Abstract
This chapter presents criteria and classification of evaporation, which include evaporation from an adiabatic wall, evaporation from a heated wall, and direct-contact evaporation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Alhusseini, K. A., Tuzla, K., & Chen, J. C. (1998). Falling film evaporation of single component liquids. Journal of Heat Transfer, 41, 1623–1632.
Carey, V. P. (2016). Liquid-vapor phase-change phenomena: An introduction to the thermophysics of vaporization and condensation processes in heat transfer equipment (3rd ed.). New York, NY: Taylor & Francis.
Chun, K. R., & Seban, R. A. (1971). Heat transfer to evaporating liquid films. Journal of Heat Transfer, 93, 391–396.
Debbissi, C., Orfi, J., & Nasrallah, S. B. (2003). Evaporation of water by free or mixed convection into humid air and superheated steam. International Journal of Heat and Mass Transfer, 46, 4703–4715.
Dombrovsky, L. A., & Sazhin, S. S. (2003). A simplified non-isothermal model for droplet heating and evaporation. International Communications in Heat and Mass Transfer, 30, 787–796.
Elperin, T., Fominykh, A., & Krasovitov, B. (2005). Modeling of simultaneous gas absorption and evaporation of large droplet. In Proceedings of 2005 International Mechanical Engineering Congress and Exposition. Orlando, FL, November 5–11, 2005.
Faghri, A. (2016). Heat pipe science & technology (2nd ed.). Columbia, MO: Global Digital Press.
Faghri, A., & Seban, R. (1985). Heat transfer in wavy liquid films. International Journal of Heat and Mass Transfer, 28, 506–508.
Faghri, A., Zhang, Y., & Howell, J. R. (2010). Advanced heat and mass transfer. Columbia, MO: Global Digital Press.
Hirschburg, R. F., & Florschuetz, L. W. (1982). Laminar wavy film flow, parts I and II. Journal of Heat Transfer, 104, 452–464.
Kapitza, P. L., & Kapitza, K. P. (1975). Wavy flow of thin layers of viscous fluid. In Collected papers of P. L. Kapitza (Vol. 2, pp. 662–709). New York: Pergamon.
Kutateladze, S. S. (1963). Fundamentals of heat transfer. New York: Academic Press Inc.
Kutateladze, S. S. (1982). Semi-empirical theory of film condensation of pure vapors. International Journal of Heat and Mass Transfer, 25, 653–660.
Lock, G. S. H. (1994). Latent heat transfer. Oxford, UK: Oxford Science Publications, Oxford University.
Rahman, M. M., & Faghri, A. (1992). Analysis of heating and evaporation from a liquid film adjacent to a horizontal rotating disk. International Journal of Heat and Mass Transfer, 35, 2644–2655.
Rice, J., Faghri, A., & Cetegen, B. M. (2005). Analysis of a free surface flow for a controlled liquid impinging jet over a rotating disk including conjugate effects, with and without evaporation. International Journal of Heat and Mass Transfer, 48, 5192–5204.
Rogovan, I. A., Olevski, V. M., & Runova, N. G. (1969). Measurement of the parameters of film type wavy flow on a vertical plane. Theoretical Foundations of Chemical Engineering, 3, 164.
Seban, R. A., & Faghri, A. (1976). Evaporation and heating with turbulent falling liquid films. Journal of Heat Transfer, 98, 315–318.
Stephan, K. (1992). Heat transfer in condensation and boiling. New York: Springer.
Yan, W. M., & Lin, T. F. (1991). Evaporative cooling of a liquid film through interfacial heat and mass transfer in a vertical channel—II. Numerical study. International Journal of Heat and Mass Transfer, 34, 1113–1124.
Yan, W. M., Lin, T. F., & Tsay, Y. L. (1991). Evaporative cooling of a liquid film through interfacial heat and mass transfer in a vertical channel—I. Experimental study. International Journal of Heat and Mass Transfer, 34, 1105–1111.
Author information
Authors and Affiliations
Corresponding author
Problems
Problems
-
8.1.
A liquid drop is on the top of a substrate at temperature Ts. Suppose the liquid drop is disk-like and the initial thickness is \(\delta_{0}\). Find the time required to completely evaporate the liquid drop. Assume that heat transfer across the disk-like liquid drop is one-dimensional conduction.
-
8.2.
A small amount of water is contained in a tray, as shown in Fig. P8.2. The bottom of the tray is maintained at a temperature, Tw, which is above the ambient temperature, T∞. The initial bulk temperature of the water in the tray, Tb, is between Tw and T∞. The rate of evaporation at the surface of the water is \(\dot{m}_{\text{evp}} \,(\text{kg}/\text{s}).\) Find the bulk temperature of the water as a function of time.
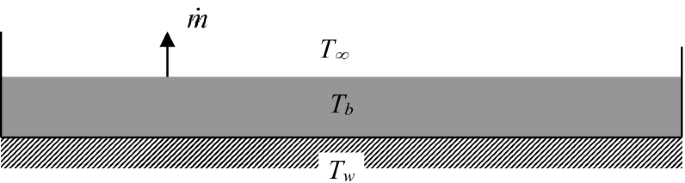
Fig. P8.2
-
8.3.
A thin layer of liquid film sits on top of a heated surface at temperature T0 (see Fig. P8.3). The top surface of the liquid film is at temperature \(T_{\delta }\) \((T_{\delta } < T_{0} )\). The mass flux on the top surface of the liquid film due to evaporation is \(\dot{m}_{\delta }^{\prime\prime }\). It is suggested that the liquid layer could be divided into upper and lower regions with linear temperature distribution in each region. Build an appropriate model to determine the location and temperature of the boundary between the two regions.
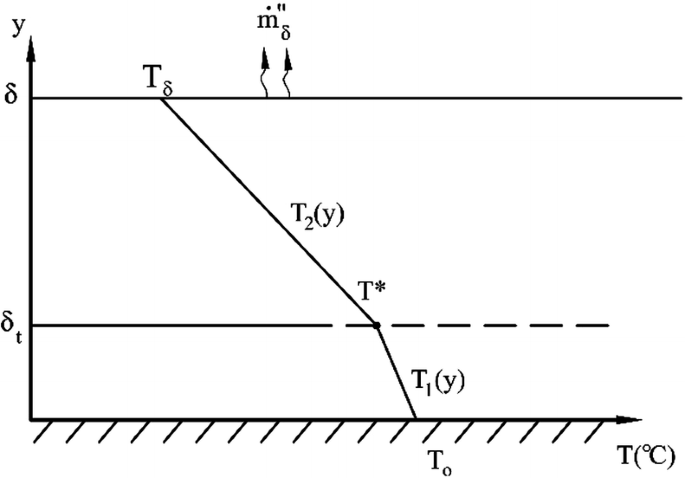
Fig. P8.3
-
8.4.
Solve Stefan’s diffusion problem (see Example 8.2) in terms of molar concentration by assuming the molar fractions of species A at liquid–vapor interface, \(x_{{\text{A},\delta }}\), and at the top of the tube, \(x_{{\text{A},\text{H}}}\), are known. The mixture concentration can be assumed constant.
-
8.5.
Do the Stefan diffusion problem (Problem 8.4) as quasi-steady rather than steady condition, and obtain the change in height with time in terms of molar concentration of mixture and initial height H.
-
8.6.
Evaporation from a horizontal thin film is described by three partial differential equations (8.12), (8.3), and (8.4). Convert these equations to ordinary differential equations using similarity variables defined in Eq. (8.13), and obtain their boundary conditions from Eqs. (8.5)–(8.10).
-
8.7.
Evaporation from a horizontal thin liquid film is described by Eqs. (8.1)–(8.6), (8.8), and (8.10). To solve the problem using an integral method, the profiles of velocity, temperature, and concentration need to be determined. If these profiles can be assumed to be second-degree polynomial functions, find velocity, temperature, and concentration distribution in the boundary layers.
-
8.8.
Solve the evaporation problem using integral approximate method. For the sake of simplicity, assume Pr = Sc = 1 so that the velocity, thermal, and concentration boundary-layer thicknesses are the same. Use the velocity, temperature, and concentration distributions in the boundary layers obtained from the previous problem. The blowing velocity at the surface due to evaporation can be neglected.
-
8.9.
Sweat cooling is a technique used to protect an intensely heated porous wall by evaporating a liquid injected from the pores to the wall surface. In order to cool a 0.5-m-long porous stainless surface, water is injected to the surface at a rate that is just sufficient to keep the surface wet. Dry air at 600 K and 1 atm flows over the plate at a velocity of 10 m/s. Find the rate of water supply needed to maintain the wall temperature at 400 K.
-
8.10.
Falling water film evaporation occurs on the inner sides of two parallel plates with a length of 1 m, and they are separated by a distance of \(2b = 3\,{\text{cm}}\). The inlet and outlet liquid film temperatures are 50 and 45 °C, respectively. Find the mass flow rate of the liquid film per unit width, \(\Gamma\).
-
8.11.
Air enters the bottom of a vertical channel formed by two parallel walls (see Fig. P8.11). A liquid water film is present on the left wall, which is maintained at a constant temperature. The right wall is adiabatic and has no liquid film on it. The liquid film on the left wall is so thin that its thickness can be neglected. The left wall can be considered as a surface where the concentration of the vapor equals the saturation concentration of the vapor at its partial pressure. Assuming that the air flow in the vertical channel is laminar and the air inlet velocity is uniform, find the governing equations and boundary conditions to describe this conjugate heat and mass transfer process.
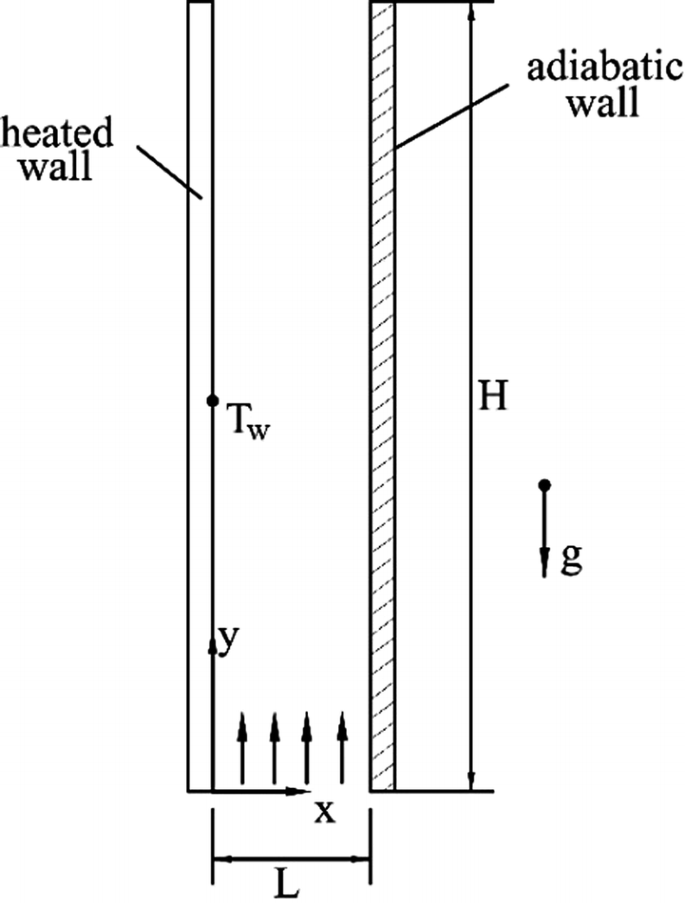
Fig. P8.11
-
8.12.
Air flows into a tube with radius R and constant wall temperature Tw. Evaporation occurs on a thin liquid film on the inner wall of the tube. The thickness of the liquid film can be neglected in comparison with the radius of the tube. The inner wall can be considered as a surface where the concentration of the vapor equals the saturation concentration of the vapor at its partial pressure. The air flow at the inlet of the tube is fully developed, but the temperature and concentration distributions at the inlet are uniform. Specify the governing equations and corresponding boundary conditions of the problem.
-
8.13.
Solve the energy and species equations of the above problem to obtain the temperature and concentration distributions in the tube. The Schmidt number, Sc, can be assumed to be equal to one. Obtain the local convective heat transfer coefficient for the system.
-
8.14.
A two-phase thermosyphon, shown in Fig. P8.14, is needed to deliver a steady-state heat rate of \(\dot{Q} = 1\,\text{kW}\) at an operating temperature of Tv = 333 K. An acceptable wall superheat is ΔT = 5 K. The inside diameter of the circular channel is D = 0.1 m, and the working fluid is water. Use classical Nusselt analysis to determine how long the evaporator should be to avoid dryout.
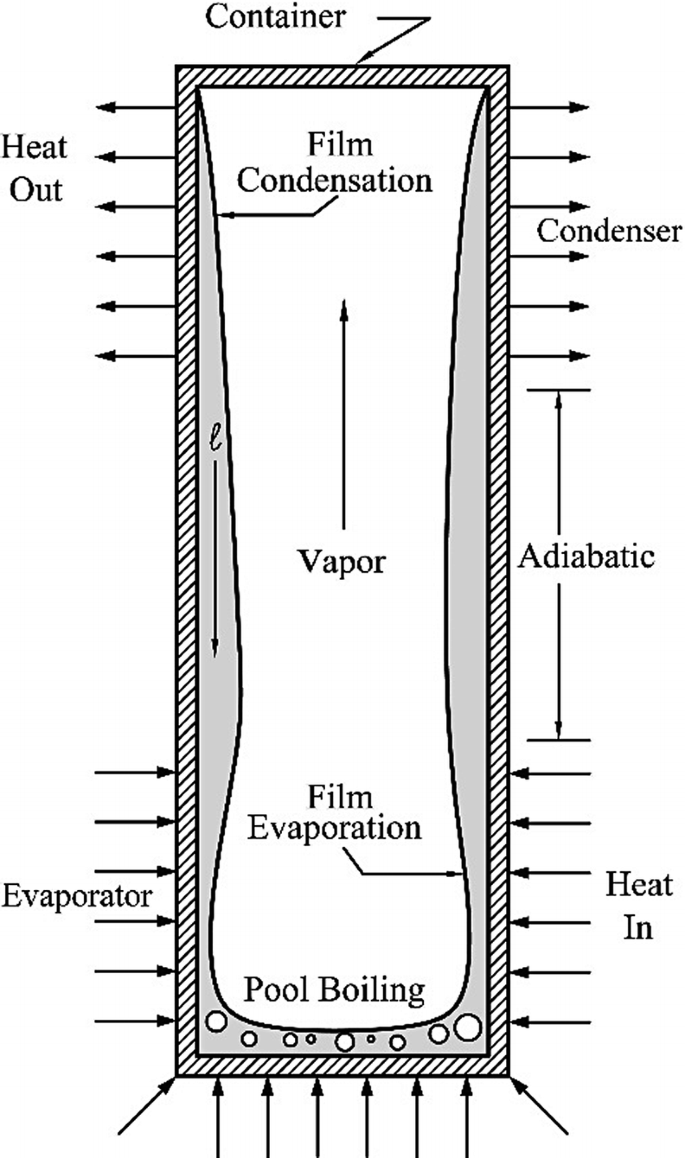
Fig. P8.14
-
8.15.
Derive Eq. (8.114) from Eqs. (8.111)–(8.112), using the transformation of variables \((x,y) \to (\xi ,\eta )\) where \(\xi = x\) and \(\eta = y/\delta (x)\).
-
8.16.
Show that Eq. (8.110) can be transformed into Eq. (8.124) by using the variables defined in Eqs. (8.113), (8.116), and (8.122)–(8.123).
-
8.17.
In a falling liquid film evaporation experiment, the temperature of the heating wall is 106 °C and the vapor pressure is at one atmosphere. The inlet mass flow rate of the liquid film per unit width is \(\Gamma_{0} = 2.24 \times 10^{ - 2} \,\text{kg}/\text{s}\, \text{m}\). Is the film flow laminar, laminar with waves, or turbulent? If the height of the vertical plate is 0.1 m, determine the average heat transfer coefficient. Please also check the overall energy balance by using Eq. (8.98).
-
8.18.
A uniformly distributed liquid water film at 0.06 kg/s is introduced at the top of a tube with an inner diameter of 4 cm and height of 3 m. The inner surface temperature of the tube is uniformly 105 °C, and the saturation temperature of the steam is 100 °C. Is the liquid film at the inlet and outlet laminar, laminar with waves, or turbulent? Find the evaporation rate and the average heat transfer coefficient.
-
8.19.
Falling film evaporation on a vertical wall is analyzed in great detail in Sect. 8.4. How would you apply the solutions of falling film evaporation on a vertical heated wall to predict heat transfer of falling film evaporation on an inclined heating wall? The inclination angle between the heating wall and the vertical direction is \(\theta\).
-
8.20.
You are asked to select the most weight-efficient evaporant for a particular evaporator. The temperature at which the evaporator will operate has not been set. Go through Appendix B.26–B.48, and rank each working fluid based on its heat of vaporization \((h_{{\ell \text{v}}} )\). Also, for each working fluid, note what the operating temperature of the evaporator would be if the operating pressure was 1 atm. Which evaporants stand out in the different temperature ranges?
-
8.21.
Consider evaporation for a constant-temperature wall rotating disk with controlled liquid jet impingement at its center (see Fig. P8.21). Rotational forces cause the film to spread across the disk and then off the outer edge. The flow field is similar to a laminar, steady falling film, except that rotation replaces gravity as the driving force and the system must be solved in cylindrical coordinates. Obtain the heat transfer coefficient for the thin liquid film evaporation on the rotating disk by neglecting inertia and convective terms.
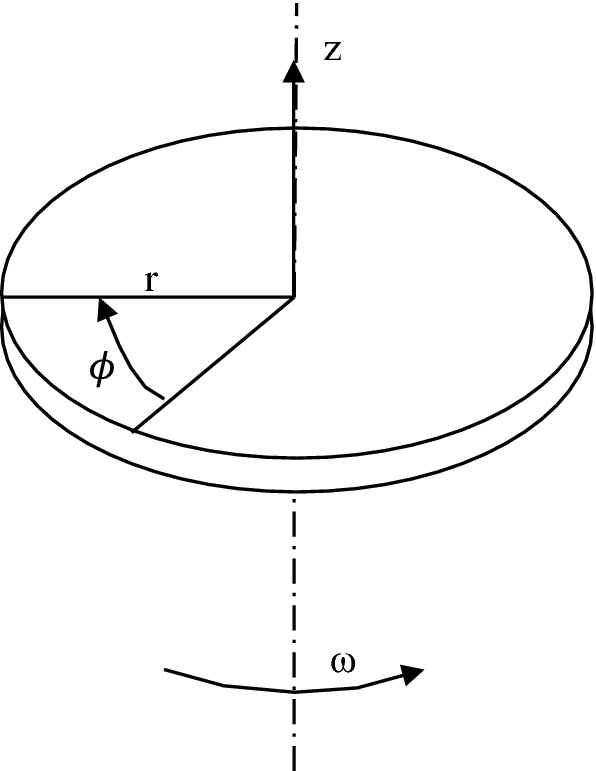
Fig. P8.21
-
8.22.
A spray of liquid water droplets with mean diameter d = 50 μm at a temperature 373 K is injected into a tank of superheated water vapor at a pressure p = 1.013 × 105 Pa held at constant T∞ = 423 K. The convective heat transfer coefficient on the surface of the droplet is 4000 W/m2-K. If the nozzle is 0.2 m from the nearest surface of the tank, what is the maximum velocity the droplets can have without making contact with the wall?
-
8.23.
The analysis presented in Sect. 8.5.1 assumed that the heat transfer coefficient on the surface of the liquid droplet was a constant. When a liquid droplet is spread into the hot gas, the following empirical correlation can be used to estimate the heat transfer coefficient: \(Nu = 2 + 0.6\,{Re}^{1/2} Pr^{1/3}\). Since the radius of the droplet is changing in the evaporation process, the heat transfer coefficient is not a constant. How will you modify Eq. (8.188) when heat transfer coefficient is not a constant?
-
8.24.
Slurry droplets and hot air are brought into contact in a slurry-drying process. During the early stage, the slurry droplet can be considered as a porous core covered by pure water (see Fig. P8.24). Evaporation takes place on the surface of the slurry droplet, the temperature of which can be assumed to be uniform all the time. The first phase of evaporation is referred to as evaporation of the pure water around the porous core. Build an appropriate model to predict the transient radius and temperature of the slurry droplet during the first phase of evaporation.
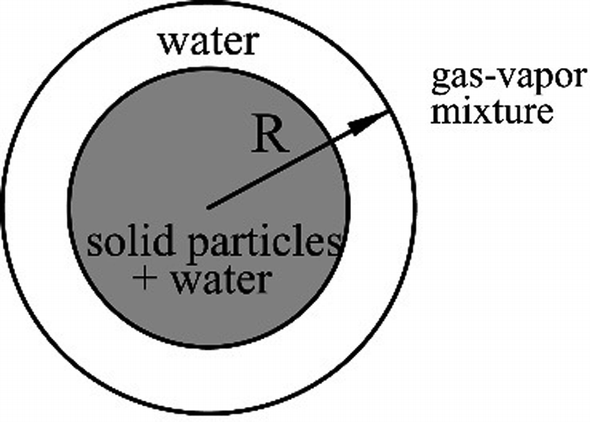
Fig. P8.24
-
8.25.
A stationary liquid fuel droplet with an initial temperature of T0 is introduced into hot gas at a temperature of \(T_{\infty }\). The initial pressures in the liquid droplet and the hot gas are \(p_{0}\) and \(p_{\infty }\) respectively. The initial mass fraction of the fuel vapor in the hot gas is \(\omega_{\infty }\). Evaporation takes place on the surface of the liquid fuel droplet, which is assumed to be in spherical shape throughout the process. Since the temperature of the fuel droplet is below the temperature of the hot gas, a laminar downward motion of hot gas is formed near the liquid fuel droplet. Write the governing conservation equations and corresponding boundary conditions for liquid and gas flow, and heat and mass transfer in both liquid fuel droplet and hot gas. The spherical coordinate system with origin located at the center of the liquid fuel droplet should be used.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faghri, A., Zhang, Y. (2020). Evaporation. In: Fundamentals of Multiphase Heat Transfer and Flow. Springer, Cham. https://doi.org/10.1007/978-3-030-22137-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-22137-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22136-2
Online ISBN: 978-3-030-22137-9
eBook Packages: EngineeringEngineering (R0)







