Abstract
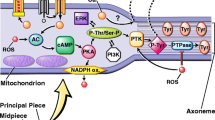
Oxygen, in its diatomic form, is essentially reduced to sustain cellular respiration and survival. This reduction leads to generation of highly reactive oxygen metabolites or reactive oxygen species (ROS), which influence various cellular functions. Endogenous ROS generation in male reproductive tissue and its seminal concentration possess both physiological and pathological significance. Seminal fluid contains several cells including immature germ cells, macrophages, and leukocytes, which may generate ROS to varying concentrations. At normal physiological levels, ROS are crucial for vital reproductive functions such as spermatogenesis, to sustain sperm viability and to mediate maturation, hyperactivation and capacitation, and sperm motility as well as acrosome reaction (AR). Excess ROS are quenched by orchestrated actions of antioxidants. However, when the balance between ROS generation and antioxidant capacity is disrupted in favor of the oxidants, an uncontrolled generation of ROS causes oxidative stress (OS) that adversely affects sperm morphology and functions through lipid peroxidation, DNA fragmentation, and apoptosis. This chapter emphasizes upon the endogenous generation of ROS in the male reproductive tract and their physiological roles in mediating sperm functions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–43.
Sengupta P, Dutta S, Krajewska-Kulak E. The disappearing sperms: analysis of reports published between 1980 and 2015. Am J Mens Health. 2017;11(4):1279–304.
Sengupta P, Borges E Jr, Dutta S, Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Hum Exp Toxicol. 2018;37(3):247–55.
Sengupta P, Dutta S, Tusimin MB, Irez T, Krajewska-Kulak E. Sperm counts in Asian men: reviewing the trend of past 50 years. Asian Pac J Reprod. 2018;7(2):87.
Sengupta P, Nwagha U, Dutta S, Krajewska-Kulak E, Izuka E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr Health Sci. 2017;17(2):418–27.
Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12.
Sengupta P. Recent trends in male reproductive health problems. Asian J Pharm Clin Res. 2014;7(2):1–5.
Ayaz A, Agarwal A, Sharma R, Kothandaraman N, Cakar Z, Sikka S. Proteomic analysis of sperm proteins in infertile men with high levels of reactive oxygen species. Andrologia. 2018;50(6):e13015.
Bui A, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50(8):e13012.
Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, Sadeghi MR. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16(1):87.
Thompson A, Agarwal A, du Plessis SS. Physiological Role of Reactive Oxygen Species in Sperm Function: A Review. In: Parekatil SJ, Agarwal A (eds.), Antioxidants in Male Infertility: A Guide for Clinicians and Researchers. New York: Springer Science+Business Media; 2013. pp. 69–89.
Agarwal A, Aitken RJ, Alvarez JG. Studies on men’s health and fertility. In: Oxidative stress in applied basic research and clinical practice: New York, Dordrecht, Heidelberg, London: Springer Science and Business Media, LLC; 2012.
Ford W. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10(5):387–99.
Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486(1):10–3.
Koppenol WH. The Haber-Weiss cycle–70 years later. Redox Rep. 2001;6(4):229–34.
Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta-Bioenerg. 1999;1411(2–3):273–89.
Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246(2):501–14.
Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24(3):249–66.
Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011;13(1):43–52.
Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288(1):191–9.
Baker MA, Krutskikh A, Aitken RJ. Biochemical entities involved in reactive oxygen species generation by human spermatozoa. Protoplasma. 2003;221(1–2):145–51.
Ford WC, Whittington K, Williams AC. Reactive oxygen species in human sperm suspensions: production by leukocytes and the generation of NADPH to protect sperm against their effects. Int J Androl. 1997;20(Suppl 3):44–9.
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Cambridge: Cambridge University Press, 2010.
Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014;12(1):45.
Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, Lewis B, Irvine S. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev. 1997;47(4):468–82.
Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:92.
Gavella M, Lipovac V. NADH-dependent oxidoreductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Arch Androl. 1992;28(2):135–41.
Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32(4):509–20.
Gelain DP, Cammarota M, Zanotto-Filho A, de Oliveira RB, Dal-Pizzol F, Izquierdo I, Bevilaqua LR, Moreira JC. Retinol induces the ERK1/2-dependent phosphorylation of CREB through a pathway involving the generation of reactive oxygen species in cultured Sertoli cells. Cell Signal. 2006;18(10):1685–94.
Zanotto-Filho A, Schröder R, Moreira JCF. Xanthine oxidase-dependent ROS production mediates vitamin A pro-oxidant effects in cultured Sertoli cells. Free Radic Res. 2008;42(6):593–601.
Hipler U, Görnig M, Hipler B, Römer W, Schreiber G. Stimulation and scavestrogen-induced inhibition of reactive oxygen species generated by rat sertoli cells. Arch Androl. 2000;44(2):147–54.
Stanczyk FZ. Estrogens: different types and properties. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause biology and pathobiology. San Diego: Academic Press; 2000. p. 421–8.
Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12(5):630–3.
Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129(4):357–67.
Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18(2):186–93.
Will MA, Swain J, Fode M, Sonksen J, Christman GM, Ohl D. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95(3):841–52.
Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89(1):124–8.
Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28(2):1–10.
Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17.
Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;1:1–11.
Agarwal A, Desai NR, Ruffoli R, Carpi A. Lifestyle and testicular dysfunction: a brief update. Biomed Pharmacother. 2008;62(8):550–3.
Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16(1):10–20.
Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78(3):491–9.
Wang M, Su P. The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: an update. Syst Biol Reprod Med. 2018;64(2):93–102.
Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36(3):353–68.
Sengupta P. Current trends of male reproductive health disorders and the changing semen quality. Int J Prev Med. 2014;5(1):1–5.
Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function – in sickness and in health. J Androl. 2012;33(6):1096–106.
Sikka SC. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci. 1996;1:e78–86.
Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997;20(2):61–9.
de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10(Suppl 1):15–21.
Lundwall Å, Bjartell A, Olsson AY, Malm J. Semenogelin I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol Hum Reprod. 2002;8(9):805–10.
Hamada A, Sharma R, Du Plessis SS, Willard B, Yadav SP, Sabanegh E, Agarwal A. Two-dimensional differential in-gel electrophoresis–based proteomics of male gametes in relation to oxidative stress. Fertil Steril. 2013;99(5):1216–26. e2
Chatterjee S, Laloraya M, Kumar P. Free radical-induced liquefaction of ejaculated human semen: a new dimension in semen biochemistry. Arch Androl. 1997;38(2):107–11.
du Plessis SS, Agarwal A, Mohanty G, Van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl. 2015;17(2):230.
Lindemann CB. Functional significance of the outer dense fibers of mammalian sperm examined by computer simulations with the geometric clutch model. Cell Motil Cytoskeleton. 1996;34(4):258–70.
Henkel RR, Defosse K, Koyro H-W, Weissmann N, Schill W-B. Estimate of oxygen consumption and intracellular zinc concentration of human spermatozoa in relation to motility. Asian J Androl. 2003;5(1):3–8.
Henkel R, Baldauf C, Bittner J, Weidner W, Miska W. Elimination of zinc from the flagella of spermatozoa during epididymal transit is important for motility. Reprod Technol. 2001;10(5):280–5.
De Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3(3):175–94.
Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci. 2003;20(9):1043–57.
López-González I, Torres-Rodríguez P, Sánchez-Carranza O, Solís-López A, Santi C, Darszon A, Treviño C. Membrane hyperpolarization during human sperm capacitation. Mol Hum Reprod. 2014;20(7):619–29.
Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991;12(5):323–30.
Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol. 2003;49(3):321–8.
Gil-Guzman E, Ollero M, Lopez M, Sharma R, Alvarez J, Thomas A Jr, Agarwal A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16(9):1922–30.
Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48(5):425–35.
Balhorn R. A model for the structure of chromatin in mammalian sperm. J Cell Biol. 1982;93(2):298–305.
Saowaros W, Panyim S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia. 1979;35(2):191–2.
Fujii J, Tsunoda S. Redox regulation of fertilisation and the spermatogenic process. Asian J Androl. 2011;13(3):420–3.
Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14(6):647–57.
Dona G, Fiore C, Tibaldi E, Frezzato F, Andrisani A, Ambrosini G, Fiorentin D, Armanini D, Bordin L, Clari G. Endogenous reactive oxygen species content and modulation of tyrosine phosphorylation during sperm capacitation. Int J Androl. 2011;34(5pt1):411–9.
Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84(10):1039–52.
Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J Androl. 2015;17(4):633–9.
Roldan E, Murase T, Shi Q-X. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266(5190):1578–81.
Khosrowbeygi A, Zarghami N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids. 2007;77(2):117–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dutta, S., Henkel, R., Sengupta, P., Agarwal, A. (2020). Physiological Role of ROS in Sperm Function. In: Parekattil, S., Esteves, S., Agarwal, A. (eds) Male Infertility. Springer, Cham. https://doi.org/10.1007/978-3-030-32300-4_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-32300-4_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32299-1
Online ISBN: 978-3-030-32300-4
eBook Packages: MedicineMedicine (R0)