Abstract
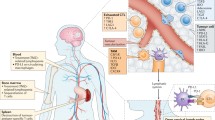
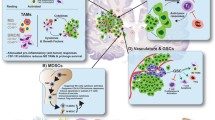
Immunotherapy has changed the landscape of treatment of many solid and hematological malignancies and is at the forefront of cancer breakthroughs. Several circumstances unique to the central nervous system (CNS) such as limited space for an inflammatory response, difficulties with repeated sampling, corticosteroid use for management of cerebral edema, and immunosuppressive mechanisms within the tumor and brain parenchyma have posed challenges in clinical development of immunotherapy for intracranial tumors. Nonetheless, the success of immunotherapy in brain metastases (BMs) from solid cancers such as melanoma and non-small cell lung cancer (NSCLC) proves that the CNS is not an immune-privileged organ and is capable of initiating and regulating immune responses that lead to tumor control. However, the development of immunotherapeutics for the most malignant primary brain tumor, glioblastoma (GBM), has been challenging due to systemic and profound tumor-mediated immunosuppression unique to GBM, intratumoral and intertumoral heterogeneity, and lack of stably expressed clonal antigens. Here, we review recent advances in the field of immunotherapy for neuro-oncology with a focus on BM, GBM, and rare CNS cancers.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Medawar, P. B. (1948). Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British Journal of Experimental Pathology, 29(1), 58–69.
Woodroofe, M. N., Bellamy, A. S., Feldmann, M., Davison, A. N., & Cuzner, M. L. (1986). Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. Journal of the Neurological Sciences, 74(2–3), 135–152.
Louveau, A., Smirnov, I., Keyes, T. J., Eccles, J. D., Rouhani, S. J., Peske, J. D., et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341.
Venur, V. A., Karivedu, V., & Ahluwalia, M. S. (2018). Systemic therapy for brain metastases. Handbook of Clinical Neurology, 149, 137–153.
Ostrom, Q. T., Wright, C. H., & Barnholtz-Sloan, J. S. (2018). Brain metastases: epidemiology. Handbook of Clinical Neurology, 149, 27–42.
Achrol, A. S., Rennert, R. C., Anders, C., Soffietti, R., Ahluwalia, M. S., Nayak, L., et al. (2019). Brain metastases. Nature Reviews Disease Primers, 5(1), 5.
Tawbi, H. A., Forsyth, P. A., Algazi, A., Hamid, O., Hodi, F. S., Moschos, S. J., et al. (2018). Combined Nivolumab and Ipilimumab in melanoma metastatic to the brain. New England Journal of Medicine, 379(8), 722–730.
Goldberg, S. B., Gettinger, S. N., Mahajan, A., Chiang, A. C., Herbst, R. S., Sznol, M., et al. (2016). Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. The Lancet Oncology, 17(7), 976–983.
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England Journal of Medicine, 372(26), 2521–2532.
Hargadon, K. M., Johnson, C. E., & Williams, C. J. (2018). Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. International Immunopharmacology, 62, 29–39.
Callahan, M. K., Wolchok, J. D., & Allison, J. P. (2010). Anti-CTLA-4 antibody therapy: Immune monitoring during clinical development of a novel immunotherapy. Seminars in Oncology, 37(5), 473–484.
Korn, E. L., Liu, P. Y., Lee, S. J., Chapman, J. A., Niedzwiecki, D., Suman, V. J., et al. (2008). Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. Journal of Clinical Oncology, 26(4), 527–534.
Callahan, M. K., Kluger, H., Postow, M. A., Segal, N. H., Lesokhin, A., Atkins, M. B., et al. (2018). Nivolumab plus Ipilimumab in patients with advanced melanoma: Updated survival, response, and safety data in a phase I dose-escalation study. Journal of Clinical Oncology, 36(4), 391–398.
Chukwueke, U., Batchelor, T., & Brastianos, P. (2016). Management of brain metastases in patients with melanoma. Journal of Oncology Practice, 12(6), 536–542.
Davies, M. A., Liu, P., McIntyre, S., Kim, K. B., Papadopoulos, N., Hwu, W. J., et al. (2011). Prognostic factors for survival in melanoma patients with brain metastases. Cancer, 117(8), 1687–1696.
Sloan, A. E., Nock, C. J., & Einstein, D. B. (2009). Diagnosis and treatment of melanoma brain metastasis: A literature review. Cancer Control, 16(3), 248–255.
Di Giacomo, A. M., Ascierto, P. A., Pilla, L., Santinami, M., Ferrucci, P. F., Giannarelli, D., et al. (2012). Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): An open-label, single-arm phase 2 trial. The Lancet Oncology, 13(9), 879–886.
Di Giacomo, A. M., Ascierto, P. A., Queirolo, P., Pilla, L., Ridolfi, R., Santinami, M., et al. (2015). Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Annals of Oncology, 26(4), 798–803.
Margolin, K., Ernstoff, M. S., Hamid, O., Lawrence, D., McDermott, D., Puzanov, I., et al. (2012). Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. The Lancet Oncology, 13(5), 459–465.
Kluger, H. M., Chiang, V., Mahajan, A., Zito, C. R., Sznol, M., Tran, T., et al. (2019). Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. Journal of Clinical Oncology, 37(1), 52–60.
Goldberg, S. B., Schalper, K. A., Gettinger, S. N., Mahajan, A., Herbst, R. S., Chiang, A. C., et al. (2020). Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. The Lancet Oncology, 21(5), 655–663.
Borghaei, H., Pluzanski, A., Caro, R. B., Provencio, M., Burgers, S., Carcereny, E., et al. (2020). Abstract CT221: Nivolumab (NIVO) + ipilimumab (IPI) as first-line (1L) treatment for patients with advanced non-small cell lung cancer (NSCLC) with brain metastases: Results from CheckMate 227. Cancer Research, 80(16 Supplement), CT221-CT.
Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J., et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine, 352(10), 987–996.
Sanai, N., & Berger, M. S. (2008). Glioma extent of resection and its impact on patient outcome. Neurosurgery, 62(4), 753–764; discussion 264–6.
Reardon, D. A., Gokhale, P. C., Klein, S. R., Ligon, K. L., Rodig, S. J., Ramkissoon, S. H., et al. (2016). Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunology Research, 4(2), 124–135.
Fecci, P. E., Ochiai, H., Mitchell, D. A., Grossi, P. M., Sweeney, A. E., Archer, G. E., et al. (2007). Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clinical Cancer Research, 13(7), 2158–2167.
Reardon, D. A., Brandes, A. A., Omuro, A., Mulholland, P., Lim, M., Wick, A., et al. (2020). Effect of Nivolumab vs Bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncology, 6(7), 1003–1010.
Rosa K. Nivolumab Plus Temozolomide/Radiotherapy Misses OS End Point in Glioblastoma Multiforme 2020. Available from: https://www.onclive.com/view/nivolumab-plus-temozolomide-radiotherapy-misses-os-end-point-in-glioblastoma-multiforme
Bristol-Myers Squibb. Bristol-Myers Squibb Announces Phase 3 CheckMate -498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme 2019, May 9. Available from: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did
Genoud, V., Marinari, E., Nikolaev, S. I., Castle, J. C., Bukur, V., Dietrich, P. Y., et al. (2018). Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology, 7(12), e1501137.
Lawrence, M. S., Stojanov, P., Polak, P., Kryukov, G. V., Cibulskis, K., Sivachenko, A., et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature, 499(7457), 214–218.
Yeung, J. T., Hamilton, R. L., Ohnishi, K., Ikeura, M., Potter, D. M., Nikiforova, M. N., et al. (2013). LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clinical Cancer Research, 19(7), 1816–1826.
Parsa, A. T., Waldron, J. S., Panner, A., Crane, C. A., Parney, I. F., Barry, J. J., et al. (2007). Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature Medicine, 13(1), 84–88.
Wainwright, D. A., Chang, A. L., Dey, M., Balyasnikova, I. V., Kim, C. K., Tobias, A., et al. (2014). Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clinical Cancer Research, 20(20), 5290–5301.
Chang, N., Ahn, S. H., Kong, D. S., Lee, H. W., & Nam, D. H. (2017). The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment. Molecular and Cellular Endocrinology, 451, 53–65.
Ceccarelli, M., Barthel, F. P., Malta, T. M., Sabedot, T. S., Salama, S. R., Murray, B. A., et al. (2016). Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell, 164(3), 550–563.
de Groot, J., Penas-Prado, M., Alfaro-Munoz, K., Hunter, K., Pei, B. L., O’Brien, B., et al. (2020). Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro-Oncology, 22(4), 539–549.
Heimberger, A. B., Sun, W., Hussain, S. F., Dey, M., Crutcher, L., Aldape, K., et al. (2008). Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro-Oncology, 10(1), 98–103.
Schartner, J. M., Hagar, A. R., Van Handel, M., Zhang, L., Nadkarni, N., & Badie, B. (2005). Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia, 51(4), 279–285.
Stevens, A., Kloter, I., & Roggendorf, W. (1988). Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer, 61(4), 738–743.
Didenko, V. V., Ngo, H. N., Minchew, C., & Baskin, D. S. (2002). Apoptosis of T lymphocytes invading glioblastomas multiforme: A possible tumor defense mechanism. Journal of Neurosurgery, 96(3), 580–584.
Wischhusen, J., Friese, M. A., Mittelbronn, M., Meyermann, R., & Weller, M. (2005). HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: Implications for immune escape in vivo. Journal of Neuropathology and Experimental Neurology, 64(6), 523–528.
Wiendl, H., Mitsdoerffer, M., Hofmeister, V., Wischhusen, J., Bornemann, A., Meyermann, R., et al. (2002). A functional role of HLA-G expression in human gliomas: An alternative strategy of immune escape. Journal of Immunology, 168(9), 4772–4780.
Huettner, C., Czub, S., Kerkau, S., Roggendorf, W., & Tonn, J. C. (1997). Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Research, 17(5A), 3217–3224.
Dix, A. R., Brooks, W. H., Roszman, T. L., & Morford, L. A. (1999). Immune defects observed in patients with primary malignant brain tumors. Journal of Neuroimmunology, 100(1–2), 216–232.
Grossman, S. A., Ye, X., Lesser, G., Sloan, A., Carraway, H., Desideri, S., et al. (2011). Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clinical Cancer Research, 17(16), 5473–5480.
Chongsathidkiet, P., Jackson, C., Koyama, S., Loebel, F., Cui, X., Farber, S. H., et al. (2018). Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nature Medicine, 24(9), 1459–1468.
Gustafson, M. P., Lin, Y., New, K. C., Bulur, P. A., O’Neill, B. P., Gastineau, D. A., et al. (2010). Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncology, 12(7), 631–644.
Bloch, O., Crane, C. A., Kaur, R., Safaee, M., Rutkowski, M. J., & Parsa, A. T. (2013). Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clinical Cancer Research, 19(12), 3165–3175.
Iorgulescu, J. B., Gokhale, P. C., Speranza, M. C., Eschle, B. K., Poitras, M. J., Wilkens, M. K., et al. (2021). Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clinical Cancer Research, 27(1), 276–287.
Bouffet, E., Larouche, V., Campbell, B. B., Merico, D., de Borja, R., Aronson, M., et al. (2016). Immune checkpoint inhibition for Hypermutant glioblastoma Multiforme resulting from germline Biallelic mismatch repair deficiency. Journal of Clinical Oncology, 34(19), 2206–2211.
Johanns, T. M., Miller, C. A., Dorward, I. G., Tsien, C., Chang, E., Perry, A., et al. (2016). Immunogenomics of Hypermutated glioblastoma: A patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discovery, 6(11), 1230–1236.
Viale, G., Trapani, D., & Curigliano, G. (2017). Mismatch repair deficiency as a predictive biomarker for immunotherapy efficacy. BioMed Research International, 2017, 4719194.
Kamiya-Matsuoka, C., Metrus, N. R., Shaw, K. R., Penas-Prado, M., Weathers, S.-P. S., Loghin, M. E., et al. (2018). The natural course of hypermutator gliomas. Journal of Clinical Oncology, 36(15_suppl), 2014-.
Omuro, A., Vlahovic, G., Lim, M., Sahebjam, S., Baehring, J., Cloughesy, T., et al. (2018). Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncology, 20(5), 674–686.
Lim, M., Omuro, A., Vlahovic, G., Reardon, D. A., Sahebjam, S., Cloughesy, T., et al. (2017). 325ONivolumab (nivo) in combination with radiotherapy (RT) ± temozolomide (TMZ): Updated safety results from CheckMate 143 in pts with methylated or unmethylated newly diagnosed glioblastoma (GBM). Annals of Oncology, 28(suppl_5), mdx366-mdx.
Nayak, L., Molinaro, A. M., Peters, K., Clarke, J. L., Jordan, J. T., de Groot, J., et al. (2021). Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clinical Cancer Research, 27(4), 1048–1057.
Cloughesy, T. F., Mochizuki, A. Y., Orpilla, J. R., Hugo, W., Lee, A. H., Davidson, T. B., et al. (2019). Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature Medicine, 25(3), 477–486.
Schalper, K. A., Rodriguez-Ruiz, M. E., Diez-Valle, R., Lopez-Janeiro, A., Porciuncula, A., Idoate, M. A., et al. (2019). Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nature Medicine, 25(3), 470–476.
Amaria, R. N., Reddy, S. M., Tawbi, H. A., Davies, M. A., Ross, M. I., Glitza, I. C., et al. (2018). Publisher correction: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nature Medicine, 24(12), 1942.
Blank, C. U., Rozeman, E. A., Fanchi, L. F., Sikorska, K., van de Wiel, B., Kvistborg, P., et al. (2018). Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nature Medicine, 24(11), 1655–1661.
Forde, P. M., Chaft, J. E., & Pardoll, D. M. (2018). Neoadjuvant PD-1 blockade in resectable lung cancer. The New England Journal of Medicine, 379(9), e14.
Larkin, J., Hodi, F. S., & Wolchok, J. D. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. The New England Journal of Medicine, 373(13), 1270–1271.
Hodges, T. R., Ott, M., Xiu, J., Gatalica, Z., Swensen, J., Zhou, S., et al. (2017). Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro-Oncology, 19(8), 1047–1057.
McGranahan, T., Li, G., & Nagpal, S. (2017). History and current state of immunotherapy in glioma and brain metastasis. Therapeutic Advances in Medical Oncology, 9(5), 347–368.
Dunn, G. P., Fecci, P. E., & Curry, W. T. (2012). Cancer immunoediting in malignant glioma. Neurosurgery, 71(2), 201–222; discussion 22–3.
Nduom, E. K., Weller, M., & Heimberger, A. B. (2015). Immunosuppressive mechanisms in glioblastoma. Neuro-Oncology, 17(Suppl 7), vii9–vii14.
Wainwright, D. A., Sengupta, S., Han, Y., & Lesniak, M. S. (2011). Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro-Oncology, 13(12), 1308–1323.
Lampson, L. A. (2011). Monoclonal antibodies in neuro-oncology: Getting past the blood-brain barrier. MAbs, 3(2), 153–160.
Gerstner, E. R., & Fine, R. L. (2007). Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: Establishing a treatment paradigm. Journal of Clinical Oncology, 25(16), 2306–2312.
Ou, A., Yung, W. K. A., & Majd, N. (2020). Molecular mechanisms of treatment resistance in glioblastoma. International Journal of Molecular Sciences, 22(1).
Desai, R., Suryadevara, C. M., Batich, K. A., Farber, S. H., Sanchez-Perez, L., & Sampson, J. H. (2016). Emerging immunotherapies for glioblastoma. Expert Opinion on Emerging Drugs, 21(2), 133–145.
Heimberger, A. B., Suki, D., Yang, D., Shi, W., & Aldape, K. (2005). The natural history of EGFR and EGFRvIII in glioblastoma patients. Journal of Translational Medicine, 3, 38.
Weller, M., Butowski, N., Tran, D. D., Recht, L. D., Lim, M., Hirte, H., et al. (2017). Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. The Lancet Oncology, 18(10), 1373–1385.
Yan, H., Parsons, D. W., Jin, G., McLendon, R., Rasheed, B. A., Yuan, W., et al. (2009). IDH1 and IDH2 mutations in gliomas. The New England Journal of Medicine, 360(8), 765–773.
Parsons, D. W., Jones, S., Zhang, X., Lin, J. C., Leary, R. J., Angenendt, P., et al. (2008). An integrated genomic analysis of human glioblastoma multiforme. Science, 321(5897), 1807–1812.
Schumacher, T., Bunse, L., Pusch, S., Sahm, F., Wiestler, B., Quandt, J., et al. (2014). A vaccine targeting mutant IDH1 induces antitumour immunity. Nature, 512(7514), 324–327.
Pellegatta, S., Valletta, L., Corbetta, C., Patane, M., Zucca, I., Riccardi Sirtori, F., et al. (2015). Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathologica Communications, 3, 4.
Michael Platten, D. S., Bunse, L., Wick, A., Bunse, T., Riehl, D., Green, E., Sanghvi, K., Karapanagiotou-Schenkel, I., Harting, I., Sahm, F., Steinbach, J., Weyerbrock, A., Hense, J., Misch, M., Krex, D., Stevanovic, S., Tabatabai, G., von Deimling, A., Schmitt, M., & Wick, W. (2018). ATIM-33. NOA-16: A first-in-man multicenter phase I clinical trial of the German neurooncology working group evaluating a mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant astrocytomas. Neuro-Oncology, 20(6), vi8–vi9.
Rampling, R., Peoples, S., Mulholland, P. J., James, A., Al-Salihi, O., Twelves, C. J., et al. (2016). A cancer research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clinical Cancer Research, 22(19), 4776–4785.
Migliorini, D., Dutoit, V., Allard, M., Hallez, N. G., Marinari, E., Widmer, V., et al. (2019). Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro-Oncology.
Keskin, D. B., Anandappa, A. J., Sun, J., Tirosh, I., Mathewson, N. D., Li, S., et al. (2019). Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature, 565(7738), 234–239.
Baratta, M. G. (2019). Glioblastoma is ‘hot’ for personalized vaccines. Nature Reviews. Cancer, 19(3), 129.
Hilf, N., Kuttruff-Coqui, S., Frenzel, K., Bukur, V., Stevanovic, S., Gouttefangeas, C., et al. (2019). Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature, 565(7738), 240–245.
Graner, M. W., & Bigner, D. D. (2005). Chaperone proteins and brain tumors: Potential targets and possible therapeutics. Neuro-Oncology, 7(3), 260–278.
Ampie, L., Choy, W., Lamano, J. B., Fakurnejad, S., Bloch, O., & Parsa, A. T. (2015). Heat shock protein vaccines against glioblastoma: From bench to bedside. Journal of Neuro-Oncology, 123(3), 441–448.
Bloch, O., Crane, C. A., Fuks, Y., Kaur, R., Aghi, M. K., Berger, M. S., et al. (2014). Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: A phase II, single-arm trial. Neuro-Oncology, 16(2), 274–279.
Ahluwalia, M. S., Reardon, D. A., Abad, A. P., Curry, W. T., Wong, E. T., Belal, A., et al. (2019). SurVaxM with standard therapy in newly diagnosed glioblastoma: Phase II trial update. Journal of Clinical Oncology, 37(15_suppl), 2016-.
Ardon, H., Van Gool, S. W., Verschuere, T., Maes, W., Fieuws, S., Sciot, R., et al. (2012). Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunology, Immunotherapy, 61(11), 2033–2044.
Liau, L. M., Ashkan, K., Tran, D. D., Campian, J. L., Trusheim, J. E., Cobbs, C. S., et al. (2018). First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. Journal of Translational Medicine, 16(1), 142.
Liau, L. M., Black, K. L., Martin, N. A., Sykes, S. N., Bronstein, J. M., Jouben-Steele, L., et al. (2000). Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report. Neurosurgical Focus, 9(6), e8.
Jena, B., Dotti, G., & Cooper, L. J. (2010). Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood, 116(7), 1035–1044.
Maher, J. (2014). Clinical immunotherapy of B-cell malignancy using CD19-targeted CAR T-cells. Current Gene Therapy, 14(1), 35–43.
Knochelmann, H. M., Smith, A. S., Dwyer, C. J., Wyatt, M. M., Mehrotra, S., & Paulos, C. M. (2018). CAR T cells in solid tumors: Blueprints for building effective therapies. Frontiers in Immunology, 9, 1740.
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, J. R., Naranjo, A., et al. (2016). Regression of glioblastoma after chimeric antigen receptor T-cell therapy. The New England Journal of Medicine, 375(26), 2561–2569.
O’Rourke, D. M., Nasrallah, M. P., Desai, A., Melenhorst, J. J., Mansfield, K., Morrissette, J. J. D., et al. (2017). A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science Translational Medicine, 9(399).
Ahmed, N., Salsman, V. S., Kew, Y., Shaffer, D., Powell, S., Zhang, Y. J., et al. (2010). HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical Cancer Research, 16(2), 474–485.
Ahmed, N., Brawley, V., Hegde, M., Bielamowicz, K., Kalra, M., Landi, D., et al. (2017). HER2-specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation TrialHER2-specific CAR-modified virus-specific T cells for progressive GlioblastomaHER2-specific CAR-modified virus-specific T cells for progressive glioblastoma. JAMA Oncology, 3(8), 1094–1101.
Weathers, S. P., Penas-Prado, M., Pei, B. L., Ling, X., Kassab, C., Banerjee, P., et al. (2020). Glioblastoma-mediated immune dysfunction limits CMV-specific T cells and therapeutic responses: Results from a phase I/II trial. Clinical Cancer Research, 26(14), 3565–3577.
John, L. B., Devaud, C., Duong, C. P., Yong, C. S., Beavis, P. A., Haynes, N. M., et al. (2013). Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clinical Cancer Research, 19(20), 5636–5646.
Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science, 331(6013), 44–49.
Nayyar, G., Chu, Y., & Cairo, M. S. (2019). Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Frontiers in Oncology, 9, 51.
Ishikawa, E., Tsuboi, K., Saijo, K., Harada, H., Takano, S., Nose, T., et al. (2004). Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Research, 24(3b), 1861–1871.
Majd, N., Rizk, M., Ericson, S., Grzegorzewski, K., Koppisetti, S., Zhu, J., et al. (2020). RTID-07. Human placental hematopoietic stem cell derived natural killer cells (CYNK-001) for treatment of recurrent glioblastoma. Neuro-Oncology, 22(Supplement_2), ii194–ii5.
Jiang, H., McCormick, F., Lang, F. F., Gomez-Manzano, C., & Fueyo, J. (2006). Oncolytic adenoviruses as antiglioma agents. Expert Review of Anticancer Therapy, 6(5), 697–708.
Jiang, H., & Fueyo, J. (2014). Healing after death: Antitumor immunity induced by oncolytic adenoviral therapy. Oncoimmunology, 3(7), e947872.
Desjardins, A., Gromeier, M., Herndon, J. E., 2nd, Beaubier, N., Bolognesi, D. P., Friedman, A. H., et al. (2018). Recurrent glioblastoma treated with recombinant poliovirus. The New England Journal of Medicine, 379(2), 150–161.
Cloughesy, T. F., Petrecca, K., Walbert, T., Butowski, N., Salacz, M., Perry, J., et al. (2020). Effect of vocimagene amiretrorepvec in combination with flucytosine vs standard of care on survival following tumor resection in patients with recurrent high-grade glioma: A randomized clinical trial. JAMA Oncology, 6(12), 1939–1946.
Lang, F. F., Conrad, C., Gomez-Manzano, C., Yung, W. K. A., Sawaya, R., Weinberg, J. S., et al. (2018). Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. Journal of Clinical Oncology, 36(14), 1419–1427.
Perez, O. D., Logg, C. R., Hiraoka, K., Diago, O., Burnett, R., Inagaki, A., et al. (2012). Design and selection of Toca 511 for clinical use: Modified retroviral replicating vector with improved stability and gene expression. Molecular Therapy, 20(9), 1689–1698.
Cloughesy, T. F., Landolfi, J., Vogelbaum, M. A., Ostertag, D., Elder, J. B., Bloomfield, S., et al. (2018). Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology, 20(10), 1383–1392.
Chiocca, E. A., Nassiri, F., Wang, J., Peruzzi, P., & Zadeh, G. (2019). Viral and other therapies for recurrent glioblastoma: Is a 24-month durable response unusual? Neuro-Oncology, 21(1), 14–25.
Harrison, R. A., Anderson, M. D., Cachia, D., Kamiya-Matsuoka, C., Weathers, S. S., O’Brien, B. J., et al. (2019). Clinical trial participation of patients with glioblastoma at The University of Texas MD Anderson Cancer Center. European Journal of Cancer, 112, 83–93.
Chiocca, E. A., Abbed, K. M., Tatter, S., Louis, D. N., Hochberg, F. H., Barker, F., et al. (2004). A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Molecular Therapy, 10(5), 958–966.
Prins, R. M., Soto, H., Konkankit, V., Odesa, S. K., Eskin, A., Yong, W. H., et al. (2011). Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clinical Cancer Research, 17(6), 1603–1615.
Yu, J. S., Liu, G., Ying, H., Yong, W. H., Black, K. L., & Wheeler, C. J. (2004). Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Research, 64(14), 4973–4979.
Yamanaka, R., Abe, T., Yajima, N., Tsuchiya, N., Homma, J., Kobayashi, T., et al. (2003). Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: Results of a clinical phase I/II trial. British Journal of Cancer, 89(7), 1172–1179.
Zhai, L., Lauing, K. L., Chang, A. L., Dey, M., Qian, J., Cheng, Y., et al. (2015). The role of IDO in brain tumor immunotherapy. Journal of Neuro-Oncology, 123(3), 395–403.
Fallarino, F., Grohmann, U., Vacca, C., Bianchi, R., Orabona, C., Spreca, A., et al. (2002). T cell apoptosis by tryptophan catabolism. Cell Death and Differentiation, 9(10), 1069–1077.
Han, J., Alvarez-Breckenridge, C. A., Wang, Q. E., & Yu, J. (2015). TGF-beta signaling and its targeting for glioma treatment. American Journal of Cancer Research, 5(3), 945–955.
Bogdahn, U., Hau, P., Stockhammer, G., Venkataramana, N. K., Mahapatra, A. K., Suri, A., et al. (2011). Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro-Oncology, 13(1), 132–142.
den Hollander, M. W., Bensch, F., Glaudemans, A. W. J. M., Enting, R. H., Bunskoek, S., Munnink, T. H. O., et al. (2013). 89zr-GC1008 PET imaging and GC1008 treatment of recurrent glioma patients. Journal of Clinical Oncology, 31(15_suppl), 2050-.
Rodon, J., Carducci, M. A., Sepulveda-Sanchez, J. M., Azaro, A., Calvo, E., Seoane, J., et al. (2015). First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clinical Cancer Research, 21(3), 553–560.
Roy, L. O., Poirier, M. B., & Fortin, D. (2018). Differential expression and clinical significance of transforming growth factor-beta isoforms in GBM tumors. International Journal of Molecular Sciences, 19(4).
Pyonteck, S. M., Akkari, L., Schuhmacher, A. J., Bowman, R. L., Sevenich, L., Quail, D. F., et al. (2013). CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine, 19(10), 1264–1272.
Butowski, N., Colman, H., De Groot, J. F., Omuro, A. M., Nayak, L., Wen, P. Y., et al. (2016). Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: An Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncology, 18(4), 557–564.
Goldberg, M. V., & Drake, C. G. (2011). LAG-3 in cancer immunotherapy. Current Topics in Microbiology and Immunology, 344, 269–278.
Harris-Bookman, S., Mathios, D., Martin, A. M., Xia, Y., Kim, E., Xu, H., et al. (2018). Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. International Journal of Cancer, 143(12), 3201–3208.
Lim, M., Ye, X., Piotrowski, A. F., Desai, A. S., Ahluwalia, M. S., Walbert, T., et al. (2019). Updated phase I trial of anti-LAG-3 or anti-CD137 alone and in combination with anti-PD-1 in patients with recurrent GBM. Journal of Clinical Oncology, 37(15_suppl), 2017-.
Pollok, K. E., Kim, Y. J., Zhou, Z., Hurtado, J., Kim, K. K., Pickard, R. T., et al. (1993). Inducible T cell antigen 4-1BB. Analysis of expression and function. Journal of Immunology, 150(3), 771–781.
Pilie, P. G., Gay, C. M., Byers, L. A., O’Connor, M. J., & Yap, T. A. (2019). PARP inhibitors: Extending benefit beyond BRCA-mutant cancers. Clinical Cancer Research, 25(13), 3759–3771.
Majd, N., Yap, T. A., Yung, W. K. A., & de Groot, J. (2020). The promise of poly(ADP-ribose) polymerase (PARP) inhibitors in gliomas. Journal of Immunotherapy and Precision Oncology, 3(4), 157–164.
Majd, N. K., Yap, T. A., Koul, D., Balasubramaniyan, V., Li, X., Khan, S., et al. (2021). The promise of DNA damage response inhibitors for the treatment of glioblastoma. Neuro-oncology Advances, 3(1), vdab015.
Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica, 131(6), 803–820.
Louis, D. N., Ellison, D. W., Brat, D. J., Aldape, K., Capper, D., Hawkins, C., et al. (2019). cIMPACT-NOW: A practical summary of diagnostic points from round 1 updates. Brain Pathology, 29(4), 469–472.
Ostrom, Q. T., Cioffi, G., Gittleman, H., Patil, N., Waite, K., Kruchko, C., et al. (2019). CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncology, 21(Supplement_5), v1–v100.
Marabelle, A., Le, D. T., Ascierto, P. A., Di Giacomo, A. M., De Jesus-Acosta, A., Delord, J. P., et al. (2020). Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. Journal of Clinical Oncology, 38(1), 1–10.
Naing, A., Meric-Bernstam, F., Stephen, B., Karp, D. D., Hajjar, J., Rodon Ahnert, J., et al. (2020). Phase 2 study of pembrolizumab in patients with advanced rare cancers. Journal for Immunotherapy of Cancer, 8(1), e000347.
Lloyd, R. V., Osamura, R. Y., Klöppel, G., & Rosai, J. (Eds.). (2017). WHO classification of tumours of endocrine organs (4th ed.). IARC Press.
Daly, A. F., Tichomirowa, M. A., & Beckers, A. (2009). The epidemiology and genetics of pituitary adenomas. Best Practice & Research. Clinical Endocrinology & Metabolism, 23(5), 543–554.
Lin, A. L., Jonsson, P., Tabar, V., Yang, T. J., Cuaron, J., Beal, K., et al. (2018). Marked response of a hypermutated ACTH-secreting pituitary carcinoma to Ipilimumab and Nivolumab. The Journal of Clinical Endocrinology & Metabolism, 103(10), 3925–3930.
Duhamel, C., Ilie, M. D., Salle, H., Nassouri, A. S., Gaillard, S., Deluche, E., et al. (2020). Immunotherapy in corticotroph and lactotroph aggressive tumors and carcinomas: Two case reports and a review of the literature. Journal of Personalized Medicine, 10(3).
Majd, N., Waguespack, S. G., Janku, F., Fu, S., Penas-Prado, M., Xu, M., et al. (2020). Efficacy of pembrolizumab in patients with pituitary carcinoma: Report of four cases from a phase II study. Journal for Immunotherapy of Cancer, 8(2).
Pajtler, K. W., Witt, H., Sill, M., Jones, D. T. W., Hovestadt, V., Kratochwil, F., et al. (2015). Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell, 27(5), 728–743.
Tapia Rico, G., Townsend, A., Price, T., & Patterson, K. (2020). Metastatic myxopapillary ependymoma treated with immunotherapy achieving durable response. BMJ Case Reports, 13(12), e236242.
Clark, V. E., Erson-Omay, E. Z., Serin, A., Yin, J., Cotney, J., Ozduman, K., et al. (2013). Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science, 339(6123), 1077–1080.
Han, S. J., Reis, G., Kohanbash, G., Shrivastav, S., Magill, S. T., Molinaro, A. M., et al. (2016). Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. Journal of Neuro-Oncology, 130(3), 543–552.
Dunn, I. F., Du, Z., Touat, M., Sisti, M. B., Wen, P. Y., Umeton, R., et al. (2018). Mismatch repair deficiency in high-grade meningioma: A rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precision Oncology, 2018.
Campbell, B. B., Light, N., Fabrizio, D., Zatzman, M., Fuligni, F., de Borja, R., et al. (2017). Comprehensive analysis of hypermutation in human cancer. Cell, 171(5), 1042-56.e10.
Kabir, T. F., Kunos, C. A., Villano, J. L., & Chauhan, A. (2020). Immunotherapy for medulloblastoma: Current perspectives. Immunotargets and Therapy, 9, 57–77.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Majd, N.K., Dasgupta, P.R., de Groot, J.F. (2021). Immunotherapy for Neuro-oncology. In: Naing, A., Hajjar, J. (eds) Immunotherapy. Advances in Experimental Medicine and Biology, vol 1342. Springer, Cham. https://doi.org/10.1007/978-3-030-79308-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-79308-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79307-4
Online ISBN: 978-3-030-79308-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)