Abstract
This chapter describes the use of the porcine ex vivo intestinal segment model. This includes the advantages and disadvantages of the segment model and a detailed description of the isolation and culture as well as the applications of the porcine ex vivo intestinal segment model in practice. Compared to the Ussing chamber (Chap. 24) the porcine ex vivo small intestinal segment model is a relatively simple to use intestinal tissue model. The main difference being that the tissue segment is not mounted in a chamber, but is freely floating in a solution. Therefore the ex vivo intestinal segment model does not distinguish between the apical and basolateral side of the tissue. The intestinal segments can be obtained from various anatomical regions of the small intestine (e.g. duodenum, jejunum, ileum or even the colon) and the segments consist of various cell types (e.g. epithelial cells, paneth cells, goblet cells, enterochromaffin cells and enteroendocrine cells). The intestinal segment model has been shown to be a suitable tool to study compound and location specific effects on the release of gastrointestinal hormones and gastrointestinal metabolism of endocannabinoids and related compounds.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 The Porcine Ex Vivo Intestinal Segment Model
Although it is possible to obtain human intestinal material for example by taking biopsies using endoscopic techniques, the availability of human tissue is often a bottleneck. Despite human biopsies from the duodenum can be obtained, biopsies from the more distal parts of the small intestine, such as the ileum, are difficult to obtain. This makes it difficult to study region specific effects. The use of tissue segments from other species (e.g. rat, mice, dogs and pigs) can provide a solution for these limitations. It is possible to obtain intestinal tissue segments from various regions of the intestine e.g. duodenum, jejunum, ileum or even the colon. Since the pig intestine shows a high degree of macroscopic and microscopic resemblance with that of humans the best alternative for human tissue is pig (Clouard et al. 2012; Miller and Ullrey 1987; Patterson et al. 2008). Despite the difference in dietary quantity between the two species, pigs and humans have the same dietary habits. Furthermore they have comparable organ sizes, and especially of importance are the similarities in the anatomy, morphology and physiology of the gastrointestinal tract (Clouard et al. 2012; Miller and Ullrey 1987; Patterson et al. 2008). The model was therefore optimized using pig tissue. The porcine ex vivo intestinal segment model will be further referred to as intestinal segment model.
The development of the intestinal segment model was necessary to obtain a more high throughput screening system compared to in vivo studies and the Ussing chamber technique. Moreover a model resembling the human in vivo situation more than single cell cultures was needed to study processes in the gastrointestinal tract, such as nutrient transport and/or hormone release. Currently different cell lines are used for this purpose; Caco-2 and HT-29 cell lines for nutrient uptake and enteroendocrine cells such as murine STC-1 cells (Abello et al. 1994; Geraedts et al. 2009; McLaughlin et al. 1998), GLUTag cells (Reimer et al. 2001) and the human NCI-H716 cell line (Brubaker et al. 1998) to study satiety hormone release. These cell lines are already discussed extensively in the previous chapters. Since intestinal segments consist of many different cell types (e.g. epithelial cells, paneth cells, goblet cells, enterochromaffin cells and enteroendocrine cells), it can be envisioned that this model can be used to study many different biological processes (e.g. nutrient uptake, immune response, hormone release). However up till now only a few applications were found and the model has to compete with a more established model, the Ussing chamber. The main difference of this model compared to the Ussing chamber is that the tissue segment is not mounted in a chamber and thus not distinguishes between the apical and basolateral side of the tissue.
Up till now the model has been used predominantly to study gastrointestinal hormone release due to its short viability (150 min). In this chapter we will therefore focus on gastrointestinal hormone release following interaction with nutrients or related compounds. The presence of nutrients and other molecules in the small intestine could be sensed and consequently stimulate the release of hormones such as Glucagon Like Peptide-1 (GLP-1), Cholecystokinin (CCK) and Peptide YY (PYY). These hormones are released following interaction of nutrients and other compounds with G-protein coupled receptors and solute carrier transporters located on enteroendocrine cells. CCK, GLP-1 and PYY are involved in generating satiety and satiation (Gribble 2012; Tolhurst et al. 2012). Enteroendocrine cells are scattered throughout the epithelial layer of the gastrointestinal tract (Tolhurst et al. 2012). Enteroendocrine I-cells mainly release CCK and are relatively abundant in the duodenum, whereas GLP-1 and PYY are released by L-cells mainly present in the distal jejunum, ileum and colon (Holst 2007; Iwasaki and Yada 2012). Satiety hormone responses depend on the type of nutrient and anatomical region of the small intestine. Nutrient type and anatomical region can both be varied when the intestinal segment model is being used.
2 General Protocol
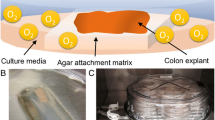
Small intestinal tissue can be obtained from sacrificed pigs. The reproducibility of the results will improve when intestine is used from pigs with comparable health status, age and diet. This is certainly true for satiety studies, however the exact effect of varying health status, age and diet on the physiology of the intestinal segment system is never studied. Furthermore, it is important to collect the intestine as quick as possible; within 5 min after sacrificing the animal. During the procedure the tissue has to be kept on ice. Various anatomical regions of the small intestine can be collected, including duodenum (starting 10 cm distal of the pylorus, total length ± 0.5 m), proximal, mid and distal jejunum (1.5, 5 and 10 m distal of the pylorus, respectively), proximal, mid and distal ileum (1.5, 1 and 0.5 m proximal from the ileal cecal valve), and colon. The intestine is stored in ice cold Krebs-Ringer Bicarbonate (KRB) buffer, which is bubbled with a O2/CO2 (95 %/5 %) gas mixture to prevent ischemia, and transported as quickly as possible to the laboratory. Upon arrival the intestinal tissue is carefully flushed and rinsed with ice cold KRB buffer, to remove luminal debris and put in a beaker with KRB on ice. The intestine is cut open in a longitudinal direction and the outer muscle layers are carefully stripped off with the basolateral side upwards. The mucosal tissue has to be placed on gauze (pores = 250 μm, Sefar Nitex 03-250/50, Sefar Heiden Switzerland) with the apical side upward, then circles of tissue (tissue segments) can be punched out (payers patches are excluded) using a biopsy punch with a diameter of 8 mm (Miltex, York, USA) (Fig. 23.1b). Tissue segment size may be varied depending on research question and amount of tissue required for analysis. After collecting all segments in the 24-wells plate filled with 500 μl ice cold KRB buffer/well (Fig. 23.1c, d), the tissue is brought to room temperature in 30 min, followed by a pre-incubation at 37 °C for 1 h in a humidified incubator at 5 % v/v CO2. To study the effects of nutrients on intestinal hormone release, incubations can be initiated by replacing the KRB buffer with 500 μl pre-warmed solution without d-glucose (0 mM) containing the test compounds and incubate for 1 h at 37 °C at 5 % v/v CO2. Add phenylmethanesulfonyl fluoride (PMSF) (final concentration 100 μM) to the collected media to inactivate serine protease activity and to avoid cleavage of active GLP-1 (7-36 and 7-37) by dipeptidyl peptidase IV (DPP-IV). The collected media as well as the tissue can be stored at 4 °C, or −80 °C until further analysis. For lactate dehydrogenase (LDH) analysis, store media samples at 4 °C for maximal 48 h, for GLP-1, PYY and CCK at −80 °C. Tissue sample has to be snap frozen immediately after the incubation and stored at 80 °C until further analysis (e.g. mRNA, or fatty acid and metabolites thereof (endocannabinoids (ECs), n-acyl ethanolamines (NAEs) or n-acyl serotonins (NAs)).
3 Sample Analysis
Satiety hormones can be analyzed using commercially available ELISA kits according to the manufacturer’s instructions. Porcine GLP-1 can be measured using the human GLP-1 kit from Millipore (EGLP-35K, Billerica, MA, USA), since the GLP-1 hormone gene sequence is highly preserved. GLP-2 can be determined using a competitive human GLP-2 (1-34) ELISA kit from Phoenix Pharmaceuticals Inc. (Belmont, CA, USA) according to the manufacturer’s instructions. There is a 92 % homology of GLP-2 protein between humans and pigs. The cross reactivity of this kit with GLP-1 is 6 %.
PYY release can be measured with a commercial available ELISA kit for total PYY (Bachem, Peninsula Laboratories, San Carlos, CA, USA). This kit measures porcine PYY, which is identical to human PYY (Adrian et al. 1987, 11).
CCK concentrations can be determined using a human EURIA-CCK radioimmunoassay (RIA) kit (Euro-diagnostica, Malmö, Sweden) according to the manufacturer’s instructions. An identical sequence of CCK-8 has been found form most mammals, among them pigs and human. The kit measures CCK-8 sulfate (CCK 26-33). Tissue concentrations of ECs (AEA and 2-AG) and NAEs (DHEA, EPEA, DLE, OEA, PEA and SEA) can be analyzed according to a method described previously (Balvers et al. 2012). For this analysis 50–100 mg freeze-dried tissue is required to be within the detection limit of LC–MS method. Although the paper describes the extraction of ileal mouse tissue, this method can also be used for porcine pig tissue (unpublished data). Tissue concentrations of NAEs can be analyzed according to the method described previously (Verhoeckx et al. 2011). For this method 50–300 mg intestinal tissue is required.
4 Monitoring Viability
The viability of the tissue segments is important to analyze, especially when secretagogue effects are studied. It has to be avoided that the release of satiety hormones is secondary to cell lysis or other nonspecific toxic effects. To analyze tissue viability macroscopic and microscopic tissue checks could be done after staining with markers for proliferation and apoptosis (e.g. hematoxylin–eosin staining). Another method to check the viability is measuring leakage of intracellular lactate dehydrogenase (LDH). LDH is stable enzyme common in all cells which can be readily detected when cell membranes are no longer intact for instance due to active proteases or mechanical forces during the preparation of the biopsies. The enzyme activity is determined with respect to the total intracellular lactate dehydrogenase. The total LDH activity of the tissue can be determined by homogenize the tissue in ice cold KRB buffer, using a Potter Elvehjem-type teflon pestle tissue grinder (Braun, Melsungen Germany) for 5 min at 200 rpm. It is also possible to use Triton-x as a positive control. Triton-x dissolves membranes and makes the tissue leaky. Samples are excluded when the LDH leakage is more than 10 % of the total intracellular LDH. The incubation time of the segments should not exceed 1 h, since at longer incubation times, LDH leakage will be >10 % (Voortman et al. 2012).
5 Incubation with Food Components and Digested Food Samples
The intestinal segment model can be used to study the effect of digested and un-digested food components (e.g. sugars, fatty acids and proteins) as long as the components are dissolved and diluted with KRB buffer (Voortman et al. 2012). When diluting compounds in the KRB buffer, care should be taken that the levels of alcohol and DMSO are below 1 %. It has been shown that the use of undiluted digested food samples from the TIM model (including bile, pancreatic enzymes and/or food components) can be applied onto the apical side of the tissue segments if mounted in a chamber (Westerhout et al. 2014). However, the use of undiluted digested food samples is not yet tested for the intestinal segment system. In this system tissue is exposed on both the apical and the basolateral side and therefore the use of digestion fluid has to be tested first.
6 Readout of the Porcine Ex Vivo Intestinal Segment Model
The intestinal segment model is used to study the effects of fatty acids, carbohydrates and proteins on the release of gastrointestinal hormones, such as GLP-1, PYY and CCK (Table 23.1). Hormone concentrations can be expressed as both the relative release compared to basal release after the negative control set to 100 % (KRB buffer without d-glucose), or as the absolute hormone release (pmol/l) per surface area (cm2). The model is also used to study the tissue concentrations of both ECs and NAEs and NAs. The concentration can be expressed per gram tissue, or as relative concentration compared to the negative control set to 100 % (KRB buffer without d-glucose).
The model can also be used to study the uptake of food compounds after labelling these compounds radioactively. The radioactivity in the tissue and in the media can be analyzed using liquid scintillation counting. However since this model does not distinguish between the apical and basolateral side, it is not possible to study transport of molecules from the apical side to the basolateral side with this model. To study this transport, the Ussing technology, or Caco-2 cells might be better systems.
7 General Advantages and Disadvantages of the Model
The advantages and disadvantages of the model are summarized in Table 23.2. The advantage of the current intestinal segment model compared to cell cultures is that this intestinal segment model consist of multiple cell types (e.g. epithelial cells, enterochromaffin cells, paneth cells, goblet cells and enteroendocrine cells) instead of one cell type only. This makes it possible to study effects which depend on cell–cell communication. Another advantage of this intestinal segment model is that it can be used to study region specific effects (duodenum, jejunum, ileum, etc.), since the anatomical region of the small intestine is an important parameter to study compound specific effects on satiety hormone release. Furthermore the intestinal segment model has a higher throughput since it is a simplified version of the Ussing chamber model. Since it is not required to mount the tissue of the intestinal segment model in a Ussing chamber, the practical skills required for the intestinal segment model are relatively simple compared to the Ussing chamber technology.
An important aspect which might be different from other systems such as organoids and Caco-2 cells is the presence of microorganisms in the intestinal segment model (Roeselers et al. 2013). Although the presence of microorganisms in the intestinal segment system was never tested, it is likely that the natural flora of the host is still present since the intestine is directly isolated from the animal. The influence of microorganisms on nutrient specific effects is still not known and has to be investigated.
One of the main disadvantages of this model is the biological variation in the release of satiety hormones per intestinal segment. The enteroendocrine cells are not homogenously distributed over the tissue and account for only 1 % of the total epithelial cells. Therefore the number of enteroendocrine cells per surface area (cm2) tissue may vary. For this reason the amount of replicates and the use of different pigs are important when using this model. The number of intestinal replicates can be determined using a power calculation, which depend on the variation of the measured parameter within the population. To study the effects of a specific compound the statistical method should test the effect within the animal (compared to the KRB buffer). The inter-tissue coefficient of variation (CV) for GLP-1 release is 33 ± 7 %, based on the average CV of incubations in triplicate from 10 pigs. The intra-animal CV for basal GLP-1 release is 78 % based on the basal GLP-1 release (after KRB buffer) from 10 pigs. For this reason the effects should be studied within the animal, and not between animals.
Another issue is the tissue source. It is recommended to use tissue from healthy animals who were kept on a controlled diet. The controlled diet is especially important when the effects of nutrients or digested compounds have to be studied. However the effects of various diets, age, weight or health status on the release on satiety hormones using the intestinal segment model are never studied.
References
Abello J, Ye F, Bosshard A, Bernard C, Cuber JC, Chayvialle JA (1994) Stimulation of glucagon-like peptide-1 secretion by muscarinic agonist in a murine intestinal endocrine cell line. Endocrinology 134:2011–2017
Adrian TE, Bacarese-Hamilton AJ, Smith HA, Chohan P, Manolas KJ, Bloom SR (1987) Distribution and postprandial release of porcine peptide YY. J Endocrinol 113:11–14
Balvers MG, Verhoeckx KC, Bijlsma S et al (2012) Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics 8:1130–1147
Batterham RL, Cowley MA, Small CJ et al (2002) Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650–654
Brubaker PL, Schloos J, Drucker DJ (1998) Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 139:4108–4114
Clouard C, Meunier-Salauen MC, Val-Laillet D (2012) Food preferences and aversions in human health and nutrition: how can pigs help the biomedical research? Animal 6:118–136
Geraedts MC, Troost FJ, Saris WH (2009) Peptide-YY is released by the intestinal cell line STC-1. J Food Sci 74:H79–H82
Gribble FM (2012) The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc Nutr Soc 71:456–462
Habib AM, Richards P, Cairns LS et al (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439
Iwasaki Y, Yada T (2012) Vagal afferents sense meal-associated gastrointestinal and pancreatic hormones: mechanism and physiological role. Neuropeptides 46:291–297
McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G (1998) Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol 513(Pt 1):11–18
Miller ER, Ullrey DE (1987) The pig as a model for human nutrition. Annu Rev Nutr 7:361–382
Patterson JK, Lei XG, Miller DD (2008) The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med (Maywood) 233:651–664
Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K (2001) A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 142:4522–4528
Ripken D, van der Wielen N, Wortelboer H, Meijerink J, Witkamp R, Hendriks H (2014) Stevia glycoside rebaudioside A induces GLP-1 and PYY release in a porcine ex vivo intestinal model. J Agric Food Chem 62:8365–8370. doi:10.1021/jf501105w
Roeselers G, Ponomarenko M, Lukovac S, Wortelboer HM (2013) Ex vivo systems to study host–microbiota interactions in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 27:101–113
Tolhurst G, Reimann F, Gribble FM (2012) Intestinal sensing of nutrients. Handb Exp Pharmacol (209):309–335
Verhoeckx KC, Voortman T, Balvers MG, Hendriks HF, Wortelboer HM, Witkamp RF (2011) Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract. Biochim Biophys Acta 1811:578–586
Voortman T, Hendriks HF, Witkamp RF, Wortelboer HM (2012) Effects of long- and short-chain fatty acids on the release of gastrointestinal hormones using an ex vivo porcine intestinal tissue model. J Agric Food Chem 60:9035–9042
Westerhout J, van de Steeg E, Grossouw D et al (2014) A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur J Pharm Sci 63:167–177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Ripken, D., Hendriks, H.F.J. (2015). Porcine Ex Vivo Intestinal Segment Model. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_23
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)