Abstract
Solid–vapor phase change, including sublimation and vapor deposition, is presented in this chapter. The discussion begins with a brief overview of solid–vapor phase change and proceeds to detailed analyses of sublimation with and without chemical reaction as well as chemical vapor deposition.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Bauerle, D. (1996). Laser processing and chemistry. New York, NY: Springer.
Bird, R. B., Stewart, W. E., & Lightfoot, E. N. (2006). Transport phenomena (2nd ed.). New York: Wiley. (Revised).
Conde, O., Kar, A., & Mazumder, J. (1992). Laser chemical vapor deposition of TiN Dot: A comparison of theoretical and experimental results. Journal of Applied Physics, 72, 754–761.
Eckert, E. R. G., & Goldstein, R. J. (1976). Measurement in heat transfer. New York, NY: McGraw-Hill.
Elyukhin, V. A., Garcia-Salgado, G., & Pena-Sierra, R. (2002). Thermodynamic model for low temperature metalorganic chemical vapor deposition of GaN. Journal of Applied Physics, 91, 9091–9094.
Evans, G., & Greif, R. (1987). A numerical model of the flow and heat transfer in a rotating disk chemical vapor deposition reactor. Journal of Heat Transfer, 109, 928–935.
Faghri, A., Zhang, Y., & Howell, J. R. (2010). Advanced heat and mass transfer. Columbia, MO: Global Digital Press.
Glassman, I., & Yetter, R. A. (2008). Combustion (4th ed.). Burlington, MA: Elsevier.
Herring, R. B. (1990). Silicon epitaxy. In W. C. O’Mara, R. B. Herring, & L. P. Hunt (Eds.), Handbook of semiconductor silicon technology (pp. 258–336). Park Ridge, NJ: Noyes Publications.
Jakubenas, K. J., Birmingham, B., Harrison, S., Crocker, J., Shaarawi, M.S., Tompkins, J. V., et al. (1997). Recent development in SALD and SALDVI. In Proceedings of 7th International Conference on Rapid Prototyping, San Francisco, CA.
Jensen, K. F., Einset, E. O., & Fotiadis, D. I. (1991). Flow phenomena in chemical vapor deposition of thin films. Annual Review of Fluid Mechanics, 23, 197–232.
Kaviany, M. (2001). Principles of convective heat transfer (2nd ed.). New York: Springer.
Kays, W. M., Crawford, M. E., & Weigand, B. (2004). Convective heat transfer (4th ed.). New York, NY: McGraw-Hill.
Kurosaki, Y. (1973). Coupled heat and mass transfer in a flow between parallel flat plate (Uniform heat flux). Journal of the Japan Society of Mechanical Engineers, Part B, 39, 2512–2521. (in Japanese).
Kurosaki, Y. (1974). Coupled heat-mass transfer of a flat plate with uniform heat flux in a laminar parallel flow. Journal of the Japan Society of Mechanical Engineers, Part B, 40, 1066–1072. (in Japanese).
Lee, Y. L., Tompkins, J. V., Sanchez, J. M., & Marcus, H. L. (1995). Deposition rate of silicon carbide by selected area laser deposition. Proceedings of Solid Freeform Fabrication Symposium, 1995, 433–439.
Mahajan, R. L. (1996). Transport phenomena in chemical vapor-deposition systems. In Advances in heat transfer. San Diego, CA: Academic Press.
Marcus, H. L., Zong, G., & Subramanian, P. K. (1993). Residual stresses in laser processed solid freeform fabrication, residual stresses in composites. In E. V. Barrera & I. Dutta (Eds.), Measurement, modeling and effect on thermomechanical properties (pp. 257–271). TMS.
Mazumder, J., & Kar, A. (1995). Theory and application of laser chemical vapor deposition. New York, NY: Plenum Publishing Co.
Patankar, S. V. (1980). Numerical heat transfer and fluid flow. Washington, DC: Hemisphere.
Powell, C., Blocher, M., & Oxley, J. (1966). Vapor deposition. New York: Wiley.
Sivaram, S. (1995). Chemical vapor deposition: Thermal and plasma deposition of electronic materials. Bordrecht, Netherlands: Kluwer Academic Publishers.
Sun, L., Jakubenas, K. J., Crocker, J. E., Harrison, S., Shaw, L. L., & Marcus, H. L. (1998). In situ thermocouples in micro-components fabricated using SALD/SALDVI techniques: II evaluation of processing parameters. Materials and Manufacturing Processes, 13, 883–907.
Taylor, C. A., Wayne, M. F., & Chiu, W. K. S. (2004). Microstructural characterization of thin carbon films deposited from hydrocarbon mixtures. Surface & Coatings Technology, 182, 131–137.
Ueda, O. (1996). Reliability and degradation of III-V optical devices. Boston: Artech House Inc.
Van Doormaal, J. P., & Raithby, G. D. (1984). Enhancements of the simple method for predicting incompressible fluid flows. Numerical Heat Transfer, 7, 147–163.
Versteeg, V. A., Avedisian, C. T., & Raj, R. (1995). Metalorganic chemical vapor deposition by pulsed liquid injection using an ultrasonic nozzle: Titanium dioxide on sapphire from titanium (IV) isopropoxide. Journal of the American Ceramic Society, 78, 2763–2768.
Zhang, Y. (2003). Quasi-steady state natural convection in laser chemical vapor deposition with a moving laser beam. Journal of Heat Transfer, 125, 429–437.
Zhang, Y. (2004). A simulation-based correlation of cross-sectional area of the thin film produced by laser chemical vapor deposition with a moving laser beam. Journal of Manufacturing Science and Engineering, 126, 796–800.
Zhang, Y., & Chen, Z. Q. (1990). Analytical solution of coupled laminar heat-mass transfer inside a tube with adiabatic external wall. In Proceedings of the 3rd National Interuniversity Conference on Engineering Thermophysics (pp. 341–345). Xi’an Jiaotong University Press, Xi’an, China.
Zhang, Y., Chen, Z. Q., & Chen, M. (1996). Local non-similarity solution of coupled heat-mass transfer of a flat plate with uniform heat flux in a laminar parallel flow. Journal of Thermal Science, 5, 112–116.
Zhang, Y., & Faghri, A. (2000). Thermal modeling of selective area laser deposition of titanium nitride on a finite slab with stationary and moving laser beams. International Journal of Heat and Mass Transfer, 43, 3835–3846.
Author information
Authors and Affiliations
Corresponding author
Problems
Problems
-
6.1.
A subcooled solid is exposed to its superheated vapor as shown in Fig. 6.2a. The temperature at the left surface of the solid is \(T_{0}\), which is below the interfacial temperature. Depending on the direction of the overall heat flux at the interface, both sublimation and deposition are possible. Derive the criteria for sublimation and deposition.
-
6.2.
Superheated vapor is brought into contact with a cold surface at a temperature of T0, and deposition takes place on the cold surface. Find the deposition rate by solving transient conduction in the deposited solid phase.
-
6.3.
The inner surface of a circular tube with radius R is coated with a layer of sublimable material, and the outer wall of the tube is kept at a constant temperature \(T_{\text{w}}\). The fully developed gas enters the tube with a uniform inlet mass fraction of the sublimable substance \(\omega_{0}\) that equals the saturation mass fraction corresponding to the inlet temperature T0. The thermal and mass diffusivities are assumed to be the same, i.e., Le = 1. Find the local Nusselt number based on convective heat flux and the total heat flux at the wall, and the local Sherwood number.
-
6.4.
A gas with a velocity of \(u_{\infty }\), a concentration of a sublimable substance, \(\omega_{\infty }\), and a temperature of \(T_{\infty }\) flows parallel to a flat plate coated with sublimable materials; the back of the flat plate is adiabatic (see Fig. P6.4). Specify the governing equations and the corresponding boundary conditions of the sublimation problem.
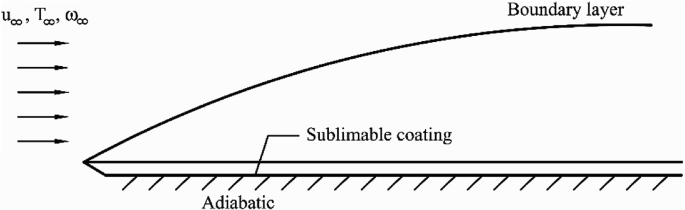
Fig. P6.4
-
6.5.
Suppose the blowing velocity on the surface of the flat plate satisfies \(v_{\text{w}} \propto x^{ - 1/2}\). Introduce appropriate similarity variables to the governing equations in Problem 6.4 and reduce the governing equations into a set of ordinary differential equations.
-
6.6.
Write a computer program to solve for the ordinary differential equations of Problem 6.5, and obtain the local Nusselt number and Sherwood number.
-
6.7.
Air with a temperature of 27 °C flows at 1 m/s over a 1-m-long solid fuel surface at a temperature of 527 °C. The average blowing velocity due to sublimation of the solid fuel is 0.01 m/s, and the heat released per unit mass of the oxidant consumed is 10,000 kJ/kg. The latent heat of sublimation for the solid fuel is 1350 kJ/kg. The sensible heat required to raise the surface temperature of the solid fuel to sublimation temperature, and heat loss to the solid fuel, can be neglected. Estimate the mass fraction of the oxidant at the solid fuel surface.
-
6.8.
Manned spacecraft and space suits reject excess thermal energy by sublimating water into the vacuum of space. The sublimator consists of a porous plate exposed to vacuum on one side and feed water on the other side. The feed water seeps into the porous plate, where it then freezes. In Fig. P6.8, the sublimator also has a separate coolant heat exchanger that interfaces with the feed water. Describe in detail how the process of sublimation keeps the astronaut or spacecraft cool.
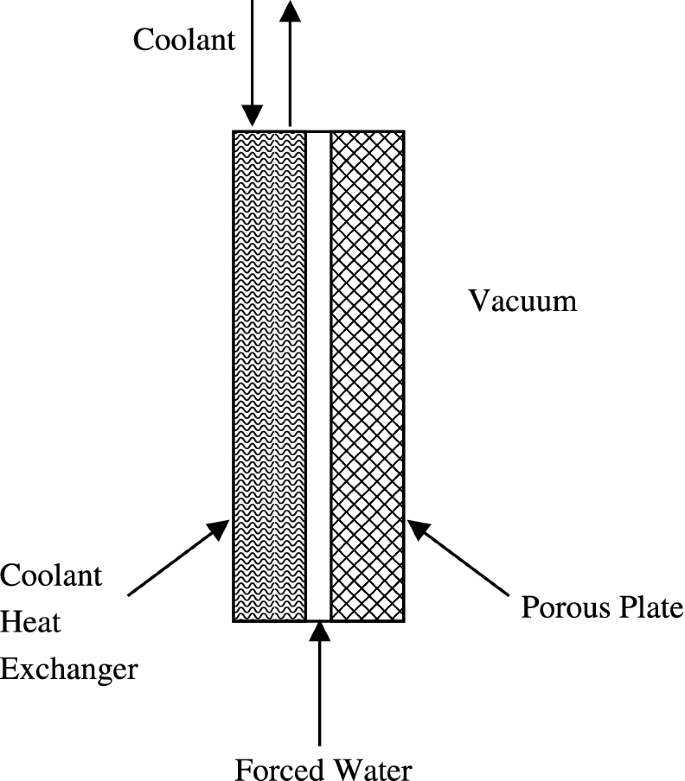
Fig. P6.8
-
6.9.
The precursor for SALD of TiN film is a mixture of titanium tetrachloride, nitrogen, and hydrogen. The total pressure in the chamber is 207 torr and the partial pressure of titanium tetrachloride is 7 torr. The partial pressures of N2 and H2 are the same. Estimate the viscosity and thermal conductivity of the precursors at 900 K.
-
6.10.
Estimate the binary mass diffusivity of titanium tetrachloride to nitrogen gas in the gaseous mixture described in Problem 6.9. The collision diameter of a nitrogen molecule is \(\sigma = 3.681\) Å, and the characteristic energy of interaction between molecules satisfies \(\varepsilon /k_{b} = 91.5{\text{ K}} .\)
-
6.11.
What is the binary mass diffusivity of titanium tetrachloride to the hydrogen gas in the gaseous mixture described in Problem 6.9? The collision diameter of the hydrogen molecule is \(\sigma = 2.915\) Å, and the characteristic energy of interaction between hydrogen molecules satisfies \(\varepsilon /k_{\text{b}} = 38.0\,{\text{K}} .\)
-
6.12.
Find the mass diffusivity of TiCl4 to a mixture of N2 and H2 used in SALD of TiN. The total pressure of the precursors is 207 torr, and the partial pressure of TiCl4 is 7 torr. The partial pressures of both N2 and H2 are at 100 torr.
-
6.13.
In an LCVD of TiN using a mixture of TiCl4, N2, and H2, a laser beam with a power of 350 W and a radius of 1 mm scans at a velocity of 1.5 mm/s. The absorptivity of the laser beam on the substrate surface is 0.23. The initial gas temperature is 338 K, and the chemical reaction temperature is 1173 K. The thermal conductivity and thermal diffusivity of the substrate at 1173 K are 24.5 W/m-K and \(4.677 \times 10^{ - 6} \,{\text{m}}^{ 2} / {\text{s}}\), respectively. Estimate the dimensional cross-sectional area of the deposited film.
-
6.14.
Laser chemical vapor infiltration (LCVI) is an additive manufacturing process that uses gas precursors and powder particles to build three-dimensional parts. The powder particles are bounded together through LCVD on the particle surface by decomposition of gas precursors. A nearly homogeneous distribution of the heat flux on the particle surface can be assumed due to multiple scattering of the radiation. Figure P6.14 illustrates a metal powder particle made of Incoloy 800 that is surrounded by a mixture of H2, N2, and TiCl4 and subjected to temporal Gaussian heat flux from a nanosecond pulsed laser beam. Because of symmetry of the spherical particle and the assumption of uniform heat flux distribution, the problem can be simplified to be one-dimensional in the r-direction. Assuming that natural convection can be neglected, give the governing equations and boundary conditions to obtain the deposited film thickness by considering heat transfer in the particle, chemical reaction on the particle surface, and mass transfer in the gas phase. The origin of time is chosen to be when the heat flux is at its maximum; thus the time-dependent heat flux is expressed as \(q^{\prime \prime } \left( t \right) = q_{0}^{{{\prime \prime }}} { \exp }[ - { \ln }\left( {2t^{2} /t_{\text{p}}^{2} } \right]\), where \(q_{0}^{{{\prime \prime }}}\) is the maximum heat flux and tp is the half-width of the laser pulse at half maximum (see Fig. P6.14).
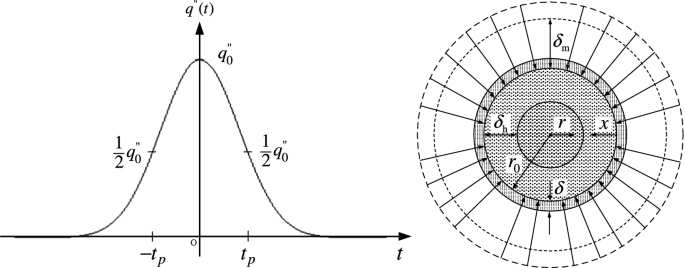
Fig. P6.14
-
6.15.
Solve heat transfer in the powder particle and mass transfer in the gas phase in Problem 6.14 using integral solution (see Chap. 5) and outline the procedure to obtain the deposited film thickness.
-
6.16.
Model a simplified catalytic reaction in Fig. P6.16 in which gas A enters the reactor and is convected to B. Assume that at the bottom surface a reaction 2A → B is being carried out steadily, irreversibly, and instantaneously in an isothermal process. Assume ideal gas and obtain the local mass flux rate of A to B.
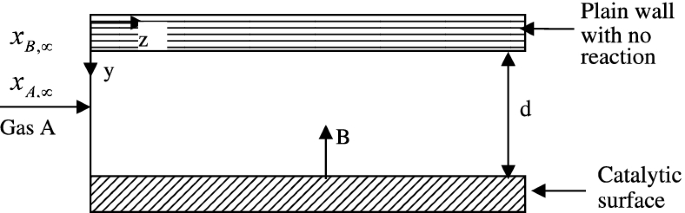
Fig. P6.16
-
6.17.
Repeat Problem 6.16, but account for the finite reaction kinetics at the catalytic surface. All other assumptions remain the same. Assume the rate at which A disappears at the catalyst surface is proportional to the molar concentration of A in the gas at the surface (first-order surface reaction).
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faghri, A., Zhang, Y. (2020). Sublimation and Vapor Deposition. In: Fundamentals of Multiphase Heat Transfer and Flow. Springer, Cham. https://doi.org/10.1007/978-3-030-22137-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-22137-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22136-2
Online ISBN: 978-3-030-22137-9
eBook Packages: EngineeringEngineering (R0)





