Abstract
A wealth of evidence indicates that plasma levels of high-density lipoprotein cholesterol (HDL-C) are inversely related to the risk of cardiovascular disease (CVD). Consequently, HDL-C has been considered a target for therapy in order to reduce the residual CVD burden that remains significant, even after application of current state-of-the-art medical interventions. In recent years, however, a number of clinical trials of therapeutic strategies that increase HDL-C levels failed to show the anticipated beneficial effect on CVD outcomes. As a result, attention has begun to shift toward strategies to improve HDL functionality, rather than levels of HDL-C per se. ApoA-I, the major protein component of HDL, is considered to play an important role in many of the antiatherogenic functions of HDL, most notably reverse cholesterol transport (RCT), and several therapies have been developed to mimic apoA-I function, including administration of apoA-I, mutated variants of apoA-I, and apoA-I mimetic peptides. Based on the potential anti-inflammatory effects, apoA-I mimetics hold promise not only as anti-atherosclerotic therapy but also in other therapeutic areas.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The beneficial consequences of lowering low-density lipoprotein cholesterol (LDL-C) on cardiovascular (CVD) events have been unequivocally shown in numerous intervention studies. A plasma LDL-C reduction of 1.0 mmol/L has been shown to reduce the risk of major cardiovascular events by approximately 20 %, irrespective of baseline cholesterol or risk. Intensive LDL-C lowering is therefore advocated in most guidelines (Baigent et al. 2010). However, despite the efficiency of established therapies, the residual burden of disease remains substantial (Roger et al. 2012). Novel targets for therapy are therefore eagerly awaited in order to decrease the residual CVD risk.
A large number of epidemiological studies have shown that levels of high-density lipoprotein cholesterol (HDL-C) are inversely associated with CVD risk. In fact, it has been calculated from these studies that a 1 mg/dL (0.03 mmol/L) increase in HDL-C would translate into a 2–3 % reduction of risk for subsequent coronary events (Gordon et al. 1989). Moreover, levels of HDL-C have been considered a stronger predictive factor of incident coronary heart disease than levels of LDL-C (Gordon et al. 1977). Even among patients who attain low levels of LDL-C while receiving LDL-C lowering therapy, levels of HDL-C remain predictive for subsequent CVD events (Barter et al. 2007; Jafri et al. 2010).
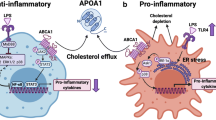
These epidemiological data do not infer a causal relation between levels of HDL-C and CVD risk. However, a number of antiatherogenic mechanisms have been ascribed to HDL. HDL has been shown to play a pivotal role in reverse cholesterol transport (RCT), a pathway by which cholesterol is transported from peripheral cells (e.g., macrophages within the vessel wall) to the liver for biliary excretion (Fielding and Fielding 1995). In addition, HDL has been shown to possess antioxidant, anti-inflammatory (Barter et al. 2004), antithrombotic (Mineo et al. 2006), and antiapoptotic properties (Suc et al. 1997).
HDL has been considered a target for therapy to lower CVD risk, based on both these epidemiological and biological arguments of atheroprotection. In recent years, a number of large clinical trial programs investigating the efficacy of drugs with an established effect on HDL-C levels have been terminated because of the inability to induce improvement in clinical outcomes. In addition, treatment-induced changes in HDL-C were not associated with CVD risk after adjusting for LDL-C (Briel et al. 2009). Moreover, a number of common genetic variants in genes coding for proteins involved in HDL metabolism have been shown to alter levels of HDL-C without showing the anticipated effect on CVD risk (Haase et al. 2011; Voight et al. 2012). These findings challenge the concept that levels of circulating HDL-C are causally related to atherosclerosis, and assessment of HDL functionality has therefore been proposed to better reflect the therapeutic potential of therapies targeting HDL (deGoma et al. 2008).
Although levels of HDL-C alone may thus be a poor target for therapies, apolipoprotein A-1 (apoA-I) itself could in fact represent a promising target. ApoA-I is the major protein component of the HDL particle and considered to play a pivotal role in many of the antiatherogenic properties attributed to HDL. The role of apoA-I in the protection against atherosclerosis has been shown in a number of rodent studies. In low-density lipoprotein receptor (LDL-R) null mice, apoA-I deficiency was shown to result in increased atherosclerosis (Moore et al. 2003). Increased atherosclerotic lesion development was also seen in apoA-I knockout mice expressing human apolipoprotein B (apoB) when fed a Western diet (WD) (Voyiaziakis et al. 1998). Additional proof of the antiatherogenic role of apoA-I was derived from animal models of human apoA-I overexpression and in mice treated with apoA-I infusions, in which it was consistently shown that apoA-I provides protection against atherosclerotic lesion formation in proatherogenic animal models (Duverger et al. 1996; Miyazaki et al. 1995; Rubin et al. 1991). Moreover, mutations in the gene coding for apoA-I have been shown to alter CVD risk in humans. Most of the carriers of mutations in the apoA-I gene are characterized by low levels of HDL-C and an increased risk of CVD (Hovingh et al. 2004). ApoA-I is widely accepted as an attractive target for therapy based on the consistent data derived in animal and human studies. Several therapies have been developed that mimic apoA-I function, including administration of full length apoA-I, mutated variants of apoA-I, and apoA-I mimetic peptides.
2 ApoA-I Mimetic Peptides
ApoA-I comprises a total of 243 amino acids and its secondary structure resembles 10 amphipathic α-helices that are crucial for its efficient interaction with lipids. Recently, there has been increasing interest in the application of peptides that resemble the amphipathic helices in apoA-I as therapeutic agents.
Anantharamaiah et al. synthesized the first apoA-I mimetic peptide, 18A, comprising 18 amino acids. Subsequently, several modifications to 18A have been made to create peptides that more closely mimic apoA-I in its antiatherogenic functions. For example, blocking the ends of 18A with an amide group and an acetyl group, thereby creating a peptide called 2 F, increased its helicity and efficiency in inducing cholesterol efflux (Venkatachalapathi et al. 1993; Yancey et al. 1995). In addition, tandem peptides composed of more than one amphipathic helix were shown to have a superior lipid affinity and ability to induce cholesterol efflux from macrophages compared to peptides that contain only one helix (Anantharamaiah et al. 1985; Garber et al. 1992; Wool et al. 2008). D’Souza et al. investigated the effect on cholesterol efflux and anti-inflammatory and antioxidant properties of 22 different bihelical apoA-I mimetic peptides. None of the compounds was superior in all antiatherogenic functions, and each of the examined antiatherogenic functions was shown to be primarily affected by specific structural features (D’Souza et al. 2010). These results indicate that apoA-I mimetic peptides more closely resembling apoA-I do not necessarily improve all various anti-atherosclerotic functions. Moreover, combining several apoA-I mimetic peptides, each mimicking different structural aspects of apoA-I, may prove to be a valuable strategy to mimic the various anti-atherosclerotic properties of apoA-I.
2.1 4F
Despite potent anti-inflammatory and antioxidant effects in vitro and in experimental models, the promise of 4 F in humans has not been a success to date (Navab et al. 2002, 2004). Two trials of 4 F in patients with CHD or at high risk of CVD assessed the effect of orally and parenterally administered 4 F on the HDL inflammatory index, a measure of HDL-mediated protection against LDL-induced monocyte chemotaxis, providing conflicting results (Bloedon et al. 2008; Watson et al. 2011). The development of 4 F by Novartis has subsequently been discontinued. Although a number of differences between the studies may partially account for the observed discrepancy, the primary outcome measure “HDL inflammatory index” has been proven to be largely irreproducible in the majority of laboratories. In fact, it has been difficult to identify an efficacy parameter for apoA-I mimetic peptides in human trials altogether, and in lack of standardization, all trials using HDL quality as readout have been disappointing.
2.2 6F
A disadvantage of many apoA-I mimetic peptides is that they require end blocking to be effective, which precludes its synthesis by living organisms. Large-scale production of the peptides for therapeutic use would therefore be too expensive. In a search for apoA-I mimetic peptides that do not require end blocking, 6 F yielded promising results.
L-6 F has been shown to possess antioxidant and anti-inflammatory properties in several mice models (Chattopadhyay et al. 2013; Navab et al. 2013). Given that the small intestine may be an important site of action for apoA-I mimetic peptides, the observed reduction in plasma levels of the proinflammatory and proatherogenic lipid lysophosphatidic acid (LPA) following administration of L-6 F is of particular interest. Feeding LDL-R knockout mice a Western diet does result in changes in intestinal gene expression and proinflammatory and hypercholesterolemic changes in serum. Similar changes were observed when mice were fed unsaturated LPA. Administration of L-6 F reduced intestinal LPA levels and prevented the Western diet-mediated proatherogenic changes in intestinal gene expression. In addition, when L-6 F was administered to mice on chow supplemented with LPA, intestinal levels of unsaturated LPA were significantly reduced, and this decrease correlated with reduced systemic inflammation and hypercholesterolemia (Chattopadhyay et al. 2013; Navab et al. 2013). Thus, reducing levels of intestinal LPA may be an important mechanism of action for L-6 F.
To enable the large-scale production of the peptide, transgenic, 6 F expressing tomatoes have been developed. Feeding LDL-R knockout mice a Western diet with and without transgenic tomatoes expressing 6 F improved an array of biomarkers of inflammation and antioxidant status and significantly reduced aortic lesion area compared to controls following 13 weeks of treatment (Chattopadhyay et al. 2013).
2.3 FX-5A
The peptide 5A is synthesized by replacing 5 amino acids of the apoA-I mimetic peptide 37pA with alanine residues, thereby decreasing its lipid affinity. Although 37pA effectively induces cholesterol efflux, its high lipid affinity is associated with cytotoxicity because of adverse effects on the integrity of the plasma membrane (Remaley et al. 2003). As a result of the amino acid substitutions, 5A is less cytotoxic and induces cholesterol efflux more specifically through ATP-binding cassette transporter A1 (ABCA1) (Sethi et al. 2008).
The 5A-phospholipid complex has been shown to induce cholesterol efflux both through ABCA1 and ATP-binding cassette transporter G1 (ABCG1). Administration of a single intravenous dose of a 5A-phospholipid complex was shown to enhance reverse cholesterol transport, as evidenced by a flux of cholesterol from peripheral cells to plasma, an increased amount of cholesterol and phospholipids in the HDL fraction, and an enhanced fecal sterol and bile acid secretion (Amar et al. 2010). In addition, 5A has been shown to possess anti-inflammatory and antioxidant properties (Tabet et al. 2010). To mimic acute inflammation, a nonocclusive collar was placed around the carotid artery in rabbits following infusion with 5A-phospholipid. Treatment with 5A-phospholipid significantly reduced the expression of proinflammatory endothelial adhesion molecules and neutrophil infiltration compared to placebo, and this effect of 5A infusion was similar to the effect observed after infusing lipid-free apoA-I or recombinant HDL. In the same rabbit model, it was shown that 5A-phospholipid reduced the production of radical oxygen species (ROS), such as O2 − (Tabet et al. 2010).
The ability of 5A-phospholipid to inhibit the formation of atherosclerosis in vivo has been demonstrated in apolipoprotein E (apoE) knockout mice. Treatment with 30 mg/kg of intravenous 5A-phospholipid three times per week for 13 weeks resulted in 30 % reduction in aortic plaque area in both young apoE knockout mice and older apoE knockout mice with established lesions. The efficiency of the complex to inhibit plaque formation was shown to be dependent on the constitution of the phospholipid component. Reconstituting 5A with a sphingomyelin-containing combination of phospholipids enhanced its anti-atherosclerotic properties. When apoE knockout mice were treated with this complex using the same dosing regimen as used in the previously described experiments, there was a superior 54 % reduction in aortic lesions compared to controls.
In addition to its potent anti-atherosclerotic effects, 5A has been shown to reduce airway inflammation in murine models of asthma (Yao et al. 2011). Thus, the therapeutic potential of 5A extends beyond atherosclerotic cardiovascular disease.
As of yet, 5A has not been tested in humans. However, the first clinical trial is planned in the near future.
2.4 ATI-5261
Bielicki et al. developed ATI-5261, a peptide consisting of 36 amino acids forming a single amphipathic helix with high aqueous solubility that induced ABCA1-mediated cholesterol efflux with similar efficiency as apoA-I (Bielicki et al. 2010). LDL-R knockout mice received a high-fat Western diet for 13 weeks while concomitantly receiving daily intraperitoneal injections of ATI-5261 during the last 6 weeks. In another model, ApoE knockout mice received a Western diet for 18 weeks, followed by a chow diet with concomitant intraperitoneal injections of ATI-5261 every other day for another 6 weeks. The aortic lesion area and plaque lipid content were significantly reduced in mice treated ATI-5261 compared to mice receiving placebo. The decrease in atherosclerosis was accompanied by increased fecal sterol excretion, which is indicative of increased RCT upon ATI-5261 treatment.
2.5 ETC-642
ETC-642 is a complex of a 22-amino acid peptide that forms an amphipathic helix and phospholipids. The complex has been shown to exert multiple favorable effects on LDL and HDL particles (Di Bartolo et al. 2011b). Following 12 weeks of treatment in rabbits, a shift in LDL subfractions toward less negatively charged particles was observed, indicating a reduction in proinflammatory oxidized LDL. Moreover, a reduction in the particularly atherogenic small dense LDL (sdLDL) subfraction was noticed. Shortly after infusion of ETC-642, there was also a shift in HDL subfractions toward the antiatherogenic pre-β fraction. Moreover, ETC-642 was shown to be a potent inducer of cholesterol efflux from human macrophages in in vitro assays. It has also been shown in vivo to increase the cholesterol content in the HDL fraction, which may indicate increased reverse cholesterol transport (Di Bartolo et al. 2011a; Iwata et al. 2011). The anti-inflammatory effects of ETC-642 have been demonstrated in rabbit models of acute and chronic inflammation (Di Bartolo et al. 2011a, b). ETC-642 reduced endothelial adhesion molecule expression both in collared carotid arteries and in the aorta of cholesterol-fed rabbits, and this reduction was similar to effect observed in animals treated with reconstituted HDL. ETC-642 has been shown to induce an anti-inflammatory effect, as HDL isolated from ETC-642-treated rabbits decreased TNF-α-induced expression of NF-kB and endothelial adhesion molecules in human coronary artery endothelial cells (HCAECs) (Di Bartolo et al. 2011b). In addition, ETC-642 inhibited TNF-α-induced monocyte adhesion in HCAECs (Di Bartolo et al. 2011a).
The efficiency of ETC-642 as an anti-atherosclerotic agent has been demonstrated using intravascular ultrasound (IVUS) in hyperlipidemic rabbits that were treated with either low or high dose (15 or 50 mg/kg, respectively) or placebo two times per week for 12 weeks. Treatment with high-dose ETC-642 significantly inhibited plaque formation compared to controls (Iwata et al. 2011).
3 ApoA-I-Based Infusion Therapy
Lipid-poor pre-β HDL is the main acceptor of cholesterol from peripheral cells, including macrophages in the subendothelial vessel wall, by means of the interaction between apoA-I and the ABCA1 (Kontush and Chapman 2006). Largely based on the findings that apoA-I overexpression and apoA-I infusion in animal models do inhibit plaque formation, it is anticipated that infusion of apoA-I-containing particles, such as lipid-poor pre-β HDL, has direct beneficial effects on atherosclerosis.
3.1 HDL-VHDL Infusions
The atheroprotective effect of infusions of apoA-I-containing particles was first shown in the 1980s by Badimon et al. Cholesterol-fed rabbits were treated with 8 weekly infusions of homologous high-density lipoprotein-very high-density lipoprotein (HDL-VHDL) obtained by ultracentrifugation. Following the 8-week treatment period, a significant reduction in the aortic surface area covered by atherosclerotic-like lipid-rich lesions was found in HDL-VHDL-treated animals, compared to controls (37.9 % vs. 14.9 %). The reduced formation of aortic lesions was accompanied by a reduced total lipid deposition in the vascular wall and liver, whereas no significant differences in plasma lipid profiles were noted between both groups (Badimon et al. 1989).
It was subsequently shown that HDL-VHDL infusions did not only inhibit plaque formation, but also reduced the extent of preexisting lesions in a model where rabbits were fed an atherogenic diet for 60 days to induce atherosclerotic lesions (group 1). Two groups were treated with an additional 30 days of cholesterol-rich diet, with or without weekly HDL-VHDL infusions during the last 30 days (groups 2 and 3). Treatment with HDL-VHDL significantly reduced the aortic surface area covered by fatty streaks (17.8 % in treated rabbits vs. 34 and 38.8 % in control groups) as well as aortic lipid accumulation (Badimon et al. 1990).
3.2 Purified ApoA-I Infusions
The initial studies performed by Badimon et al. showed that infusions of HDL-VHDL did result in protection against atherosclerosis. Miyazaki et al. subsequently tested the hypothesis that infusions of purified apoA-I would have similar effects. In their first experiment, rabbits were fed an atherogenic diet for 90 days. During the last 30 days, half of the rabbits received weekly injections of purified apoA-I. Treatment with purified apoA-I resulted in a significant reduction in the proportion of the aortic surface area covered by fatty streaks, to a similar extent to treatment with HDL-VHDL (46.0 % vs. 23.9 %). In a second experiment, Miyazaki et al. studied whether purified apoA-I infusions induced regression of established plaques. Rabbits were fed an atherogenic diet for 105 days. Group 1 was sacrificed on day 105. Groups 2, 3, and 4 were fed a chow diet for an additional 60 days. Group 3 received infusions of purified apoA-I every other day, and group 4 received weekly infusions of a higher dose, while rabbits in group 2 served as untreated controls. The aortic surface area covered by atherosclerotic lesions progressed from day 105 to day 165 despite chow diet (50.0 % vs. 86.2 % for groups 1 and 2, respectively). Compared to the controls in group 2, both groups 3 and 4 showed less aortic surface area covered by atherosclerosis (86.2 % vs. 70.2 % and 65.7 %, respectively). However, apoA-I infusions did not induce plaque regression (Miyazaki et al. 1995).
3.3 ApoA-IMilano Infusions
Carriers of the apoA-IMilano mutation are characterized by reduced levels of HDL-C without the anticipated increased risk of CVD (Franceschini et al. 1980). Compared to native apoA-I, apoA-IMilano has been shown to induce more ABCA1-mediated cholesterol efflux, and, in addition, apoA-IMilano has been shown to exert superior anti-inflammatory and plaque stabilizing properties (Ibanez et al. 2012). Ameli et al. showed that infusion of recombinant apoA-IMilano effectively reduced the formation of intimal lesions in cholesterol-fed rabbits following balloon-induced vascular injury. Twenty rabbits on an atherogenic diet underwent balloon injury of the femoral and iliac arteries. Eight animals received injections with a complex of apoA-IMilano and phospholipids on alternating days during a 10-day period, starting 5 days prior to the balloon injury. Eight other animals received only the phospholipid carrier, and 4 animals served as controls; they did not receive any treatment. Infusions of apoA-IMilano were found to significantly inhibit intimal thickening following balloon injury (0.49 mm2 vs. 1.14 mm2 and 1.69 mm2) and to reduce intimal macrophage content. This effect was present while plasma cholesterol levels remained similar among the groups (Ameli et al. 1994). Subsequent animal studies have confirmed the ability of infusion of recombinant apoA-IMilano to inhibit plaque progression and reduce plaque area of established lesions in models of arterial injury and in apoE-deficient mice (Chiesa et al. 2002; Shah et al. 1998, 2001; Soma et al. 1995). A reduction in plaque lipid and macrophage content has been demonstrated after only 48 h of a single infusion (Shah et al. 2001).
In the “ApoA-I Milano Trial,” 57 patients with an acute coronary syndrome (ACS) were randomized to 5 weekly infusions of either a high (45 mg/kg) or a low dose (15 mg/kg) of a complex of recombinant apoA-IMilano with phospholipid carriers (ETC-216) or placebo, and the treatment began within 2 weeks after ACS. Percentage and total atheroma volume and plaque thickness were significantly reduced in patients treated with apoA-IMilano compared to baseline as assessed by IVUS. The infusions with ETC-216 were generally well tolerated. The small pilot study lacked power to detect dose effects and differences between the treatment and placebo groups (Nissen et al. 2003). Additional analysis revealed that treatment with apoA-IMilano had a beneficial effect on arterial wall remodeling (Nicholls et al. 2006). In conclusion, regression of atherosclerosis was demonstrated following short-term infusions, and the results of larger studies with longer follow-up and clinically relevant end points are eagerly awaited.
3.4 CSL-111 and CSL-112 Infusions
Two subsequent studies investigated the safety and efficacy of infusion of reconstituted HDL (rHDL), consisting of native apoA-I and phospholipids, on coronary atherosclerosis. CSL-111 is composed of human apoA-I and soybean phosphatidylcholine and thereby resembles native HDL. In the “Effect of rHDL on Atherosclerosis-Safety and Efficacy” (ERASE) trial, a total of 183 patients was randomized to 4 weekly infusions of either 40 or 80 mg/kg of CSL-111 or placebo, and this treatment was started within 2 weeks after an ACS. An IVUS and a quantitative coronary angiography were obtained prior to randomization and 2 weeks after the last infusion. Administration of high-dose CSL-111 was associated with a high incidence of transaminase elevations, which led to early discontinuation of the 80 mg/kg study arm. Treatment with 4 weekly infusions of 40 mg/kg of CSL-111 significantly reduced atheroma volume compared to baseline, to a similar extent as seen in the ApoA-I Milano Trial. However, the differences in atheroma volume between the study groups did not reach statistical significance. Nonetheless, the authors conclude that CSL-111 may exert a favorable treatment effect, as treatment improved plaque characterization index on IVUS and coronary score on quantitative angiography (Tardif et al. 2007).
Further development of CSL-111 was discontinued after the observation that the therapy induced a high incidence of liver function abnormalities, possibly related to the high cholate content of the CSL-111 particle.
CSL-112, a second-generation compound with no liver toxicity (Krause and Remaley 2013), has been tested in two phase 1 studies comprising a total of 93 healthy individuals. In the single-dose study, subjects received doses of up to 135 mg/kg, and in the multiple-dose study, 36 subjects were divided into four groups. Groups 1 and 2 received 4 weekly infusions of either low- or high-dose CSL-112, group 3 received a low dose of CSL-112 twice weekly for 4 weeks, and group 4 received placebo. Infusion of CSL-112 resulted in a dose-dependent increase in apoA-I, which remained above baseline levels for 3 days. A rapid 36-fold increase in pre-β HDL levels was noted, which was accompanied by increased ABCA1-mediated cholesterol efflux (maximum increase 270 % compared to baseline). Importantly, CSL-112 did not cause clinically relevant elevations of liver function parameters (Easton et al. 2013). A phase 2a study of CSL-112 in patients with stable CVD was recently completed, and the results are eagerly awaited.
3.5 CER-001 Infusions
CER-001, an engineered rHDL particle comprising recombinant human wild-type apoA-I and diphosphatidylglycerol and sphingomyelin, was designed to mimic nascent pre-β HDL. CER-001 infusions were shown to reduce atherosclerotic plaque size and aortic macrophage and lipid content in atherosclerosis-prone mice (Goffinet et al. 2012). A phase 1 randomized crossover study evaluated dose ranges from 0.25 to 45 mg/kg in 32 dyslipidemic volunteers. CER-001 infusions were well tolerated and did not show any safety issue at any dose; in particular there were no liver enzyme elevations. Following administration of a single infusion of low doses of CER-001, plasma total cholesterol and cholesterol in the HDL fraction were significantly elevated (almost 700 % increase in the HDL fraction at the 45 mg/kg dose), which may indicate mobilization of cholesterol from peripheral tissues (Keyserling et al. 2011). Cholesterol esterification was also observed, consistent with LCAT activation. Three phase 2 trials are currently investigating the effects of CER-001 on atherosclerotic plaques. The “Can HDL Infusions Significantly Quicken Atherosclerosis Regression” (CHI-SQUARE) trial is designed to investigate whether CER-001 infusions induce plaque regression in patients with ACS. In total, 507 patients have been randomized to 6 weekly infusions of three ascending doses of CER-001, or placebo. The primary outcome measure is the absolute change in plaque volume, as determined by IVUS, from baseline to 3 weeks after the last infusion (NCT01201837). In parallel, two open-label trials are currently evaluating the effects of CER-001 on plaque volume. In the Modifying Orphan Disease Evaluation (MODE) trial (NCT01412034), a total of 23 patients with homozygous familial hypercholesterolemia (FH) is treated for 6 months with biweekly infusions with CER-001. The primary outcome measure in this trial is the percent change in carotid mean vessel wall area as assessed by 3Tesla MRI. A similar regimen is followed in the investigator-driven SAMBA trial, conducted in patients with very low or absent HDL due to genetic defects—familial primary hypoalphalipoproteinemia (FPHA) (EudraCT number 2011-006188-23). Preliminary results presented at the 2013 PACE Snapshot session (ESC, Amsterdam) indicate that CER-001 increases RCT and may effectively reduce aortic atheroma volume.
These studies will provide valuable information about the clinical utility of CER-001 infusions in patients at increased CVD risk.
3.6 Modified ApoA-I
The rapid renal clearance of lipid-poor apoA-I may limit the therapeutic potential of apoA-I-based therapies. TripA-I represents a trimeric apoA-I analogue that functionally resembles wild-type apoA-I, with a prolonged half-life in plasma due to its increased size (Graversen et al. 2008; Ohnsorg et al. 2011). Intravenous treatment with tripA-I decreased the progression of atherosclerotic lesion size in LDL-R knockout mice on an atherogenic diet, although tripA-I did not affect aortic atherosclerotic lesion size in uremic apoE knockout mice (Graversen et al. 2008; Pedersen et al. 2009).
4 Selective Delipidation
Given that cholesterol-poor HDL particles are particularly effective in inducing reverse cholesterol transport, a procedure has been developed that selectively delipidates large, cholesterol-rich HDL particles, thereby creating small, lipid-poor HDL particles similar to small α- and pre-β-like HDL (HDL selectively delipidated or HDL-sdl). The technique involves mixing plasma with two delipidation reagents, followed by bulk separation by gravity and subsequent passage through a charcoal column to remove residual reagents (Sacks et al. 2009). Potential benefits of this technique are the fact that autologous particles are reinfused, which is anticipated to limit potential toxicity, and that the reinfused pre-β HDL particles are deemed particularly effective in promoting cholesterol efflux. Sacks et al. showed that 12 weekly infusions of selectively delipidated HDL reduced diet-induced atherosclerosis in monkeys, as assessed using IVUS. Delipidated small HDL particles were entirely converted into large α-HDL particles after infusion. Combined with the finding that selectively delipidated plasma induced a seven-fold increase in ABCA1-mediated cholesterol efflux, this may indicate enhanced reverse cholesterol transport (Sacks et al. 2009). Waksman et al. subsequently randomized 28 patients undergoing cardiac catheterization for ACS to 7 weekly infusions with either selectively delipidated HDL or control plasma to evaluate the safety of the Lipid Sciences Plasma Delipidation System-2 (LS PDS-2) and to explore the potential effects on atheroma volume. The treatment was well tolerated by all patients. On average, there was a 28-fold increase in pre-β-like HDL following delipidation compared to baseline. IVUS was obtained during cardiac catheterization for the ACS and after 2 weeks of the last treatment. A trend toward regression of total atheroma volume was found in patients treated with selective delipidation, while a small increase in atheroma volume was observed in the control group. Although the sample size was too small to detect significant effects on atheroma volume, the trend toward plaque regression can be considered as a beneficial effect. Additional studies in larger cohorts of patients will provide a more definite answer to the question whether delipidation is a potential benefit for patients suffering from an ACS (Waksman et al. 2010).
Conclusion
The development of apoA-I analogues has been driven by the observation that apoA-I plays a pivotal role in the antiatherogenic capacity of HDL particles.
A large number of apoA-I analogue proteins have been studied in vitro and some did show beneficial effects. However, “the proof of the pudding will be in the eating:” well-designed clinical trials in patients are indispensable to address the role of apoA-I mimetics and alike in atherosclerosis regression.
The clinical potential of rHDL particles reaches beyond the treatment of atherosclerosis. For example, rHDL particles labeled with contrast-generating agents, such as gadolinium or 125I, can be used as natural nanoparticles in medical imaging to visualize natural targets such as macrophages in atherosclerotic plaques or other targets by attaching targeting molecules (Ryan 2010; Stanley 2014). In addition, rHDL can be used as a vector for drug transport by incorporating lipophilic drugs into rHDL particles, which may decrease drug toxicity and increase bioavailability (Stanley 2014).
Abbreviations
- ACS:
-
Acute coronary syndrome
- ABCA1:
-
ATP-binding cassette transporter A1
- ABCG1:
-
ATP-binding cassette transporter G1
- ApoA-I:
-
Apolipoprotein A-1
- ApoB:
-
Apolipoprotein B
- ApoE:
-
Apolipoprotein E
- CVD:
-
Cardiovascular disease
- FH:
-
Familial hypercholesterolemia
- FPHA:
-
Familial primary hypoalphalipoproteinemia
- HCAEC:
-
Human coronary artery endothelial cells
- HDL-C:
-
High-density lipoprotein cholesterol
- HDL-sdl:
-
HDL selectively delipidated
- HDL-VHDL:
-
High-density lipoprotein-very high-density lipoprotein
- IVUS:
-
Intravascular ultrasound
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDL-R:
-
Low-density lipoprotein receptor
- LPA:
-
Lysophosphatidic acid
- RCT:
-
Reverse cholesterol transport
- rHDL:
-
Reconstituted HDL
- ROS:
-
Radical oxygen species
- sdLDL:
-
Small dense LDL
- WD:
-
Western diet
References
Amar MJ, D’Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT (2010) 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther 334:634–641. doi:10.1124/jpet.110.167890
Ameli S, Hultgardh-Nilsson A, Cercek B, Shah PK, Forrester JS, Ageland H, Nilsson J (1994) Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation 90:1935–1941
Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, Bhown AS, Segrest JP (1985) Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 260:10248–10255
Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V (1989) High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest 60:455–461
Badimon JJ, Badimon L, Fuster V (1990) Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 85:1234–1241. doi:10.1172/jci114558
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376:1670–1681. doi:10.1016/s0140-6736(10)61350-5
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM (2004) Antiinflammatory properties of HDL. Circ Res 95:764–772. doi:10.1161/01.res.0000146094.59640.13
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC (2007) HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357:1301–1310. doi:10.1056/NEJMoa064278
Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S (2010) A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res 51:1496–1503. doi:10.1194/jlr.M003665
Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ (2008) Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 49:1344–1352. doi:10.1194/jlr.P800003-JLR200
Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O’Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH (2009) Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ 338:b92. doi:10.1136/bmj.b92
Chattopadhyay A, Navab M, Hough G, Gao F, Meriwether D, Grijalva V, Springstead JR, Palgnachari MN, Namiri-Kalantari R, Su F, Van Lenten BJ, Wagner AC, Anantharamaiah GM, Farias-Eisner R, Reddy ST, Fogelman AM (2013) A novel approach to oral apoA-I mimetic therapy. J Lipid Res 54:995–1010. doi:10.1194/jlr.M033555
Chiesa G, Monteggia E, Marchesi M, Lorenzon P, Laucello M, Lorusso V, Di Mario C, Karvouni E, Newton RS, Bisgaier CL, Franceschini G, Sirtori CR (2002) Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res 90:974–980
deGoma EM, deGoma RL, Rader DJ (2008) Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol 51:2199–2211. doi:10.1016/j.jacc.2008.03.016
Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, Barter PJ, Bursill C (2011a) The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 217:395–400. doi:10.1016/j.atherosclerosis.2011.04.001
Di Bartolo BA, Vanags LZ, Tan JT, Bao S, Rye KA, Barter PJ, Bursill CA (2011b) The apolipoprotein A-I mimetic peptide, ETC-642, reduces chronic vascular inflammation in the rabbit. Lipids Health Dis 10:224. doi:10.1186/1476-511x-10-224
D’Souza W, Stonik JA, Murphy A, Demosky SJ, Sethi AA, Moore XL, Chin-Dusting J, Remaley AT, Sviridov D (2010) Structure/function relationships of apolipoprotein a-I mimetic peptides: implications for antiatherogenic activities of high-density lipoprotein. Circ Res 107:217–227. doi:10.1161/circresaha.110.216507
Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P (1996) Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation 94:713–717
Easton R, Gille A, D’Andrea D, Davis R, Wright SD, Shear C (2013) A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J Clin Pharmacol. doi:10.1002/jcph.194
Fielding CJ, Fielding PE (1995) Molecular physiology of reverse cholesterol transport. J Lipid Res 36:211–228
Franceschini G, Sirtori CR, Capurso A 2nd, Weisgraber KH, Mahley RW (1980) A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest 66:892–900. doi:10.1172/jci109956
Garber DW, Venkatachalapathi YV, Gupta KB, Ibdah J, Phillips MC, Hazelrig JB, Segrest JP, Anantharamaiah GM (1992) Turnover of synthetic class A amphipathic peptide analogues of exchangeable apolipoproteins in rats. Correlation with physical properties. Arterioscler Thromb 12:886–894
Goffinet M, Tardy C, Bluteau A, Boubekeur N, Baron R, Keyserling C, Barbaras R, Lalwani N, Dasseux J (2012) Anti-atherosclerotic effect of CER-001, an engineered HDL-mimetic, in the high-fat diet-fed LDLr knock-out mouse. Circulation 126, A18667
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62:707–714
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA (1989) High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79:8–15
Graversen JH, Laurberg JM, Andersen MH, Falk E, Nieland J, Christensen J, Etzerodt M, Thøgersen HC, Moestrup SK (2008) Trimerization of apolipoprotein A-I retards plasma clearance and preserves antiatherosclerotic properties. J Cardiovasc Pharmacol 51:170–177. doi:10.1097/FJC.0b013e31815ed0b9
Haase CL, Frikke-Schmidt R, Nordestgaard BG, Kateifides AK, Kardassis D, Nielsen LB, Andersen CB, Kober L, Johnsen AH, Grande P, Zannis VI, Tybjaerg-Hansen A (2011) Mutation in APOA1 predicts increased risk of ischaemic heart disease and total mortality without low HDL cholesterol levels. J Intern Med 270:136–146. doi:10.1111/j.1365-2796.2011.02381.x
Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JH, Petersen W, Dullaart RP, Stroes ES, Zwinderman AH, de Groot E, Hayden MR, Kuivenhoven JA, Kastelein JJ (2004) A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol 44:1429–1435. doi:10.1016/j.jacc.2004.06.070
Ibanez B, Giannarelli C, Cimmino G, Santos-Gallego CG, Alique M, Pinero A, Vilahur G, Fuster V, Badimon L, Badimon JJ (2012) Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type). Atherosclerosis 220:72–77. doi:10.1016/j.atherosclerosis.2011.10.006
Iwata A, Miura S, Zhang B, Imaizumi S, Uehara Y, Shiomi M, Saku K (2011) Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis 218:300–307. doi:10.1016/j.atherosclerosis.2011.05.029
Jafri H, Alsheikh-Ali AA, Karas RH (2010) Meta-analysis: statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann Intern Med 153:800–808. doi:10.7326/0003-4819-153-12-201012210-00006
Keyserling CH, Hunt TL, Klepp HM, Scott RA, Barbaras R, Schwendeman A, Lalwani N, Dasseux J-L (2011) CER-001, a synthetic HDL-mimetic, safely mobilizes cholesterol in healthy dyslipidemic volunteers. Circulation 124, A15525
Kontush A, Chapman MJ (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58:342–374. doi:10.1124/pr.58.3.1
Krause BR, Remaley AT (2013) Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol 24:480–486. doi:10.1097/mol.0000000000000020
Mineo C, Deguchi H, Griffin JH, Shaul PW (2006) Endothelial and antithrombotic actions of HDL. Circ Res 98:1352–1364. doi:10.1161/01.res.0000225982.01988.93
Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M et al (1995) Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 15:1882–1888
Moore RE, Kawashiri MA, Kitajima K, Secreto A, Millar JS, Pratico D, Rader DJ (2003) Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor. Arterioscler Thromb Vasc Biol 23:1914–1920. doi:10.1161/01.atv.0000092328.66882.f5
Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM (2002) Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105:290–292
Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM (2004) Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109:3215–3220. doi:10.1161/01.cir.0000134275.90823.87
Navab M, Hough G, Buga GM, Su F, Wagner AC, Meriwether D, Chattopadhyay A, Gao F, Grijalva V, Danciger JS, Van Lenten BJ, Org E, Lusis AJ, Pan C, Anantharamaiah GM, Farias-Eisner R, Smyth SS, Reddy ST, Fogelman AM (2013) Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res 54:3403–3418. doi:10.1194/jlr.M042051
Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE (2006) Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol 47:992–997. doi:10.1016/j.jacc.2005.11.040
Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R (2003) Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290:2292–2300. doi:10.1001/jama.290.17.2292
Ohnsorg PM, Mary JL, Rohrer L, Pech M, Fingerle J, von Eckardstein A (2011) Trimerized apolipoprotein A-I (TripA) forms lipoproteins, activates lecithin: cholesterol acyltransferase, elicits lipid efflux, and is transported through aortic endothelial cells. Biochim Biophys Acta 1811:1115–1123. doi:10.1016/j.bbalip.2011.09.001
Pedersen TX, Bro S, Andersen MH, Etzerodt M, Jauhiainen M, Moestrup S, Nielsen LB (2009) Effect of treatment with human apolipoprotein A-I on atherosclerosis in uremic apolipoprotein-E deficient mice. Atherosclerosis 202:372–381. doi:10.1016/j.atherosclerosis.2008.04.041
Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB (2003) Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res 44:828–836. doi:10.1194/jlr.M200475-JLR200
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2012) Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125:e2–e220. doi:10.1161/CIR.0b013e31823ac046
Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM (1991) Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353:265–267. doi:10.1038/353265a0
Ryan RO (2010) Nanobiotechnology applications of reconstituted high density lipoprotein. J Nanobiotechnol 8:28. doi:10.1186/1477-3155-8-28
Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, Rothblat G, de la Llera-Moya M, Asztalos B, Perlman T, Zheng C, Alaupovic P, Maltais JA, Brewer HB (2009) Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J Lipid Res 50:894–907. doi:10.1194/jlr.M800622-JLR200
Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, Remaley AT (2008) Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem 283:32273–32282. doi:10.1074/jbc.M804461200
Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B (1998) Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation 97:780–785
Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B (2001) High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation 103:3047–3050
Soma MR, Donetti E, Parolini C, Sirtori CR, Fumagalli R, Franceschini G (1995) Recombinant apolipoprotein A-IMilano dimer inhibits carotid intimal thickening induced by perivascular manipulation in rabbits. Circ Res 76:405–411
Stanley S (2014) Biological nanoparticles and their influence on organisms. Curr Opin Biotechnol 28:69–74. doi:10.1016/j.copbio.2013.11.014
Suc I, Escargueil-Blanc I, Troly M, Salvayre R, Negre-Salvayre A (1997) HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler Thromb Vasc Biol 17:2158–2166
Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G (2010) The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30:246–252. doi:10.1161/atvbaha.109.200196
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J (2007) Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 297:1675–1682. doi:10.1001/jama.297.15.jpc70004
Venkatachalapathi YV, Phillips MC, Epand RM, Epand RF, Tytler EM, Segrest JP, Anantharamaiah GM (1993) Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins 15:349–359. doi:10.1002/prot.340150403
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S et al (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380:572–580. doi:10.1016/s0140-6736(12)60312-2
Voyiaziakis E, Goldberg IJ, Plump AS, Rubin EM, Breslow JL, Huang LS (1998) ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apoB transgenic mice. J Lipid Res 39:313–321
Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB Jr (2010) A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 55:2727–2735. doi:10.1016/j.jacc.2009.12.067
Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A (2011) Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res 52:361–373. doi:10.1194/jlr.M011098
Wool GD, Reardon CA, Getz GS (2008) Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J Lipid Res 49:1268–1283. doi:10.1194/jlr.M700552-JLR200
Yancey PG, Bielicki JK, Johnson WJ, Lund-Katz S, Palgunachari MN, Anantharamaiah GM, Segrest JP, Phillips MC, Rothblat GH (1995) Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry 34:7955–7965
Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJ, Remaley AT, Levine SJ (2011) 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J Immunol 186:576–583. doi:10.4049/jimmunol.1001534
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Stoekenbroek, R.M., Stroes, E.S., Hovingh, G.K. (2015). ApoA-I Mimetics. In: von Eckardstein, A., Kardassis, D. (eds) High Density Lipoproteins. Handbook of Experimental Pharmacology, vol 224. Springer, Cham. https://doi.org/10.1007/978-3-319-09665-0_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-09665-0_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09664-3
Online ISBN: 978-3-319-09665-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)