including use of fertilizers in copious quantities to increase agricultural output. Contamination sources from household activities, such as sewage and large-scale livestock feedlots also appear to contribute to the contamination, because the area is devoid of proper sewage system. The concentration of F− in groundwaters varied significantly from 0.22 to 1.37 mg/L (mean 0.47 mg/L), contributing 0.12–0.87 % (mean 0.32 %) to the total anions. The F− in drinking water has a narrow optimum concentration range in relation to human health. While a moderate concentration range between 0.7 and 1.2 mg/L prevents dental caries, higher concentrations are responsible for dental and skeletal fluorosis (Marimon et al. 2007). Mere 5 % of the samples exceeded drinking water quality limit of 1.0 mg/L recommended by BIS (2003). Elevated concentration in some of the groundwater samples could be attributed to the weathering of F−-bearing minerals such as micas and amphiboles in the rocks.
The concentrations of major cations such as Ca2+, Mg2+, Na+ and K+ ions in the groundwater samples varied between 49.19–119.62 mg/L, 20.31–41.93 mg/L, 26.95–94.00 mg/L and 4.51–119.91 mg/L, with mean values of 78.26, 31.67, 37.10 and 20.99 mg/L, respectively. Their concentrations (on an equivalent basis) represent 28.34–53.07 %, 20.93–39.11 %, 12.38–27.60 % and 1.03–22.80 % with mean values ~46 %, ~31 %, ~18 % and ~5 % of the total cations, respectively. The observed excess of Na+ over K+ is because of the greater resistance of K+ to chemical weathering and its adsorption on clay minerals restricting its mobility. Although K+ is the least dominant cation in the groundwater of the study area, its concentration in most of the samples were detected often modest to high for the study period. Dissolution of K-bearing minerals and ion-exchange reactions may be involved. But high K+ concentrations in the basin cannot exclusively be linked to groundwater-rock interaction as its natural occurrence in higher concentrations is rare and may be the result of increased K-rich fertilizer used for agricultural land uses. The occurrence of K+ in groundwater, especially in higher concentrations reflects manure spreading and fertilization on the surface as well as a short flow path and infiltration length of the shallow aquifers in the area. The results suggest that K+ is the potential indicator of anthropogenic impacts on groundwater, namely agricultural practices in the recharge area of the aquifers. High K+ concentration in several groundwater samples also seems to be derived from domestic effluents. The elevated concentrations of Ca and Mg may be attributed to the dissolution of carbonate minerals. Dissolved silica (H4SiO4) concentration in groundwater is most conveniently related to interaction with solid phases, as it enters the system during the weathering of silicate minerals. The groundwater has high concentrations of H4SiO4 ranging between 27.35 and 41.00 mg/L (mean 34.49 mg/L). The range reflects both high groundwater temperatures and reaction of fine-grained (e.g. clays) and easily weathered (e.g. feldspar) silicate minerals in the aquifers. Further, H4SiO4 shows dominance over other nutrients such as NO3 − and PO4 3−.
3.1.1 Weathering Processes and Evolution of Groundwater Chemistry
Reactions between CO2 and carbonate and silicate minerals, containing the common alkali and alkaline earth metals (Na, K, Mg and Ca), produce the bicarbonate and carbonate anions that neutralize the positive cation charges and generate alkalinity. The computed effective partial pressure of carbon dioxide (pCO2) in groundwater samples range from 10−1.70 to 10−2.67 atm (mean 10−2.21 atm), which are higher than that in the atmosphere (10−3.50 atm) (Table 10.1). The trend indicates that groundwater is in disequilibrium with the atmosphere. The groundwater pCO2 in the study area are higher than those reported in case of surface waters (Sharma and Subramanian 2008). This increase in pCO2 would typically be counterbalanced by mineral weathering reactions in the aquifer. Thus groundwater pCO2, significantly higher (upto ~10−1.7 atm) than atmospheric as well as surface waters, could possibly be caused by biologic activity leading to the decay of organic matter in the sediments through sequence of redox reactions including reductive dissolution of Fe(III)–oxyhydroxides in the solid phase and root respiration in the vadose zone during recharge. The observed high pCO2 values suggest that, in general, redox reactions in the aquifers are responsible for the generation of CO2 which, in turn, results in the elevated level of HCO3 − in the groundwater samples, as observed in the study area.
In order to understand the foremost functional sources of major dissolved ions in the groundwater, the chemical data of groundwater samples of the study area are discussed below taking into account stoichiometric relations among dissolved ions during weathering process. The predominance of Ca2+ and Mg2+ cations and high (Ca2+ + Mg 2+)/(Na+ + K+) ratio is the characteristic feature of prevailing carbonate weathering in the basin (Sharma and Subramanian 2008). The average mill equivalent ratio of (Ca2+ + Mg 2+)/(Na+ + K+) worked out to be 3.66 (range 1.06–5.65). The observed higher ratio values indicate the dominance of carbonate weathering and suggest that chemical composition of groundwater in the parts of middle-Gangetic alluvial aquifers is controlled by carbonate lithology of the Ganga basin. Lower (Ca2+ + Mg 2+)/(Na+ + K+) ratios obtained in few cases suggest additional source of Ca2+ and Mg2+ ions, which might have been balanced by Cl− and SO4 2− and/or partly supplied by silicate weathering in addition to the carbonate weathering. The Ca2+/Mg 2+ molar ratio was calculated for further justification. In case of dolomite dissolution, the Ca2+/Mg 2+ molar ratio equals to 1 whereas, the higher ratio indicates the source from calcite (Maya and Loucks 1995). The observed higher Ca2+/Mg2+ ratios (>1) varying between 1.10 and 1.82 (mean 1.49) suggest dissolution of calcite favoured over dolomite in the aquifers.
Most of the samples (75 %) have Na+/Cl− molar ratio >1, which indicate that Na+ is released from silicate weathering (Meybeck 1987). If it is so, the groundwater would have HCO3 − as the most dominant anion (Rogers 1989), as observed in the study area. This also suggests that higher concentration of alkalies is from sources other than precipitation. At few locations, the ratio was found less than 1 which implies for halite dissolution as the source of Na+, indicating the influence of secondary evaporate deposits in the aquifers. However, the Cl−-enriched samples reveal that surplus chlorides are likely to result in aqueous species such as CaCl2 and MgCl2. The (Na+ + K+)/TZ+ ratio is an index to assess the contribution of the cations via silicate weathering (Stallard and Edmund 1987). The (Na+ + K+)/TZ+ ratios ranging from 0.15 to 0.48 (mean 0.24) suggest inputs from the weathering of aluminosilicates such as Na- and K-feldspars and/or from soils. Thus the results, taking into account hydrogeochemical processes discussed above, reveal that groundwater quality in the study area is primarily affected by natural mineralization with secondary inputs from anthropogenic sources in terms of major dissolved constituents. The Piper plot of major ions (in %milli equivalents) shown in Fig. 10.2 allows identification of groundwater chemical types and presence of chemical evolutionary trends that may exist during processes of water-rock interaction in the study area.
Groundwater in the study area is of predominantly Ca-HCO3 type, reflecting the initial impacts of water-rock interaction in the soil zone and shallow bedrock environments. The occurrence of Cl− in the groundwater cannot be explained through water-rock interaction, since it derives mainly from a non-lithological source and it also tends to prefer to form compounds with alkalis rather than Ca2+ and Mg 2+. Therefore, it may be inferred that Cl− has been consumed in the formation of alkali chlorides. Further, the observed alkali concentrations in most of the samples are much higher than those required for the formation of chlorides. It is, therefore, logical to expect the existence of aqueous species such as alkali bicarbonates in the groundwater. The corollary to this situation is presented by the Cl-enriched samples in which surplus chlorides are likely to result in aqueous species such as CaCl2 and MgCl2 as discussed above. The diamond shaped field (Fig. 10.2) further indicates that with very few exceptions, no cation-anion pair exceeds 50 % of the total ions which, in turn, reflects that the chemical properties are dominated by alkaline earths (Ca2+ + Mg2+) and weak acids (HCO3 − + CO3 2−). Thus it may be concluded that the groundwater in the parts of middle-Gangetic alluvial plain is primarily of bicarbonate alkaline earth type. The rock-water interaction processes tend to release cations (especially Ca2+ and Na+) and anions (especially HCO3 −) with raising pH condition. Subsequent ion-exchange reactions may modify the groundwater chemistry, while redox condition may lead to the dissolution of minor and trace elements in groundwater. Solutes which are subject to these processes may have been derived from water-rock interaction and/or from leachates coming with the rainfall recharge.
3.2 Source and Distribution of Redox-Sensitive Parameters
The redox-sensitive characteristics of groundwater are presented in Table 10.1. The concentration of DOC in groundwater varied considerably from 0.98 mg/L to as high as 5.25 mg/L, with a mean value of 3.22 mg/L. Abandoned channels, swamps and active channels in MGP are perennially or seasonally water filled where the aquifers are presumed to be enriched in biomass and thereby organic carbon (Shah 2008). During recharging of aquifers, organic carbon of surface water is also transported into the aquifers upto a certain depth depending on hydrological conditions. The reported high DOC concentration in these shallow alluvial aquifers is attributed to the sedimentary biomass contribution via microbial degradation in the aquifers and surficial contribution during groundwater recharge. Saline ammonia (NH4 +) is a useful indicator for sewage pollution. Another possible source of NH4 + is through natural anaerobic decomposition of sedimentary organic matter. The concentrations of NH4 + are fairly low to moderate with few exceptions, ranging between 0.20 and 4.08 mg/L, with an average of 1.48 mg/L. Mobility of As could be affected by the redox reactions involving either aqueous or adsorbed As (Manning and Goldberg 1997). The total dissolved As (AsT) concentrations in the shallow aquifers in the study area significantly varied from 21.96 to 190.21 μg/L (mean 83.57 μg/L), which far exceed drinking water guideline of 10 μg/L (BIS 2003; WHO 2011).
Thick sequences of young sediments are quite often the sites of high groundwater As concentration. The variability may be attributed both to the variations in the As content of the soils, rocks and the aquifer minerals that serve as the proximate source of As in the groundwater and to the variable extent of processes that release or sequester As from or to solid phases. The exceptionally elevated As concentrations at few locations could be attributed to the local dissolution of Fe(III)–oxyhydroxides in perhaps more reducing condition. Reducing conditions of the aquifers are also reflected by sizeable presence of Fe and Mn in most of the groundwater samples ranging between 0.23 and 4.73 (mean 1.04) and 0.04–0.23 (mean 0.10), respectively. The AsT and other important redox parameters such as Fe, PO4 3−, DOC and NH4 + related to its release into groundwater were plotted against depth of the wells monitored (Fig. 10.3). Variations in concentration of these redox characteristics with the depth indicated some trend, generally high values being found in the shallowest wells upto the depth of ~20 m with maximum concentrations at ~15 m below the ground level and then declined towards the deeper part of the shallow aquifers. These variations may be attributed to varying redox states at shallow depths within the aquifer. The trend suggests that microbial degradation of sedimentary organic matter in the shallow aquifers under anoxic condition is perhaps responsible for the release and mobilization of As in groundwater. Correlation between As and Fe or Mn is often observed in groundwater because, under certain conditions, the presence of Fe/Mn oxyhydroxides could lead to desorption of As, Fe and Mn. In order to infer their role in mobilization of As into groundwater, the As concentrations were plotted against selected physico-chemical parameters showing meaningful correlations. The highest As concentrations, in general, were detected in groundwaters with pH values exceeding 8. This may be attributed to As desorption from the oxide surfaces at pH above 8.0 (Smedley and Kinniburgh 2002). The positive correlation between pH and As justifies the desorption processes at higher pH values (r = 0.62; p < 0.01; Fig. 10.4a). A significant positive correlation between As and Fe concentrations in the groundwater (r = 0.80; p < 0.01; Fig. 10.4b) suggests that As is released in groundwater due to reductive dissolution of Fe(III)–oxyhydroxides. The As and Mn were negatively correlated (r = −0.46; p < 0.01), suggesting their different sources in the wells, while generally low concentrations of PO4 3− detected in the groundwater do not lead to any conclusion regarding its role in the mobilization and enrichment of As in the aquifers.
It has also been confirmed by the experimental desorption of As sorbed to mineral surfaces by PO4 3− (Manning and Goldberg 1997), that phosphorus in groundwater cannot contribute to As pollution. No clear correlation between As and SO4 2− was observed in present study, which suggests that pyrite oxidation is not the basic mechanism for As mobilization in these aquifers. The relationship between As and HCO3 − is also well documented because this ion can play an important role in the mobilization of As through the competition for adsorption sites and through the formation of arseno-carbonate complexes. However absence of any correlation between these two parameters nullifies the hypothesis for the present case.
Strong positive correlations of As with DOC (r = 0.81; p < 0.01) and NH4 + (r = 0.71; p < 0.01) signify their role in mobilizing As through microbial degradation of organic matter in the aquifer sediments (Fig. 10.4c, d). Significant negative correlation between Eh and As (r = −0.73; p < 0.01) supports the phenomenon of As mobilization in the moderately reducing aquifers impacted by frequent flooding in the area (Fig. 10.4e). Tremendous amount of groundwater pumped out especially during dry seasons leading to flow in of monsoonal rain and local surface water loaded with surficial organic matter, which upon reduction, dissolves Fe-oxides bearing As and thus may trigger the release of As from sediments in the study area. The positive correlation of As with Fe and elevated HCO3 − concentrations in groundwater are similar to those recorded over broader geographical areas (McArthur et al. 2001). Thus the results from present study support the hypothesis that desorption of As accompanied with reductive dissolution of Fe(III)–oxyhydroxides in the aquifer sediments is the principal mechanism by which As is released to the groundwater. Presence of elevated As concentration despite Fe concentration being low at few locations may be attributed to the precipitation of Fe-carbonates (Nickson et al. 2000). Slow groundwater flow also contributes to an elevated As concentration of water (Smedley et al. 2003). The aquifers in newer alluvial section of MGP have low hydraulic gradient with less fluctuation suggesting a sluggish flow regime in the area (Saha 2009). Hence groundwater As enrichment in the study area may also be partly attributed to the influence of longer groundwater residence time due to its limited flow in the alluvial aquifers of MGP. Based on the above discussion, it can be explicitly figured out that mobilization and enrichment of As in this part of middle-Gangetic plain is due to the influence of the sediments’ geochemical and hydrogeological characteristics of the alluvial aquifers with partial inputs from anthropogenic sources.
4 Conclusions
Weathering, mineral dissolution, leaching and other processes related to anthropogenic activities generally control groundwater chemistry in this part of MGP. Groundwater is of predominantly Ca-HCO3 type produced mainly by the dissolution of carbonate minerals, which reflects the influence of their host rock in the study area. The predominance of Ca2+ and Mg2+ cations and high (Ca2+ + Mg2+)/(Na+ + K+) ratio in the groundwater indicate the prevailing carbonate weathering in the aquifers. The elevated concentrations of NO3 − and Cl− ions are caused by intensive agricultural practices in the basin. Thus it can be concluded that the chemical solutes of groundwater samples are influenced by anthropogenic inputs in addition to the natural chemical weathering. The hydrogeochemical characteristics of the alluvial aquifers govern the mobility of As in groundwater. Positive correlations of As with Fe, NH4 + and DOC, as well as the negative correlation with Eh, typically characterizes anoxic aquifers where reductive dissolution of Fe-phases and release of surface bound As is the principal source of dissolved As in groundwater of the middle-Gangetic alluvial plain. The As contamination in groundwater presents significant threats to health of the local communities in the area. Nevertheless, groundwater is being widely used for drinking and other domestic works due to the lack of potable water supply networks or other clean water resources in these rural localities. Noticeable variations with well-depth were observed with higher As concentrations being found in the shallowest wells. Therefore, from mitigation and policy point of view, installation of deep wells in the affected villages can provide a reliable source of drinking water.
References
Acharyya SK (2002) Arsenic contamination in groundwater affecting major parts of southern West Bengal and parts of western Chhattisgarh: source and mobilization process. Curr Sci 82:740–744
Acharya SK (2005) Arsenic trends in groundwater from quaternary alluvium in the Ganga plain and the Bengal basin, Indian subcontinent: insights into influences of stratigraphy. Gondwana Res 8(1):55–66
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam MA, Bhuyian H et al (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19:181–200
Andre L, Franceschi M, Pouchan P, Atteia O (2005) Using geochemical data and modelling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, south-west of France. J Hydrol 305:40–62
Bhattacharya P, Chatterjee D, Jacks G (1997) Occurrence of arsenic contamination of groundwater in alluvial aquifers from Delta Plain, Eastern India: option for safe drinking supply. Int J Water Resour Dev 13:79–92
Bhattacharya P, Welch AH, Ahmed KM, Jacks G, Naidu R (2004) Arsenic in groundwater of sedimentary aquifers. Appl Geochem 19(2):163–167
Bhattacharya P, Aziz Hasan M, Sracek O, Smith E, Ahmed KM, von Bromssen M, Huq SMI, Naidu R (2009) Groundwater chemistry and arsenic mobilization in the Holocene flood plains in south-central Bangladesh. Environ Geochem Health 31:23–43
Bhowmick S, Nath B, Halder D, Biswas A, Majumder S, Mondal P, Chakraborty S, Nriagu J, Bhattacharya P, Iglesias M, Roman-Ross G, Guha Mazumder D, Bundschuh J, Chatterjee D (2013) Arsenic mobilization in the aquifers of three physiographic settings of West Bengal, India: understanding geogenic and anthropogenic influences. J Hazard Mater 262:915–923
BIS (2003) Indian standard: drinking water–specifications (first revisions). Bureau of Indian Standard, New Delhi
Chae GT, Kim K, Yun ST, Kim KH, Kim SO, Choi BY, Kim HS, Rhee CW (2004) Hydrogeochemistry of alluvial groundwaters in an agricultural area: an implication for groundwater contamination susceptibility. Chemosphere 55:369–378
Chauhan VS, Nickson RT, Chauhan D, Iyengar L, Sankararamakrishnan N (2009) Groundwater geochemistry of Ballia district, Uttar Pradesh, India and mechanism of arsenic release. Chemosphere 75:83–91
Clescerl LS, Greenberg AE, Eaton AD (eds) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Handa BK (1983) The effect of fertilizers use on groundwater quality in the phreatic zone with special reference to Uttar Pradesh. In: Proceedings of the seminar on assessment development and management of groundwater research. Central Groundwater Board, New Delhi
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water. Scientific Publishers, Jodhpur
Kumar P, Kumar M, Ramanathan AL, Tsujimura M (2010) Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: a source identification perspective. Environ Geochem Health 32:129–146
Lave J, Avouac JP (2000) Active fold of fluvial terraces across the Siwaliks Hills, Himalayas of central Nepal. J Geophys Res 105(B3):5735–5770
Mandal BK, Roy Choudhury T, Samanta G, Basu GK, Chowdhury PP, Chandra CR, Lodh D, Karan NK, Dhar RK, Tamili DK, Das D, Saha KC, Chakroborti D (1996) Arsenic in groundwater in seven districts of West Bengal, India—the biggest arsenic calamity in the world. Curr Sci 70:976–986
Manning BA, Goldberg S (1997) Adsorption and stability of arsenic(III) at the clay-mineral water interface. Environ Sci Technol 31:200–201
Marimon MPC, Knöller K, Roisenberg A (2007) Anomalous fluoride concentration in groundwater – is it natural or pollution? A stable isotope approach. Isotopes Environ Health Stud 43(2):165–175
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
McArthur JM, Ravencroft P, Safiullah S, Thirlwall MF (2001) Arsenic in groundwater: testing pollution mechanism for sedimentary aquifers in Bangladesh. Water Resour Res 37:109–117
Meybeck M (1987) Global chemical weathering of surficial soils estimated from river dissolved loads. Am J Sci 287:401–428
Mukherjee A, von Bromssen M, Scanlon BR, Bhattacharya P, Fryar AE, Hasan Md A, Ahmed KM, Chatterjee D, Jacks G, Sracek O (2008) Hydrogeochemical comparison and effects of overlapping redox zones on groundwater arsenic near the Western (Bhagirathi sub-basin, India) and Eastern (Meghna sub-basin, Bangladesh) margins of the Bengal Basin. J Contam Hydrol 99:31–48
N’egrel P, Pauwels H (2004) Interaction between different groundwaters in Brittany catchments (France): characterizing multiple sources through strontium and sulphur isotope tracing. Water Air Soil Pollut 151:261–285
Nath B, Stüben D, Basu Mallik S, Chatterjee D, Charlet L (2008) Mobility of arsenic in West Bengal aquifers conducting low and high groundwater arsenic. Part I: Comparative hydrochemical and hydrogeological characteristics. Appl Geochem 23:977–995
Nickson RT, McArthur J, Ravenscroft P, Burgess W, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Nordstrom DK (2002) Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Ritzi RW, Wright SL, Mann B, Chen M (1993) Analysis of temporal variability in hydrogeochemical data used for multivariate analyses. Ground Water 31:221–229
Rogers RJ (1989) Geochemical comparison of groundwater in areas of New England, New York and Pennsylvania. Ground Water 27:690–712
Saha D (1999) Hydrogeological framework and groundwater resources of Bihar. Central Ground Water Board, Patna
Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Curr Sci 97(6):753–755
Saha D, Sarangam SS, Dwivedi SN, Bhartariya KG (2010) Evaluation of hydrogeochemical processes in arsenic-contaminated alluvial aquifers in parts of Mid-Ganga Basin, Bihar, Eastern India. Environ Earth Sci 61(4):799–811
Shah BA (2008) Role of quaternary stratigraphy on arsenic contaminated groundwater from parts of middle Ganga Plain, UP–Bihar, India. Environ Geol 35:1553–1561
Sharma SK, Subramanian V (2008) Hydrochemistry of the Narmada and Tapti Rivers, India. Hydrol Process 22:3444–3455
Singh IB (2001) Proxy records of neotectonics, climate changes and anthropogenic activity in the late Quaternary of Ganga plain. In: Proceedings of the national symposium on role of earth sciences in integrated development and societal issues, 2–4 November 2001, Lucknow, India. Special Publication 65, vol 1. Geological Survey India, Calcutta
Sinha R, Tandon SK, Gibling MR, Bhattacharjee PS, Dasgupta AS (2005) Late Quaternary geology and alluvial stratigraphy of the Ganga basin. Himal Geol 26(1):223–240
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smedley PL, Zhang M, Zhang G, Luo Z (2003) Mobilisation of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl Geochem 18:1453–1479
Stallard RF, Edmond JM (1987) Geochemistry of the amazon: weathering chemistry and limits to dissolved inputs. J Geophys Res 92:8293–8302
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York
Subba Rao N (2007) Groundwater quality as a factor for identification of recharge zones. Environ Geosci 14:79–90
Subramanian V (1984) River transport of phosphorous and genesis of ancient phosphorites. Geol Survey India (Special Publication) 17:11–15
Thornton I (1996) Sources and pathways of arsenic in the geochemical environment: health implications. Geol Soc Lond (Special Publications) 113:153–160
Todd DK (1980) Groundwater hydrology. Wiley, New York
WHO (2004) Guidelines for drinking-water quality, vol 1, 3rd edn. World Health Organization, Geneva
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Zheng Y, Stute M, van Geen A, Gavriela I, Dhar R, Simpson H, Schlosser P, Ahmed KM (2004) Redox control of arsenic mobilization in Bangladesh groundwater. Appl Geochem 19:201–214
Acknowledgements
The University Grant Commission (UGC) is gratefully acknowledged for awarding research fellowship (to the first author) which has been helpful for carrying out the present study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Capital Publishing Company
About this chapter
Cite this chapter
Sharma, S.K., Ramanathan, A., Subramanian, V. (2015). Chemical Characteristics of Arsenic Contaminated Groundwater in Parts of Middle-Gangetic Plain (MGP) in Bihar, India. In: Ramanathan, A., Johnston, S., Mukherjee, A., Nath, B. (eds) Safe and Sustainable Use of Arsenic-Contaminated Aquifers in the Gangetic Plain. Springer, Cham. https://doi.org/10.1007/978-3-319-16124-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-16124-2_10
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16123-5
Online ISBN: 978-3-319-16124-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)