Abstract
A coherent and effective remote sensing (RS) contribution to biodiversity monitoring requires careful consideration of scale in all its dimensions, including spatial, temporal, spectral, and angular, along with biodiversity at different levels of biological organization. Recent studies of the relationship between optical diversity (spectral diversity) and biodiversity reveal a scale dependence that can be influenced by the RS methods used, vegetation type, and degree and nature of disturbance. To better understand these issues, we call for multi-scale field campaigns that test the effect of sampling scale, vegetation type, and degree of disturbance on the ability to detect different kinds of biodiversity, along with the development of improved models that incorporate both physical and biological principles as well as ecological and evolutionary theory. One goal of these studies would be to more closely match instrumentation and sampling scales to biological definitions of biodiversity and so improve optical diversity (spectral diversity) as a proxy for biodiversity. The ultimate goal would be to design and implement a truly effective, “scale-aware” global biodiversity monitoring system employing RS methods. Such a system could improve our understanding of the distribution and functional importance of biodiversity and enhance our ability to manage ecosystems for resilience and sustainability in a changing world.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Scale
- Scaling
- Spatial scale
- Temporal scale
- Spectral scale
- Angular scale
- Optical remote sensing
- Optical diversity
- Spectral diversity
- Biological diversity
16.1 Introduction
Biodiversity is critical to ecosystem function and provides many goods and services essential to human well-being (Hooper et al. 2012; Tilman et al. 2012). Despite centuries of effort, we lack a comprehensive account of global biodiversity, at a time the world is facing a sixth mass extinction due to human disturbance and climate change (Barnosky et al. 2011). Effective management of biological resources to preserve diversity and maintain ecosystem function in a rapidly changing world remains difficult, in part due to sampling challenges and lack of globally consistent data sets. Sampling biodiversity using traditional field methods alone simply cannot address this need, leading to recent calls for remote sensing (RS) as part of a global biodiversity monitoring system (Scholes et al. 2012; Pereira et al. 2013; Turner 2014; Jetz et al. 2016; Geller et al. Chap. 20).
In response to this need for a more complete accounting of biodiversity, there have been several recent attempts to define Essential Biodiversity Variables (EBVs), many of which involve RS (Pereira et al. 2013; Turner 2014; Vihervaara et al. 2017; Fernández et al., Chap. 18). However, most of the appeals for a global biodiversity monitoring system involving RS have not fully addressed the topic of how RS would be used or what aspects of biodiversity would be measured. A review of the literature on biodiversity assessment via RS reveals a wide array of methods and definitions of biodiversity (Table 16.1), most of which are strongly scale-dependent in the measurements and/or in the definitions of biodiversity. Many of these RS studies do not directly address standard biological metrics of species diversity (e.g., alpha or beta diversity; Whittaker 1972; see also Chap. 1), but may be indirectly related to biodiversity through characterization of habitat, dominant vegetation, or vegetation functional traits, some of which can, in principle, be captured with proposed EBVs (Pereira et al. 2013; Kissling et al. 2018), but often involve mismatches between sampling scales and the biodiversity variables being sampled.
With the advent of hyperspectral sensors and imaging spectrometers, a growing number of studies have utilized optical diversity, or the variability in vegetation optical properties (also called spectral diversity) to assess species diversity (typically alpha or beta diversity), or to address plant traits related to functional diversity. These methods offer the opportunity to directly detect species and functional diversity, but also require close attention to scale (Asner et al. 2015). In this chapter, our primary focus is on these latter RS methods involving optical RS, with the understanding that other RS methods can also make important contributions to our understanding of biodiversity.
16.1.1 Why a Chapter on Scale?
A key thesis of this chapter is that much of the uncertainty in the RS of biodiversity arises from the lack of attention to sampling scale, which affects both traditional biodiversity metrics and our ability to detect biodiversity remotely. In most cases, the sampling scales of typical satellite or airborne RS methods do not match those of our biological definitions or biodiversity measurements on the ground, confounding our interpretation of biodiversity from RS. In part because of scale mismatches, the interpretation of remotely sensed data from one time or place often cannot be applied to another, and we lack a universal, operational approach to RS of biodiversity. For RS of biodiversity to be meaningful, a careful consideration of scale is essential.
The purpose of this chapter is to address this need, with the goal of contributing to the design of an effective, operational global biodiversity monitoring system. Our primary focus is on optical diversity (a.k.a. “spectral diversity”) using passive optical RS in the visible to shortwave-infrared (VIS-SWIR) range (400–2500 nm) because this approach allows species and functional diversity assessment. However, we acknowledge that other methods, including lidar (Asner et al. 2012; Lausch et al., Chap. 13, this volume), can also make important contributions to our understanding of biodiversity. Our key examples involve optical studies of terrestrial vegetation primarily at the level of alpha and beta diversity (Whittaker 1972), with the underlying assumption that vegetation diversity may be related to the diversity of other trophic levels via surrogacy (Magurran 2004) or to belowground diversity via biogeochemical cycling (Madritch et al. 2014; Madritch et al., Chap. 8). Similarly, in aquatic environments optical diversity (often expressed as “ocean color”) can reveal dynamic structure related to the distribution of phytoplankton (Moses et al. 2016; Muller-Karger et al. 2018) and benthic organisms (Goodman and Ustin 2007), and scaling principles discussed here may apply in these cases. While a comprehensive assessment of all aspects of biodiversity in all environments is beyond the scope of this review, our hope is that the principles discussed here with a primary focus on terrestrial vegetation will enable progress toward an operational global biodiversity monitoring system involving RS.
16.1.2 What Is Optical (Spectral) Diversity?
Optical diversity can be defined and measured in many ways. It is often based on spectral reflectance of leaves and canopies, in which case the term “spectral diversity” is often applied. One definition is based on the number of different kinds of reflectance spectra (“spectral types,” “spectral species,” or “spectral signatures,” Fig. 16.1) present in a given area, a direct analogy to the biological concept of species diversity (Féret and Asner 2014). The categorical spectral type concept presumes distinct and stable spectral patterns exist for a given species. However, this is usually not the case, in part because species’ spectra are dynamic and in part because intraspecific spectral variation may approach or exceed that of interspecific variation, particularly when the full range of environmental conditions is expressed (Roth et al. 2015). For these reasons, the number of distinguishable spectral types does not always match the number of species in a given area.
Top panel: contrasting canopy reflectance spectra of boreal tree seedlings (Picea glauca, an evergreen conifer, and Populus tremuloides, a deciduous angiosperm) illustrating spectral regions (arrows) influenced by leaf traits (categorized here as pigments, biochemicals, water, and structural features). Also shown are examples of green, red, and near-infrared (NIR) bands to illustrate the more limited spectral coverage provided by most airborne cameras and many satellite sensors. Middle panel: mean (+/− SD) spectral for experimental tree plots of varying species richness (SR: 1, 2, 5, and 8). Species include Acer negundo, Fraxinus pennsylvanica, Picea glauca, Pinus contorta, Populus balsamifera, Populus tremuloides, Larix sibirica, and Prunus virginiana. Bottom panel: coefficient of variation (CV) spectra for the same plots. Inset: average CV for each level of species richness (1, 2, 5, and 8). (Data from canopy reflectance spectra sampled in Edmonton, Alberta, (summer 2013) using a full-range spectrometer (PSR 3500, Spectral Evolution, North Andover, MA, USA.). From (DeLancey 2014))
An alternate definition refers to the amount of spectral variability in a given area using statistical metrics of spectral information content, which can be measured many ways (Table 16.2). This concept has an early expression in the spectral variability hypothesis, which stated that variation in spectral characteristics scales with species richness (Palmer et al. 2000, 2002). In recent years, this concept has been further developed in many studies that explore the links between expressions of spectral variability and metrics of biodiversity, typically at the level of alpha or beta diversity (Baldeck et al. 2014; Féret and Asner 2014; Wang et al. 2016; Wang et al. 2018a; Gholizadeh et al. 2019).
Many spectral variability methods derive from information theory, which provides a rich array of methods for assessing the abstract “information content” or “entropy” in a given data set (Table 16.2). One simple method expresses spectral variation as the coefficient of variation (CV) spectrum for a given region, which can then be averaged into a single metric (Fig. 16.1). At this point, it is not entirely clear if there is a “best” method, because most of these methods work to some degree and their strength of correlation may vary with the circumstances (e.g., Gholizadeh et al. 2018).
The fundamental reason spectral patterns reveal underlying biological diversity is that plant reflectance spectra contain information on plant structure and chemical composition (Ustin and Jacquemoud, Chap. 14) that can differ slightly between species or functional types (Ustin and Gamon 2010) and can indicate different evolutionary histories (Schweiger et al. 2018; Meireles et al., Chap. 7, this volume). Thus, another way to utilize spectral information is to directly relate spectral patterns to plant functional traits (Serbin and Townsend, Chap. 3), providing a link between optical diversity and functional diversity (Cavender-Bares et al. 2017; Schweiger et al. 2018).
The topic of how to measure spectral diversity remains an active area of research, and the “best” metric is likely to vary depending upon the particular context and purpose. Rather than provide a detailed summary of all the possible methods used, we present the concept of spectral information content as a viable proxy for multiple dimensions of biodiversity at the species, functional, or genetic and phylogenetic levels (Cavender-Bares et al. 2017; Schweiger et al. 2018; Cavender-Bares et al., Chap. 2, this volume) and note that recent reviews (e.g., Wang and Gamon 2019) consider this topic in more detail.
16.2 What Is “Scale” and Why Is It Important?
“Scale” has several definitions and is used as both a noun and a verb. As a noun, it refers to a level of observation and can have several dimensions, including spatial, temporal, spectral, and biological. As a verb, it refers to the act of examining a phenomenon at multiple levels, usually referring to transcending spatial scales, as in “upscaling” (extrapolation from fine-scale data to a coarser scale) or “downscaling” (interpreting underlying patterns or mechanisms from coarse-scale data).
Discussions of scale have a rich history in both biology and RS. In biology, scale typically refers to levels of organization (genetic, cellular, organismal, species or population, community or ecosystem, etc.). Biodiversity can be defined at many of these levels, requiring different study approaches (Bonar et al. 2011). Biological systems typically exhibit complex, nonlinear interactions, and feedbacks, resulting in emergent properties and thus requiring evaluation across multiple levels of organization (Heffernan et al. 2014). Scale can also refer to sampling scale, as in the grain size and extent of field sampling (Turner et al. 1989). There is an abundant literature on the importance of considering scale when exploring ecological phenomena (Ehleringer and Field 1993; Levin 1992) and a number of “scaling rules” have emerged. For example, Levin (1992) stated the importance of matching the scale of observation to the phenomenon (and grain size or patch size) in question, and specific rules of sample grain size have been developed (e.g., O’Neill et al. 1996). However, these rules are often violated when remotely sensing biodiversity.
In RS, scale has several aspects or dimensions (Malenovský et al. 2007). The spatial dimension typically refers to the pixel size (grain size) and spatial extent of a remotely sensed image. The temporal dimension can refer to the time of sampling, repeat frequency, or temporal extent of sampling. The spectral dimension includes band position, bandwidth (full width half maximum, FWHM, and sampling interval), and range. The directional or angular dimension, including the illumination or viewing angle, leading to variations in anisotropic reflectance and the bidirectional reflectance distribution function (BRDF), is also an important scale dimension in optical RS, as it strongly influences the ability to detect signals present in reflectance spectra (Schaepman-Strub et al. 2006; Malenovský et al. 2007; Gamon 2015). These scale dimensions often interact, with the effects of one influencing the effects of another, so it is often best to consider multiple scale dimensions together. A consideration of sampling scale in all these dimensions is relevant to a discussion of RS of biodiversity. Sampling across scales often reveals critical information that is not apparent from a single-scale observation alone. Below, we consider these scale effects in more detail by providing examples of how these dimensions impact biodiversity assessment from RS.
16.2.1 Biological Scale
Biodiversity exists across multiple scales of organization from genes to biomes. A detailed discussion of the various “dimensions” of biodiversity at different scales of organization is beyond the scope of this chapter and has been reviewed elsewhere (e.g., Magurran 2004; see also Cavender-Bares et al., Chap. 2, this volume). In the context of this chapter, a key challenge lies in matching the scale of the measurement approach to the biological scale of organization, a topic considered in the sections below.
16.2.2 Spatial Scale
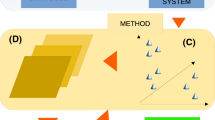
Optical instruments used for sampling biodiversity range from laboratory spectrometers and proximal field spectrometers to airborne and satellite-based imaging spectrometers, with grain sizes (pixel sizes) covering approximately six orders of magnitude (Fig. 16.2). If we include the molecular cross-section of DNA (e.g., when determining genetic diversity), our grain sizes span an even wider range, from roughly 2 nm (in the case of the DNA double helix; van Holde 1989) to roughly 1 km (for “Pando,” a large clonal aspen stand (DeWoody et al. 2008), a range of approximately 12 orders of magnitude). A rule of thumb for distinguishing spectral differences is that the sampling grain size should be smaller than the cross-section of the target (e.g., leaf or individual canopy crown in the case of individual plants; Woodcock and Strahler 1987). These numbers imply that the sampling grain size needs to span an extremely wide range to properly match all our definitions of biodiversity. Clearly, this is not possible from a single instrument, but can be considered in multi-scale field campaigns employing multiple instruments and platforms. Additional challenges arise when designing a field campaign to validate remotely sensed biodiversity data. Many field sampling methods for species richness entail quadrat or transect sampling (Bonar et al. 2011), neither of which is well-matched to the size, shape, and location of typical airborne or satellite pixels. Moreover, our classical definitions of biodiversity at different scales (alpha, beta, or gamma) reflect relative rather than absolute spatial scales and often poorly match the scale of both field sampling and RS. These challenges of scale mismatch abound and require careful attention to definitions and sampling protocols.
Schematic of a proposed, multi-scale, global biodiversity monitoring system. Satellite imaging spectrometry would provide the context for understanding patterns in time and space, and regional and proximal sampling would provide sampling at progressively finer scales. This design would be replicated systematically around the world, using field sampling plots for different biomes (indicated by parallelograms). Note that spatial scale (sampling grain size, typically measured as optical cross-section or pixel size) associated with various optical sampling methods span roughly six orders of magnitude
Similar issues of scale mismatch arise when exploring plant traits with RS. It is unclear how well the ability to detect leaf traits transcends spatial scales, with some studies suggesting certain leaf traits (nitrogen content) cannot be detected at the sampling scale of a typical aircraft or satellite pixel (Knyazikhin et al. 2013). However, many leaf traits (if not all) can be detected at the canopy scale (i.e., the scale of an individual plant crown), particularly when appropriate sampling and scaling methods are followed, suggesting that scale-appropriate RS methods can often resolve trait differences associated with functional or species diversity (Townsend et al. 2013; Asner et al. 2015). This is particularly relevant to airborne data, where pixel sizes often approximate those of individual tree crowns, allowing individual traits to be distinguished (Asner et al. 2015; Singh et al. 2015), but calls into question the accuracy of trait retrievals when the pixel sizes are much larger than individual canopies, as is the case for most satellite RS.
Not surprisingly, a review of the recent literature suggests strong effects of spatial scale on the ability to detect biodiversity using optical RS. Most of this research has been conducted in North American prairie, with relatively short-statured vegetation, so may be biased by the relatively small plant crown sizes (roughly 10 cm). One set of studies, conducted using tallgrass prairie species at the Cedar Creek Ecosystem Science Reserve in Minnesota (USA), used pixel sizes ranging from 1 mm (sampled on the ground) to 1 m (sampled from aircraft) and found a significant correlation between optical diversity and alpha diversity at finer scales (1 mm to 10 cm), but most of the information on alpha diversity was lost at pixel sizes larger than about 10 cm, roughly the size of many plant crowns (Fig. 16.3, red line). Another study conducted at Wood River in Nebraska (USA) using airborne data found a strong relationship (R2) between optical diversity and alpha diversity for prairie species at pixel sizes of 0.5–1 m and noted that the optical diversity-alpha diversity relationship was markedly weakened at progressively larger spatial scales (up to 6 m) (Fig. 16.3, black line). A third study, conducted at Mattheis Ranch in southern Alberta (Canada), found an intermediate relationship between the two other prairie sites (Fig. 16.3, blue square).
Scale dependence of the optical diversity-biodiversity relationship from three experimental studies in prairie grasslands: Cedar Creek, Minnesota, USA (red), Wood River, Nebraska, USA (black), and Mattheis Ranch, Alberta, Canada (blue). (Data combined from Wang et al. (2016) and Gholizadeh et al. (2018, 2019))
These studies have significant implications for attempts to sample optical diversity from aircraft or satellite sensors and illustrate the importance of matching sampling scale (pixel size) to crown size when designing airborne campaigns. Clearly, there is a strong scale-dependence of the optical diversity-biodiversity relationship, but this scale-dependence varies between study sites, even for the same biome (prairie grassland, in this case). These site differences have been attributed to a number of possible factors, including the degree of disturbance (fire regimes, invasion by exotic weeds, or subsequent weed removal), the size of the plot (sampling extent) relative to the pixel (grain) size, and the differences in species richness between studies. The Wood River site was less disturbed, with less bare ground and larger, more species-rich plots than the Cedar Creek site (Gholizadeh et al. 2019). It is likely that multiple features influence the scale dependence of the optical diversity-biodiversity relationship, illustrating that the larger context and experimental design of a study can matter.
We know less about the scale dependence of these relationships for other biomes where explicit scaling experiments involving the RS of biodiversity have not yet been conducted. Despite unanswered questions, these experiments in prairie ecosystems demonstrate the importance of spatial scale and support the idea that canopy crown size is an important factor in scale dependence. These findings have clear implications for studies using airborne and satellite platforms where the pixel sizes often exceed the crown sizes of common plant species.
16.2.3 Temporal Scale
The temporal dimension is rarely considered in most RS campaigns; many RS studies are based on a single overpass (e.g., a single aircraft image or satellite image), or at best a few overpasses, limiting the opportunities for examining temporal effects. A typical field campaign might focus on an optimal time to collect data from a RS perspective (e.g., the dry season in the tropics or the summer growing season in higher latitudes). However, biological communities are inherently dynamic, and the visibility of different species changes with ontogeny, season, and over longer time spans due to disturbance, invasion, succession, climate change, and other processes. Consequently, our ability to detect species richness will vary with time, often in ways that are poorly understood. The few studies that have investigated the temporal aspect of biodiversity using RS or spectral reflectance demonstrate the importance of temporal dynamics when examining the optical diversity-biodiversity links.
A study of California chaparral (Zutta 2003) found a clear seasonal dependence in the ability to distinguish plant functional types using reflectance spectra (Fig. 16.4a). Different methods involving contrasting spectral indices and bands yielded distinct seasonal patterns, indicating the importance of the spectral dimension (further discussed below) and illustrating interactions between temporal and spectral dimensions. In that study, photosynthetic and flowering phenology contributed to the seasonal patterns observed. Similarly, when evaluating several functional leaf traits with spectral reflectance in tropical species, Chavana-Bryant et al. (2017) found a clear seasonal effect on the trait retrievals using PLSR, again emphasizing an interaction between temporal and spectral dimensions. A study of optical diversity for prairie species also revealed strong phenological effects that were different at the leaf and canopy scales, demonstrating an interaction between temporal and spatial scale (Fig. 16.4b). Clearly, the temporal dimension should be considered in any study of the RS of biodiversity, yet most studies have been limited to a single time frame, limiting the power to distinguish biodiversity. These examples also demonstrate that measurement technique (e.g., instrument foreoptics) and interacting scale effects can influence optical diversity.
(a) Ability (% accuracy) of spectral variability to distinguish plant phenological types (evergreen, winter deciduous, drought deciduous, and annual) in California chaparral (Santa Monica Mountains, California, USA, Dec 1998–Sept 1999). Input variables include reflectance at all wavelengths (450–1000 nm), three physiological indices derived from reflectance (Photochemical Reflectance Index, PRI; Water Band Index WBI, and Normalized Difference Vegetation Index, NDVI) and indices derived from the coefficients produced in discriminant analysis. (From Zutta (2003).) (b) Phenology of optical diversity (convex hull area in spectral space) at the leaf (black) and canopy (red) scale for prairie vegetation sampled at the Cedar Creek Ecosystem Science Reserve, Minnesota, USA, in summer 2014. Leaf-scale data sampled with a leaf clip and canopy-scale data sampled with a straight fiber yielding a field-of view of approximately 10 cm diameter. Canopy data are available as “Phenology Canopy Spectra Big Biodiversity Experiment Cedar Creek LTER 2014” on EcoSIS (doi: https://doi.org/10.21232/C2Z070)
16.2.4 Spectral Scale
The advent of hyperspectral sensors, both imaging and nonimaging, provides rich opportunities for exploring spectral features related to biodiversity. Approaches range from detection of species or functional traits to methods based on the information content of the spectra themselves (Fig. 16.1; Table 16.2). All of these methods require attention to spectral scale, including spectral resolution and range, which influence biodiversity detection in complex ways. Furthermore, our methods of analysis, ranging from simple vegetation indices to more complex full-spectral statistical methods (Table 16.2), explore the spectral dimension in different ways and to varying degrees. To date, relatively few studies have explicitly addressed spectral scale in the context of biodiversity detection, but most show that more spectral information is generally better than less (e.g., Asner et al. 2012). Consequently, hyperspectral sensors are more informative than multiband sensors, and full-range (VIS-SWIR) detectors are usually more useful than limited range (e.g., VIS-NIR) detectors for detecting plant traits or biodiversity. The importance of spectral scale in biodiversity detection can be readily seen when comparing multiband data (measured from a drone) to hyperspectral data (measured from a tram system) for Cedar Creek; in this case, multiband drone imagery failed to detect different alpha diversity levels, despite pixel sizes (2.3 cm) that were intermediate between those of the hyperspectral sensor (Fig. 16.5).
Effect of spatial and spectral scale on the relationship between optical diversity (measured as the coefficient of variation) and Simpson Index, a measure of alpha diversity combining species richness and evenness (Simpson 1949). Data collected by a hyperspectral sensor on a tram, sampled at two resolutions (1 cm = red dots, 10 cm = blue dots) (for methods see Wang et al. 2018a) and a multispectral sensor on a drone (resolution = 2.3 cm, black circles) (Parrot Sequoia, Parrot Drones, Paris, France). All data measured at the Cedar Creek Ecosystem Science Reserve
A number of studies have demonstrated that some wavebands provide more information than others, and this information can vary seasonally (Zutta 2003; Chavana-Bryant et al. 2017; see also Fig. 16.4). Similarly, visible bands (revealing pigment composition) and NIR bands (revealing structural information) respond differently to spatial scale (Wang et al. 2018b) or sampling angle (Gamon 2015) illustrating the functionally distinct responses of these different spectral regions (Fig. 16.1) and providing further evidence of interactions between the spectral, spatial, and angular dimensions of scale.
16.2.5 Angular Scale
Illumination and view angle both interact with vegetation structure to influence the shape and intensity (brightness) of reflectance spectra. While these effects have been well-studied in RS and can be characterized by the BRDF for a particular surface (Malenovský et al. 2007), angular information has generally not been employed in detection of biodiversity. A few studies have noted that the BRDF response can help distinguish vegetation types (Gamon et al. 2004), suggesting that angular information may be useful in biodiversity detection. Sensor view angle affects different spectral regions differently, illustrating an interaction between angular scale and spectral scale (Gamon 2015). With the advent of lidar (Asner et al. 2012) and structure-from-motion (SFM; Wallace et al. 2016) to characterize plant 3-D structure, angular information can now be better understood and effectively integrated with optical RS to improve biodiversity or plant trait detection.
16.3 Implementing Scaling Approaches
Properly addressing scale effects in biodiversity detection requires explicit attention to scale in all its dimensions and designing a study approach that transcends scale limitations. Scaling methods can be empirical or theoretical. Typical empirical methods involve a multi-scale sampling strategy, often using multiple RS instruments operating at different scales, along with traditional field sampling for validation. Often, a goal of such field campaigns is to aggregate fine-scale data to be used as validation for coarse-scale data (e.g., Cohen and Justice 1999; Wehlage et al. 2016), yet aggregation tends to obscure spectral variability at the scale of individual leaves or plant canopies and undercuts the goal of detecting local (alpha) biodiversity with optical diversity. On the other hand, sampling at progressively larger scales often involves transitions from local (alpha) to regional (beta) diversity, creating the opposite effect of increasing optical diversity with increasing pixel size, and these transitions can themselves be scale-dependent and vary with vegetation type (Fig. 16.6).
Optical diversity, expressed as coefficient of variation (CV) derived from reflectance spectra, along a transect (yellow line, top panel) crossing forest and prairie communities at the Cedar Creek Ecosystem Science Reserve, Minnesota. This image cube (top panel) was collected on July 22, 2016, using an imaging spectrometer (AISA Eagle, Specim, Oulu, Finland) mounted on a fixed-wing aircraft (Piper Saratoga, Piper Aircraft, Vero Beach, Florida, USA) operated by the University of Nebraska Center for Advanced Land Management Information Technologies (CALMIT) Hyperspectral Airborne Monitoring Program (CHAMP). Images were collected from a height of approximately 1435 m and a speed of approximately 177 km/h, providing a ground pixel size of approximately 1 m2. The imaging spectrometer provided hyperspectral images covering 400–970 nm with 10 nm spectral resolution (FWHM). Airborne data were collected and preprocessed by Rick Perk from CALMIT. A–G indicate particular points of interest, including transition points between communities (A, C, E, grassland (B), forest (G), and the Cedar Creek biodiversity experiment (F). CV has been calculated at various pixel sizes (3 m × 3 m to 96 m × 96 m); bottom panels to illustrate the effect of spatial resolution on retrieved optical diversity, illustrating a general increase in CV with pixel size, but this pattern varies between woodland (G) and natural (B) vs. experimental (F) grassland plots
The complexity of scaling effects involving the transition from alpha to beta diversity is briefly illustrated in Fig. 16.6, which illustrates optical diversity (CV in this case) calculated for different regions along a transect crossing several plant communities, including woodland, grassland, and experimental grassland plots at the Cedar Creek Ecosystem Science Reserve, Minnesota (USA). In this case, the highest optical diversity values occur at edges, points of abrupt landscape transitions marking the boundaries between adjacent communities (i.e., ecotones), an effect commonly seen in remotely sensed images (Paz-Kagan et al. 2017). In this complex landscape, (and in contrast to the plot-level patterns shown in Fig. 16.3), CV generally increases with spatial scale, reflecting a transition from alpha to beta diversity with increasing spatial lag (pixel sizes). Interestingly, this transition occurs at about 10 m × 10 m in the manipulated grassland (see arrow and blue curve, Fig. 16.6), matching the plot sizes in this experiment, but occurs more gradually in the natural grassland (black curve, Fig. 16.6). These patterns seem to contradict findings of declining optical diversity in grasslands with increasing pixel size obtained at finer scales (1 mm to 1 m in Wang et al. 2018a) (see also Fig. 16.3). This comparison illustrates that the patterns of scale dependence are themselves scale dependent and can differ within the same landscape or for different communities depending upon both individual crown size and larger landscape structure. These observations agree with a body of RS literature that show wide variation in patterns of scale dependency for different landscapes using geostatistical approaches, with the local variance a function of the relative sizes of the pixels and the discrete targets themselves (e.g., Woodcock and Strahler 1987). For these reasons, the spatial aggregation approaches often used in other fields (e.g., plant productivity) may actually confound the detection of optical diversity at certain scales. Consequently, optical diversity (alpha or beta diversity) or variation in plant traits from RS requires particular attention to scaling methodologies (e.g., Asner et al. 2015).
By providing both fine-scale patterns (Fig. 16.3) and the larger context of landscape structure, imaging spectroscopy (Fig. 16.6) provides a valuable tool for further, more detailed studies, and can help define the proper scale at which alpha and beta diversity can be most effectively sampled. Remotely sensed data can also be used to define patterns of geodiversity, the physical template influencing biodiversity (Record et al., Chap. 10, this volume). Geostatistical approaches that examine optical diversity as a function of distance, analogous to the use of semivariograms (Curran 1988), can help design appropriate sampling methodologies by illustrating the influence of landscape features (e.g., ecotones or different vegetation types) on optical diversity (Fig. 16.6). Imaging spectrometry can also reveal temporal dynamics and disturbance patterns that can provide additional context for understanding both the drivers and the consequences of biodiversity changes. Furthermore, image spectrometry can be used to assess the relationships between optical diversity and ecosystem function (e.g., productivity) over large areas (Wang et al. 2016; Schweiger et al. 2018). For these reasons, satellite and airborne RS can be a powerful complement to local and regional field studies of biodiversity.
Other approaches to scaling involve the use of models for upscaling or downscaling, including radiative transfer models and statistical models (Knyazikhin et al. 2013; Malenovský et al. 2019; Verrelst et al. 2019). While potentially powerful, such models are generally limited by the lack of suitable data, bringing us back to the need for experimental studies incorporating empirical methods. Ultimately, a combined empirical and theoretical framework that explicitly considers multiple dimensions of scale is needed to advance our knowledge of biodiversity detection with RS.
16.4 Designing a Scale-Aware Biodiversity Monitoring System
To be truly robust, a global biodiversity monitoring system will need to consider scale in all the dimensions discussed here. Biological concepts (e.g., grain size and extent) often have a direct analog to RS concepts (e.g., pixel size and extent). Ideally, we should design our instrument and optical sampling protocols to match our sampling scale to a particular organizational scale of biodiversity. While specific sampling rules have been proposed (e.g., Justice and Townshend 1981), the general rule of thumb that the pixel size must be smaller than the size of the individual sampling unit (e.g., typical plant canopy) is a good starting point, and this has been supported by studies discussed above (see Fig. 16.3).
However, matching instrument to organizational scale remains a challenge; we typically have a poor match between the pixel size and sampling extent and the organism size and distribution on the ground, often due to the practical constraints of field sampling (in the case of biological studies) and instrument design (in the case of RS). Most remote sensing instruments are designed for a particular airborne or spaceborne platform with physics and engineering requirements in mind. The detector response is constrained by the amount of electromagnetic energy available, which in turn determines the sensor design, pixel size, and spectral resolution needed to achieve a given signal-to-noise ratio. Greater signal-to-noise ratios can be attained by reducing the spectral resolution (combining narrow bands into broadbands, e.g., via spectral binning), or by reducing spatial resolution (e.g., pixel binning), but these choices limit the ability to distinguish individuals, species, and functional types due to the degradation of spectral and spatial information. Orbital and altitudinal considerations also determine the pixel size obtainable from a particular sensor platform. Together, these constraints often reduce the ability to properly distinguish individual organisms or vegetation functional types. Adding to this mismatch, field sampling (including plot size, transect size, and location) is often limited by practical considerations of personnel, time, and budget and is rarely designed with RS in mind.
Improvements in sensor design and novel sampling platforms can relax these impediments, but with trade-offs. For example, flexible airborne platforms, emerging unmanned aerial vehicles (UAV) systems, or robotic ground-based systems (Wang et al. 2018a, b) provide useful platforms for testing the effects of spatial scale, but may not always have the temporal or spectral coverage desired for biodiversity detection (Fig. 16.5). Plans to deploy global satellite imaging spectrometers with frequent repeat visits (Schimel et al., Chap. 19) offer new ways to explore the temporal dimension with high spectral resolution and wide spectral range, although at relatively coarse spatial resolution. Consequently, a key application of satellite RS can be to provide the larger context within which effective sampling regimes can then be defined at finer scales.
Due to these inherent limitations and trade-offs between scale dimensions, we suggest that the ideal global monitoring system would be an integrated, multi-component system, combining RS at different scales (satellite and aircraft sensors) with proximal sensing (ground optical sensors and field sampling of biodiversity) (Fig. 16.2). Such a system would operate within a clearly defined scaling framework, incorporating empirical and modeling approaches, with explicit attention to sampling scale in each of the dimensions mentioned above. Although rarely used in RS, explicit experimental approaches involving cross-scale and cross-instrument comparisons should be a key capability of an effective global biodiversity monitoring system. With a multi-scale system, it would be possible to express results (e.g., spectral variability) as a function of sampling scale (pixel or grain size; see Figs. 16.3 and 16.6), much in the way that semivariograms are used to express landscape structure (Curran 1988). These patterns could be compared to the driving variables, including the underling patterns of “geodiversity,” (Record et al., Chap. 10, this volume). Such a system would reveal ideal spatial scales for sampling alpha and beta diversity and would help reveal the causes of biodiversity patterns at multiple scales, as illustrated in Figs. 16.3 and 16.6.
Such a scale-aware global biodiversity monitoring system could incorporate and integrate many aspects of existing networks such as NEON (Hopkin 2006), Forest GEO (Anderson-Teixeira et al. 2014), and many others (see Geller et al., Chap. 20), but would provide many benefits currently not provided by existing biodiversity monitoring efforts. The system would require global imaging spectrometry with repeat coverage, revealing global patterns in time and space and providing essential context for more detailed studies at higher spatial resolution (see Schimel et al. Chap. 19). More detailed resolution could be achieved by a fleet of regional aircraft carrying imaging spectrometers at a spatial resolution matching the crown sizes of many shrub and tree species (e.g., Kampe et al. 2010). For even more detailed sampling resolution needed for smaller statured vegetation or for resolving individual leaf traits, UAVs, robotic, or tower-mounted imaging spectrometers could be deployed at key sites. These methods would be coupled to systematic ground sampling of species composition (alpha and beta diversity) using traditional field sampling methods, along with leaf and canopy optical properties (using field spectrometry) for a detailed assessment of plant traits. Radiative transfer models and statistical scaling methods (Serbin and Townsend, Chap. 3) could provide a framework for integrating and analyzing data across scales.
A systematic global evaluation of optical diversity across multiple scales could readily detect dynamics in biodiversity, identify causes biodiversity changes, adapt to these changes, and help to identify monitoring and conservation priorities. By integrating diversity metrics with measures of ecosystem function, our understanding of the ecosystem impacts of biodiversity would be enhanced, allowing better management for resilience. On our rapidly changing planet, such a system would enable the monitoring required for sustainable management of ecosystems globally.
References
Anderson-Teixeira KJ et al (2014) CTFS-ForestGEO: a worldwide network monitoring forests in an era of global change. Glob Chang Biol 21(2):528–549
Asner GP, Martin RE (2009) Airborne spectranomics: mapping canopy chemical and taxonomic diversity in tropical forests. Front Ecol Environ 7:269–276. https://doi.org/10.1890/070152
Asner GP et al (2012) Carnegie Airborne Observatory-2: increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens Environ 124:454–465. https://doi.org/10.1016/j.rse.2012.06.012
Asner GP, Martin RE, Anderson CB, Knapp DE (2015) Quantifying forest canopy traits: imaging spectroscopy versus field survey. Remote Sens Environ 158:15–27. https://doi.org/10.1016/j.rse.2014.11.011
Baldeck CA, Colgan MS, Feret JB, Levicx SR, Martin RE, Asner GP (2014) Landscape-scale variation in plant community composition of an African savanna from airborne species mapping. Ecol Appl 24:84–93. https://doi.org/10.1890/13-0307.1
Barnosky AD et al (2011) Has the Earth’s sixth mass extinction already arrived? Nature 471:51–57. https://doi.org/10.1038/nature09678
Bonar S, Fehmi J, Mercado-Silva N (2011) An overview of sampling issues in species diversity and abundance surveys. In: Magurran AE, McGill BJ (eds) Biological diversity: Frontiers in measurement and assessment. Oxford University Press, Oxford, England, p 376
Cavender-Bares J, Gamon JA, Hobbie SE, Madritch MD, Meireles JE, Schweiger AK, Townsend PA (2017) Harnessing plant spectra to integrate the biodiversity sciences across biological and spatial scales. Am J Bot 104:966–969. https://doi.org/10.3732/ajb.1700061
Chadwick KD, Asner GP (2016) Organismic-scale remote sensing of canopy foliar traits in lowland tropical forests. Remote Sens 8. https://doi.org/10.3390/rs8020087
Chavana-Bryant C et al (2017) Leaf aging of Amazonian canopy trees as revealed by spectral and physiochemical measurements. New Phytol 214:1049–1063. https://doi.org/10.1111/nph.13853
Cohen WB, Justice CO (1999) Validating MODIS terrestrial ecology products: linking in situ and satellite measurements. Remote Sens Environ 70:1–3. https://doi.org/10.1016/s0034-4257(99)00053-x
Curran PJ (1988) The semivariogram in remote sensing: an introduction. Remote Sens Environ 24:493–507. https://doi.org/10.1016/0034-4257(88)90021-1
Dahlin KM (2016) Spectral diversity area relationships for assessing biodiversity in a wildland-agriculture matrix. Ecol Appl 26:2756–2766. https://doi.org/10.1002/eap.1390
DeLancey E (2014) Hyperspectal remote sensing of boreal forest tress diversity at multiple scales. Master’s Thesis, University of Alberta
DeWoody J, Rowe CA, Hipkins VD, Mock KE (2008) “Pando” lives: molecular genetic evidence of a giant aspen clone in Central Utah. West N Am Nat 68:493–497. https://doi.org/10.3398/1527-0904-68.4.493
Ehleringer JR, Field CB (1993) Scaling physiological processes: leaf to globe, 1st edn. Academic Press, New York
Fairbanks DHK, McGwire KC (2004) Patterns of floristic richness in vegetation communities of California: regional scale analysis with multi-temporal NDVI. Glob Ecol Biogeogr 13:221–235. https://doi.org/10.1111/j.1466-822X.2004.00092.x
Fava F, Parolo G, Colombo R, Gusmeroli F, Della Marianna G, Monteiro AT, Bocchi S (2010) Fine-scale assessment of hay meadow productivity and plant diversity in the European Alps using field spectrometric data. Agric Ecosyst Environ 137:151–157. https://doi.org/10.1016/j.agee.2010.01.016
Féret JB, Asner GP (2014) Mapping tropical forest canopy diversity using high-fidelity imaging spectroscopy. Ecol Appl 24:1289–1296
Franklin SE, Ahmed OS (2018) Deciduous tree species classification using object-based analysis and machine learning with unmanned aerial vehicle multispectral data. Int J Remote Sens 39:5236–5245. https://doi.org/10.1080/01431161.2017.1363442
Gaitán JJ et al (2013) Evaluating the performance of multiple remote sensing indices to predict the spatial variability of ecosystem structure and functioning in Patagonian steppes. Ecol Indic 34:181–191. https://doi.org/10.1016/j.ecolind.2013.05.007
Gamon JA (2015) Reviews and syntheses: optical sampling of the flux tower footprint. Biogeosciences 12:4509–4523. https://doi.org/10.5194/bg-12-4509-2015
Gamon JA et al (2004) Remote sensing in BOREAS: lessons leamed. Remote Sens Environ 89:139–162. https://doi.org/10.1016/j.rse.2003.08.017
Gholizadeh H, Gamon JA, Zygielbaum AI, Wang R, Schweiger AK, Cavender-Bares J (2018) Remote sensing of biodiversity: soil correction and data dimension reduction methods improve assessment of alpha-diversity (species richness) in prairie ecosystems. Remote Sens Environ 206:240–253. https://doi.org/10.1016/j.rse.2017.12.014
Gholizadeh H et al (2019) Detecting prairie biodiversity with airborne remote sensing. Remote Sens Environ 221:38–49. https://doi.org/10.1016/j.rse.2018.10.037
Goodman JA, Ustin SL (2007) Classification of benthic composition in a coral reef environment using spectral unmixing. J Appl Remote Sens 1:011501. https://doi.org/10.1117/1.2815907
Gould W (2000) Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecol Appl 10:1861–1870. https://doi.org/10.2307/2641244
Heffernan JB et al (2014) Macrosystems ecology: understanding ecological patterns and processes at continental scales. Front Ecol Environ 12:5–14. https://doi.org/10.1890/130017
Hooper DU et al (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108. https://doi.org/10.1038/nature11118
Hopkin M (2006) Spying on nature. Nature 444:420–421
Jetz W et al (2016) Monitoring plant functional diversity from space. Nature Plants 2. https://doi.org/10.1038/nplants.2016.24
Justice CO, Townshend JRG (1981) Integrating ground data with remote sensing. In: Townshend JRG (ed) Terrain analysis and remote sensing. Allen and Unwin, London, pp 38–101
Kampe TU, Johnson BR, Kuester M, Keller M (2010) NEON: the first continental-scale ecological observatory with airborne remote sensing of vegetation canopy biochemistry and structure. J Appl Remote Sens 4:043510. https://doi.org/10.1117/1.3361375
Kerr JT, Southwood TRE, Cihlar J (2001) Remotely sensed habitat diversity predicts butterfly species richness and community similarity in Canada. Proc Natl Acad Sci U S A 98:11365–11370. https://doi.org/10.1073/pnas.201398398
Kissling WD et al (2018) Towards global data products of Essential Biodiversity Variables on species traits. Nat Ecol Evol 2:1531–1540. https://doi.org/10.1038/s41559-018-0667-3
Knyazikhin Y et al (2013) Hyperspectral remote sensing of foliar nitrogen content. Proc Natl Acad Sci U S A 110:E185–E192. https://doi.org/10.1073/pnas.1210196109
Kruse FA, Lefkoff AB, Boardman JW, Heidebrecht KB, Shapiro AT, Barloon PJ, Goetz AFH (1993) The spectral image processing system (SIPS) - interactive visualization and analysis of imaging spectrometer data. Remote Sens Environ 44:145–163. https://doi.org/10.1016/0034-4257(93)90013-n
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967. https://doi.org/10.2307/1941447
Lucas KL, Carter GA (2008) The use of hyperspectral remote sensing to assess vascular plant species richness on Horn Island, Mississippi. Remote Sens Environ 112:3908–3915. https://doi.org/10.1016/j.rse.2008.06.009
Madritch MD, Kingdon CC, Singh A, Mock KE, Lindroth RL, Townsend PA (2014) Imaging spectroscopy links aspen genotype with below-ground processes at landscape scales. Philos Trans R Soc B Biol Sci 369:20130194. https://doi.org/10.1098/rstb.2013.0194
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
Malenovský Z, Bartholomeus HM, Acerbi-Junior FW, Schopfer JT, Painter TH, Epema GF, Bregt AK (2007) Scaling dimensions in spectroscopy of soil and vegetation. Int J Appl Earth Obs Geoinf 9:137–164. https://doi.org/10.1016/j.jag.2006.08.003
Malenovský Z et al. (2019) Variability and uncertainty challenges in upscaling imaging spectroscopy observations from leaves to vegetation canopies Surv Geophys 40:631–656
Moses WJ, Ackleson SG, Hair JW, Hostetler CA, Miller WD (2016) Spatial scales of optical variability in the coastal ocean: implications for remote sensing and in situ sampling. J Geophys Res Oceans 121:4194–4208. https://doi.org/10.1002/2016jc011767
Muller-Karger FE et al (2018) Satellite sensor requirements for monitoring essential biodiversity variables of coastal ecosystems. Ecol Appl 28:749–760. https://doi.org/10.1002/eap.1682
Nagendra H, Lucas R, Honrado JP, Jongman RHG, Tarantino C, Adamo M, Mairota P (2013) Remote sensing for conservation monitoring: assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol Indic 33:45–59. https://doi.org/10.1016/j.ecolind.2012.09.014
O’Neill RV, Hunsaker CT, Timmins SP, Jackson BL, Jones KB, Riitters KH, Wickham JD (1996) Scale problems in reporting landscape pattern at the regional scale. Landsc Ecol 11:169–180. https://doi.org/10.1007/bf02447515
Oldeland J, Wesuls D, Rocchini D, Schmidt M, Jurgens N (2010) Does using species abundance data improve estimates of species diversity from remotely sensed spectral heterogeneity? Ecol Indic 10:390–396. https://doi.org/10.1016/j.ecolind.2009.07.012
Palmer MW, Wohlgemuth T, Earls PG, Arévalo JR, Thompson SD (2000) Opportunities for long-term ecological research at the Tallgrass Prairie Preserve, Oklahoma. In: Lajtha K, Vanderbilt K (eds) Cooperation in long term ecological research in central and eastern Europe: proceedings of the ILTER Regional Workshop, Budapest, Hungary, 22–25 June 1999. Oregon State University, Corvallis, pp 123–128
Palmer MW, Earls PG, Hoagland BW, White PS, Wohlgemuth T (2002) Quantitative tools for perfecting species lists. Environmetrics 13:121–137. https://doi.org/10.1002/env.516
Paz-Kagan T, Caras T, Herrmann I, Shachak M, Karnieli A (2017) Multiscale mapping of species diversity under changed land use using imaging spectroscopy. Ecol Appl 27:1466–1484. https://doi.org/10.1002/eap.1540
Pereira HM et al (2013) Essential biodiversity variables. Science 339:277–278. https://doi.org/10.1126/science.1229931
Psomas A, Kneubuhler M, Huber S, Itten K, Zimmermann NE (2011) Hyperspectral remote sensing for estimating aboveground biomass and for exploring species richness patterns of grassland habitats. Int J Remote Sens 32:9007–9031. https://doi.org/10.1080/01431161.2010.532172
Rey-Benayas JM, Pope KO (1995) Landscape ecology and diversity patterns in the seasonal tropics from Landsat TM imagery. Ecol Appl 5:386–394. https://doi.org/10.2307/1942029
Rocchini D, McGlinn D, Ricotta C, Neteler M, Wohlgemuth T (2011) Landscape complexity and spatial scale influence the relationship between remotely sensed spectral diversity and survey-based plant species richness. J Vegetation Sci 22:688–698. https://doi.org/10.1111/j.1654-1103.2010.01250.x
Roth KL, Roberts DA, Dennison PE, Alonzo M, Peterson SH, Beland M (2015) Differentiating plant species within and across diverse ecosystems with imaging spectroscopy. Remote Sens Environ 167:135–151. https://doi.org/10.1016/j.rse.2015.05.007
Schaepman-Strub G, Schaepman ME, Painter TH, Dangel S, Martonchik JV (2006) Reflectance quantities in optical remote sensing-definitions and case studies. Remote Sens Environ 103:27–42. https://doi.org/10.1016/j.rse.2006.03.002
Schäfer E, Heiskanen J, Heikinheimo V, Pellikka P (2016) Mapping tree species diversity of a tropical montane forest by unsupervised clustering of airborne imaging spectroscopy data. Ecol Indic 64:49–58. https://doi.org/10.1016/j.ecolind.2015.12.026
Scholes RJ et al (2012) Building a global observing system for biodiversity. Curr Opin Environ Sustain 4:139–146. https://doi.org/10.1016/j.cosust.2011.12.005
Schweiger AK et al (2018) Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat Ecol Evol 2:976–982. https://doi.org/10.1038/s41559-018-0551-1
Simpson EH (1949) Measurement of diversity. Nature 163:688–688. https://doi.org/10.1038/163688a0
Singh A, Serbin SP, McNeil BE, Kingdon CC, Townsend PA (2015) Imaging spectroscopy algorithms for mapping canopy foliar chemical and morphological traits and their uncertainties. Ecol Appl 25:2180–2197. https://doi.org/10.1890/14-2098.1.sm
Somers B, Asner GP, Martin RE, Anderson CB, Knapp DE, Wright SJ, Van De Kerchove R (2015) Mesoscale assessment of changes in tropical tree species richness across a bioclimatic gradient in Panama using airborne imaging spectroscopy. Remote Sens Environ 167:111–120. https://doi.org/10.1016/j.rse.2015.04.016
Tilman D, Reich PB, Isbell F (2012) Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc Natl Acad Sci U S A 109:10394–10397. https://doi.org/10.1073/pnas.1208240109
Townsend PA, Serbin SP, Kruger EL, Gamon JA (2013) Disentangling the contribution of biological and physical properties of leaves and canopies in imaging spectroscopy data. Proc Natl Acad Sci U S A 110:E1074. https://doi.org/10.1073/pnas.1300952110
Tuanmu MN, Jetz W (2015) A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modelling. Glob Ecol Biogeogr 24:1329–1339. https://doi.org/10.1111/geb.12365
Turner W (2014) Sensing biodiversity. Science 346:301–302. https://doi.org/10.1126/science.1256014
Turner MG, Dale VH, Gardner RH (1989) Predicting across scales: theory development and testing. Landsc Ecol 3:245–252. https://doi.org/10.1007/bf00131542
Ustin SL, Gamon JA (2010) Remote sensing of plant functional types. New Phytol 186:795–816. https://doi.org/10.1111/j.1469-8137.2010.03284.x
van Holde KE (1989) Chromatin. Springer, New York
Verrelst J et al (2019) Quantifying vegetation biophysical variables from imaging spectroscopy data: a review on retrieval methods. Surv Geophys 40:589–629. https://doi.org/10.1007/s10712-018-9478-y
Vihervaara P et al (2017) How Essential Biodiversity Variables and remote sensing can help national biodiversity monitoring. Glob Ecol Conserv 10:43–59. https://doi.org/10.1016/j.gecco.2017.01.007
Wallace L, Lucieer A, Malenovsky Z, Turner D, Vopenka P (2016) Assessment of forest structure using two UAV techniques: a comparison of airborne laser scanning and structure from motion (SfM) point clouds. Forests 7. https://doi.org/10.3390/f7030062
Wang R, Gamon JA (2019) Remote sensing of biodiversity. Remote Sens Environ 231:111218 https://doi.org/10.1016/j.rse.2019.111218
Wang L, Sousa WP, Gong P, Biging GS (2004) Comparison of IKONOS and QuickBird images for mapping mangrove species on the Caribbean coast of Panama. Remote Sens Environ 91:432–440. https://doi.org/10.1016/j.rse.2004.04.005
Wang R, Gamon JA, Emmerton CE, Hitao L, Nestola E, Pastorello G, Menzer O (2016) Integrated analysis of productivity and biodiversity in a Southern Alberta prairie. Remote Sens 8:214. https://doi.org/10.3390/rs8030214
Wang R, Gamon JA, Cavender-Bares J, Townsend PA, Zygielbaum AI (2018a) The spatial sensitivity of the spectral diversity-biodiversity relationship: an experimental test in a prairie grassland. Ecol Appl 28:541–556. https://doi.org/10.1002/eap.1669
Wang R, Gamon JA, Schweiger AK, Cavender-Bares J, Townsend PA, Zygielbaum AI, Kothari S (2018b) Influence of species richness, evenness, and composition on optical diversity: a simulation study. Remote Sens Environ 211:218–228. https://doi.org/10.1016/j.rse.2018.04.010
Wehlage DC, Gamon JA, Thayer D, Hildebrand DV (2016) Interannual variability in dry mixed-grass prairie yield: a comparison of MODIS, SPOT, and field measurements. Remote Sens 8. https://doi.org/10.3390/rs8100872
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251. https://doi.org/10.2307/1218190
Woodcock CE, Strahler AH (1987) The factor of scale in remote sensing. Remote Sens Environ 21:311–332. https://doi.org/10.1016/0034-4257(87)90015-0
Zutta B (2003) Assessing vegetation functional type and biodiversity in Southern California using spectral reflectance. Master’s Thesis, California State University, Los Angeles
Acknowledgments
The authors wish to acknowledge funding from a NSF and NASA Dimensions grant to JCB (DEB 1342872), PT (DEB 1342778), and JG (DEB 1342823), as well as the Cedar Creek NSF Long-Term Ecological Research grant (DEB 1234162) for supporting many of the examples cited here. We also thank NIMBioS and the Keck Institute of Space Studies (KISS) for sponsoring meetings that helped develop some of the ideas in this chapter. We also acknowledge additional funding support from NSERC, CFI, and from Rangeland Research Institute (U. Alberta) grants to JG. Thanks to Evan Delancey for spectral data (Fig. 16.1), Greg Crutsinger for access to the Parrot Drone data (Fig. 16.5), and to Rick Perk and Brian Leavitt (CALMIT, University of Nebraska-Lincoln) for technical assistance in airborne data collection and processing (Figs. 16.3 and 16.6).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Gamon, J.A., Wang, R., Gholizadeh, H., Zutta, B., Townsend, P.A., Cavender-Bares, J. (2020). Consideration of Scale in Remote Sensing of Biodiversity. In: Cavender-Bares, J., Gamon, J.A., Townsend, P.A. (eds) Remote Sensing of Plant Biodiversity. Springer, Cham. https://doi.org/10.1007/978-3-030-33157-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-33157-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33156-6
Online ISBN: 978-3-030-33157-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)