Abstract
Due to its particular “silent” metabolic state, without transcription or translation, and a low level of cytosolic protective activities, mature sperm is a cellular type of aerobic organisms particularly at risk of oxidative damage. Despite the efforts of the male genital tract to treat this problem, a subcellular compartment of the sperm, the nucleus, and consequently, the paternal DNA cannot be effectively protected. There is an accumulation of evidence that oxidative damage to sperm DNA is quite common in male infertilities/subfertilities with potential harmful impacts on reproductive success, including the transgenerational inheritance of a paternal chromosomal lot carrying mutations.
Similar content being viewed by others
Keywords
Observations that the sperm cells of a large proportion of infertile men have high levels of reactive oxygen species (ROS) and that their antioxidant content is low compared to fertile men have been reported for several years (Aitken et al. 2012; Henkel 2011; Lewis et al. 1995). At present, it is fairly commonly accepted that oxidative stress interferes with the fertilization potential of sperm, damages DNA, and even affects the epigenetic profile of sperm cells (Aitken et al. 2014; Aitken 2016). Despite this recognized situation, oxidative DNA lesions in semen are still neglected, even though they could have significant clinical consequences for male fertilizing potential, optimal embryonic development, and the health and well-being of the offspring. In this chapter, we first summarize why the nucleus and DNA of sperm cells are so sensitive to oxidative damage, when and where sperm cells are at risk of oxidative damage to DNA, where this damage is concentrated in the sperm cell, whether oxidative damage can also affect epigenetic information transmitted by sperm cells, and what are the possible consequences when a sperm with an oxidatively damaged nucleus fertilizes an oocyte. In a second section, we present how sperm cells are protected from oxidative DNA damage and discuss the relevance of a therapeutic strategy focused on oral antioxidant supplementation.
Why Spermatozoa DNA Is Susceptible to Oxidative Damage?
Due to its particular architecture and biology, the sperm cell is at greater risk of oxidative damage to DNA than any other cell. The main reason for this vulnerability is the spermatozoon’s inability to fight oxidative insults because the cell is “silent” by nature (it does not transcribe and, despite some reports to the contrary, is generally assumed to be translationally inactive or to translate poorly). In this respect, the spermatozoa cannot mount a stress response as any other cell would. In addition, the sperm cell is generally lacking in cytosolic antioxidant protective capacity in the form of enzymes (such as catalase, superoxide dismutase, and glutathione peroxidase) and nonenzymatic small molecular mass scavengers (such as polyamines, taurine, lipoic acid, and vitamins C and E) that counteract ROS because uniquely, this cell type divests itself of most of its cytoplasm prior to its release from the germinal epithelium. We add to this picture additional facts such as the following: 1) having lost most of its cytosol, the nuclear compartment of the sperm is readily exposed to environmental ROS; 2) the plasma membrane of the sperm cell has a particular lipid composition rich in polyunsaturated fatty acids (PUFAs) that are very susceptible to a peroxidative process that generates even more aggressive ROS and other toxic metabolites (Jones et al. 1978; Jones et al. 1979); and 3) the sperm cells themselves produce ROS. It is therefore not surprising to note that the sperm nucleus and its DNA should be at risk of oxidative damage.
As a consequence of such factors, spermatozoa under conditions of oxidative stress easily generate cytotoxic lipid aldehydes such as malondialdehyde (MDA) and, above all, 4- hydroxynonenal (4-HNE) as a result of membranous lipid peroxidation (Lenzi et al. 1996; Aitken et al. 2012; Ayala et al. 2014; Moazamian et al. 2015). 4-HNE is a very toxic lipid aldehyde that has been shown to efficiently alkylate proteins, induce DNA damage, stimulate the production of inflammatory markers and, also, interfere with mitochondria function leading to more ROS generation entertaining and amplifying the situation of oxidative stress in spermatozoa as in any other cells (Aitken et al. 2012; Shoeb et al. 2014). A very recent review stresses the importance of 4-HNE production in male infertility (Walters et al. 2018) and how it could eventually be circumvented via either antioxidant supplementation or a direct action on lipoxygenase enzymes that contribute to 4-HNE such as the arachidonate 15-lipoxygenase (ALOX15). It turns out that ALOX15 could well be a pertinent target to manipulate in order to decrease sperm oxidative damage as it was shown elsewhere to mediate oxidative stress in mouse spermatozoa (Brütsch et al. 2015).
Where Are Sperm Cells at Risk of DNA Oxidative Damage?
In a physiological context, from its genesis in the germinal epithelium to the moment of fertilization in the female genital tract, spermatozoa may experience oxidative attacks. Spermatozoa themselves are good producers of ROS, especially hyperactivated ones at the onset of capacitation since ROS are known to facilitate this process (Aitken et al. 1995. Aitken et al. 1998b; Aitken and Curry 2011). Briefly, because ROS can inhibit tyrosine phosphatase activity, promote cAMP generation, and facilitate sperm plasma membrane cholesterol efflux via the oxidation in oxysterols, they are key actors in this sperm maturation process which is an essential requirement for fertilization to occur (Aitken 1997; Leclerc et al. 1997; Aitken et al. 1998a; Lewis and Aitken 2001; O’Flaherty et al. 2006; Awda and Buhr 2010; Aitken and Curry 2011; Aitken 2011; Brouwers et al. 2011; Donà et al. 2011). The dark side of this process is the self- damaging effect of ROS produced by capacitated spermatozoa affecting the plasma membrane, mitochondria function, and ultimately the sperm DNA. Thus, even in a physiological context, mature spermatozoa may endure DNA oxidative damage especially when the condensation of the sperm nucleus is not optimized (De Iuliis et al. 2009).
Outside this physiological situation, in both men and women, oxidative stress within the genital tract can have several origins, being either grossly systemic or local. Inflammatory and infectious situations are classically associated with increasing level of ROS in the vicinity of sperm cells since hydrogen peroxide (H2O2), a harmful ROS, is a key player in the resolution of such situations. It was therefore not surprising to see in clinical practice that leukocytospermia is strongly associated with sperm DNA oxidative damage (Vorilhon et al. 2018). To a lesser extent, asthenozoospermia was also found associated with increased sperm DNA oxidative damage in line with what could be expected in situations of defective mitochondrial functions and superoxide anion leakage. Exposures to environmental toxicants, as well as environmental physical and mechanical stressors such as radiation and heat, are classical sources of oxidative stress that spermatozoa could face (Houston et al. 2016; Houston et al. 2018). A non-balanced diet and many medical treatments are also sources of oxidative stress that could easily affect spermatozoa during their life span whether it is during their generation in the testis or during their post-testicular life. The multiplicity of situations leading to systemic or local oxidative stress around sperm cells whether there are physiological, pathological, or even artificial as in the case of assisted reproductive technologies (ART) lets us suspect that oxidative DNA damage should be a rather common feature of this particular cell type (Aitken 2016). This is exactly what was observed in a clinical context at the Clermont-Ferrand public infertility clinic where a cohort of males entering the andrology lab for fertility assessment or/and an ART program was monitored for the presence of 8-OHdG, a precocious marker of sperm DNA oxidative damage. The result of such a survey was that 2/3 of men showed moderate to high levels of 8-OHdG whatever the origin of the couple infertility and whatever their individual fertility status as measured by the standard WHO parameters (Cooper et al. 2010) addressing sperm count, morphology, and motility (Vorilhon et al. 2018). If this local observation reflects on the worldwide situation, sperm DNA oxidative damage would appear to be a very common form of insult to the paternal DNA; even more frequent than sperm DNA fragmentation which is estimated to involve about 1 out of 7 males from couple having difficulties to conceive (Giwercman et al. 2010).
Sperm Nuclear Oxidative Damage and Its Developmental Impacts (Putative Mutational Risks and Transgenerational Effects)
Sperm Nuclear and DNA Damage
Oxidative damage to the sperm nucleus can take multiple forms. Firstly, oxidative alterations can promote nuclear decondensation and DNA fragmentation. Nuclear DNA is, to a large extent, protected from oxidative damage because it has been condensed to the point of crystallization as a result of the remodeling of sperm chromatin that occurs during spermiogenesis. During this process, about 85% of the human sperm histones are eliminated and replaced by small basic proteins, rich in arginine, called protamines . These molecules, due to their net positive charge, are able to neutralize the negative charges carried by phosphate groups in the DNA skeleton and, by overcoming electrostatic repulsion between adjacent DNA strands, allow the high level of compaction of the DNA typical of the spermatic nucleus. The DNA of the mitochondria of the semen is not conditioned in this way and is therefore more vulnerable to oxidative attacks (Kocer et al. 2015). This may not be relevant in terms of embryonic developmental potential because paternal mitochondria are destroyed after fertilization to make way for the maternal mitochondrial line. However, it should be noted that the increased vulnerability of mitochondrial DNA to oxidative attack provides an opportunity to monitor such oxidative DNA damage in spermatozoa for a diagnostic perspective (Sawyer et al. 2003).
It is not entirely clear at this stage whether the oxidation of sperm DNA and its fragmentation are closely correlated. Obviously, the fragmentation of sperm DNA, to some extent, has an oxidative origin since the high concentration of ROS (especially hydrogen peroxide) has the ability to cause single and double strand breaks (SSB, DSB) in most cell types. Furthermore, human and mouse spermatozoa have been shown to lack a fully functional DNA repair machinery, as they only possess the first enzyme in the base-excision repair pathway, OGG1, which removes the oxidized base leaving a vulnerable abasic site. The second step in this base excision repair pathway, which utilizes APE1 to create a nick in the phosphodiester backbone of the AP site in readiness for the insertion of a new base, is missing from these cells. However, APE1 and other downstream constituents of the base-excision repair pathway are present in abundance within the oocyte. It has therefore been concluded that the effective repair of oxidative DNA damage in spermatozoa involves a collaboration between the male and female germ lines; the spermatozoon removing the oxidized base, while the oocyte completes the repair process, through the insertion of a new base into the damaged site, following fertilization (Smith et al. 2013a, b). Therefore, the oxidation of sperm DNA could lead to paternal DNA fragmentation and other forms of defect, as a result of inadequate or aberrant oocyte repair after fertilization, a situation that cannot be measured by solely evaluating the fragmentation of the sperm nucleus in a semen sample.
When H2O2 levels around sperm cells are very high, it could directly lead to DNA breaks, and, in that sense when sperm DNA fragmentation is recorded, it may be partly due to excessive DNA oxidation. However, it is important to keep in mind that absence of DNA fragmentation in the semen should not be interpreted as absence of oxidative DNA alterations, a shortcut frequently made in the clinic, because DNA oxidation, if not monitored, may be moderate to high without leading to dramatic sperm DNA fragmentation. This situation, in which sperm DNA oxidation is seen to be disconnected from sperm DNA fragmentation, has been clearly demonstrated in several mouse models of post-testicular oxidative stress in which high levels of DNA oxidation were recorded in cauda epididymis sperm without any evidence of increased DNA fragmentation (Chabory et al. 2009; Noblanc et al. 2013; Kocer et al. 2015). In these models where spermatozoa were challenged by mild reducing condition, low nuclear condensation and/or increased susceptibility to nuclear decondensation was recorded (Noblanc et al. 2013). Thus, nuclear oxidation and DNA fragmentation of semen are two conditions that should be considered separately to avoid misleading diagnoses.
On the contrary, sperm nuclear condensation and oxidation are quite well-associated parameters. This is due to the fact that sperm nuclear compaction is completed in the epididymis via an oxidative process involving the creation of inter- and intramolecular cross bridges between, and within, nuclear protamines. These events result in the further condensation of the sperm nucleus ultimately locking it into a compacted state. Disulfide bridging of sperm protamines is enabled by the presence of a well-controlled luminal concentration of hydrogen peroxide in the epididymis (Drevet 2006) and enzymes (protein disulfide isomerase and glutathione peroxidase) in the sperm nucleus (Chabory et al. 2009). This finely controlled process can be challenged by systemic or local factors, which can lead to the production of ROS, as mentioned above. There is, therefore, a delicate balance between physiological oxidation which will allow optimal nuclear compaction of the sperm and harmful nuclear oxidation. Excessive production of ROS by mature sperm cells, in addition to affecting sperm membranes and amplifying ROS production, may transiently increase nuclear condensation of sperm cells but will quickly promote spontaneous DNA fragmentation resulting in nuclear decondensation and increased oxidative damage to DNA and proteins associated with chromatin.
Despite the compaction of nuclear DNA into dense doughnut-shaped structures called toroids (which comprise 50 to 100 kb of DNA), there are still areas of the mammalian nuclear genome that are vulnerable to oxidative attacks. These correspond to the less dense genomic regions still organized into nucleosomes (where only 146 bp of DNA is associated per histone octamer). Depending on the species looked at, the share of paternal DNA still loosely compacted in nucleosomes is very different. In the mouse, only 1 to 2% of histones are left after spermiogenesis, while in the human this figure goes up to 12 to 15% (although there is some controversy about it with some authors claiming that it could be less and closer to 5 to 7%). Whatever the precise level of histone retention, these findings reveal that the human sperm nucleus is rather less compacted than the mouse counterpart since there are a lot more chromosomal regions maintained in nucleosomal arrangements making them more prone to suffer from oxidative DNA damage (De Iuliis et al. 2009; Noblanc et al. 2013). This concept was supported by the finding that human sperm DNA oxidation is a very common phenomenon affecting 66% of the men tested, whether they were classified fertile or infertile following the WHO criteria (Vorilhon et al. 2018). A fundamental difference between human and mouse spermatozoa was also suggested by the fact that 8-OHdG oxidative marks were found to affect the entire nuclear compartment in human sperm, while the regions affected by oxidative damage in mouse spermatozoa were a lot more discrete (Vorilhon et al. 2018; Noblanc et al. 2013; Kocer et al. 2015; Champroux et al. 2018a). Experimentally, a quantitative PCR technique has been used to demonstrate conclusively that the nuclear genes of human spermatozoa are more vulnerable to oxidative attack than the murine equivalent (Bennetts and Aitken 2005). In the mouse nucleus, it has recently been shown that persistent regions of histone-bound sperm DNA belong to two categories (Johnson et al. 2011; Noblanc et al. 2013). In the first category, nucleosomes were found at irregular intervals in large regions of DNA associated with protamines within nuclear toroids. In the second category, histone-rich DNA regions have also been found in the small DNA strands connecting protamine toroids to one another – the so- called interlinker regions (Kocer et al. 2015). It is interesting to note that these short DNA strands were also attached to the nuclear matrix of the sperm, anchoring the chromosomes at the periphery and at the base of the sperm head (Noblanc et al. 2013). The peripheral location of these nucleosomal nuclear domains and their less condensed nature make them particularly vulnerable to oxidative DNA damage (Noblanc et al. 2013). In addition, because of the way sperm chromosomes are organized in the sperm head, some chromosomes have interconnected regions that are more vulnerable to damage than others (Noblanc et al. 2013; Kocer et al. 2015). From a quantitative perspective, the oxidation of DNA bases is not a minor problem. As an illustration, in the mouse models of mild post-testicular oxidative damage we analyzed, even though the level of luminal epididymal oxidative stress is rather low (Chabory et al. 2009; Noblanc et al. 2013), the numbers of oxidized regions on mouse chromosomes were considerable. In one model, more than 15,000 DNA regions (with an average length of 300 bp each) were found significantly oxidized, including a set of 1000 highly oxidized regions (Kocer et al. 2015). This situation has to be compared with the observation that in a wild-type mouse, less than 60 sperm chromosomal regions were found significantly oxidized on average (Kocer et al. 2015). A theoretical calculation estimates that, in the mildly oxidized transgenic context, about one million guanine residues could be oxidized and will have to be replaced by the BER pathway of the oocyte. Moreover, this is only the visible tip of the iceberg since while guanine is the most sensitive base to oxidation, it is not the only base affected since all other bases can suffer oxidative damage. The replacement of all these oxidized bases puts great pressure on the oocyte repair systems, increasing the risk of errors or/and the impossibility of repair. In the absence of 8-OHdG repair, an increased risk of de novo mutations by transversion (following Hoogsteen base pairing between 8-oxoG with adenine) in embryonic cells and their transmission in offspring will occur (Ohno et al. 2014). These issues can have enormous implications for the optimal completion of the embryonic developmental program and, potentially, the health of the offspring and beyond.
Oxidative Alteration of the Sperm Nucleus and its Possible Consequences on Epigenetic Information
In addition to DNA, there are reasons to suspect that the proteins and RNA components of the sperm nucleus may also be affected by oxidation, thus altering the epigenetic information carried by the paternal nucleus (Champroux et al. 2018b).
Oxidation of Nuclear Proteins
Oxidation of sperm protamines does not seem to be a major problem because these proteins will be quickly removed from the paternal nucleus after fertilization. On the other hand, oxidative alteration of the sperm DNA regions still bound to nucleosomes will activate the oocyte BER pathway that may not faithfully replace the appropriate histone/histone variants in these sites of oxidative attack. It is therefore not difficult to imagine that changes in the composition of paternal nucleosomes (the so-called “histone” code) may lead to subtle changes in the expression of the concerned genes in the developing embryo. This aspect has not yet been studied.
Oxidation of Methylated Cytosine Residues
Another obvious example where alterations in the nuclear oxidation of sperm can influence epigenetic information concerns the different chemical modifications of the conventional methylcytosine (meC) mark. Immediately after fertilization, there is a particular cycle of demethylation that mainly concerns the paternal nucleus (McLay and Clarke 2003). Demethylation begins with the oxidation of meC to a hydroxy methyl-cytosine residue (hmeC) by the action of the TET enzyme machinery. HmeC will then be transformed into formylmethyl-cytosine (fmC), carboxymethyl-cytosine (camC), and finally cytosine (Wu and Zhang, 2017). The activity of TET is nothing less than an oxidation of meC. Spontaneous post-testicular oxidative alterations of mature sperm cells can therefore generate excessive transformations of meC into hmeC (M. Gentil, unpublished data), possibly modifying the kinetics and landscape of paternal DNA demethylation. Considering the fact that some regions of the male pronucleus are meant to escape the post-fertilization demethylation process, it is likely that if the meC pattern of these regions has been transformed into hmeC, a significant change in the pattern of imprinting within the male genome will result. The involvement of oxidative stress in CpG mutagenesis in the male germ line could therefore have a profound effect on the expression of paternally imprinted genes later in development. In addition, it has also been reported that the presence of 8-OHdG in a CpG doublet may impair the methylation process of the adjacent cytosine by interfering with methyl transferase activity, again leading to changes in the pattern of paternally imprinted regions (Wachsman 1997; Wu and Ni, 2015).
Oxidation of Sperm-Associated RNAs
Recently, it has been reported that the contribution of sperm to the embryo is not limited to paternal DNA. Spermatozoa also provides a complex set of noncoding short and long RNAs (ncRNAs) that have been assigned regulatory functions. These ncRNAs constitute another form of paternal epigenetic inheritance. In 2016, two research groups reported that male sperm cells under specific environmental conditions such as unbalanced nutrition or behavioral stress have different RNA loads that are, in turn, responsible for transmission of the father’s phenotype to the offspring (Sharma et al. 2016, Chen et al. 2016). Current unpublished studies from our team suggest that post-testicular (i.e., epididymal) exposure to moderate oxidizing conditions alters the profile of sperm ncRNAs, which may potentially affect embryonic development.
The consequences of such oxidative damage to sperm DNA and RNA for the health and well-being of the offspring, are extremely important issues that still needs to be assessed. One important feature to keep in mind is that sperm DNA oxidation (eventually leading to sperm DNA fragmentation) does not, of itself, impair the fertilizing ability of spermatozoa, particularly when ICSI (intra-cytoplasmic sperm injection) is used as the insemination procedure (Aitken et al. 1998a, b). Therefore, it is quite possible that a sperm cell with high levels of oxidative DNA damage could fertilize an oocyte and disrupt normal embryonic development. We may see the consequences of this mechanism in action in the high rates of cancer observed in the offspring of heavy male smokers (Lee et al. 2009). Unfortunately, we may see other examples of this association with the unrestricted use of ICSI. Already, there is an increase in the rate of spontaneous abortions associated with ICSI, but not with IVF, which may be partly due to oxidative damage to the sperm nucleus (Zhao et al. 2014). The risk of seeing further impacts on fetuses that develop into adults should be considered.
Endogenous and Exogenous Defense of Spermatozoa Against Oxidative DNA Damage
As mentioned above, mature sperm cells are not very well equipped to counteract the harmful effects of ROS. The low cytosol content, the particular lipid composition of plasma membranes that promote the production of ROS in the event of oxidation (Moazamian et al. 2015), and their inability to mount a genetically mediated antioxidant stress response make them easily vulnerable to oxidative damage. They therefore depend essentially on their environment to protect themselves from oxidative stress. As the focus of this chapter is on oxidative damage to the DNA of sperm cell, the following section will focus on the intrinsic and extrinsic arsenal of enzymes and scavengers protecting sperm from nuclear oxidative alterations. The first factors that come to mind when we think of the nuclear protection of sperm against oxidative damage are protamines. Protamines with their high content of cysteine residues and their associated thiol groups are involved in protecting sperm DNA from oxidative damage (Liang et al. 1999).
Although, during epididymal maturation, many thiol groups of protamines are oxidized to disulfide bonds, which stiffens the organization of the toroids and encloses the nucleus of the sperm in a condensed state, there are still free thiol groups that have the ability to mitigate oxidative attacks by blocking the action of ROS. Zinc also contributes directly to the nuclear protection of sperm. Zinc is incorporated into the nucleus of the sperm cell during spermiogenesis and the zinc content of the sperm chromatin is estimated to be one to one with protamine (Bjorndhal and Kvist 2010). In its conventional tertiary zinc finger structure, zinc mobilizes cysteine and histidine residues to form inter- and intramolecular zinc bridges (as is the case between proteins and DNA). By blocking thiol groups on protamines, zinc can therefore, to some extent, help protect them from oxidation to disulfide bridges, as a salt bridge involving zinc, thiol groups of histidine cysteine, and imidazole groups are rather stable and are not very sensitive to oxidation or reduction (Bjorndhal and Kvist 2010).
Apart from the anti-ROS action of protamine and zinc, sperm cells are poorly equipped to fight insults caused by ROS. It is the role of the epididymal environment and, later, of the seminal plasma, to ensure this protection. To this end, it has been demonstrated that the mouse caput epididymis synthesizes and secretes a significant amount of a H2O2 recycling enzyme belonging to the glutathione peroxidase family. The importance of this activity in protecting sperm (and in particular the paternal nucleus) from oxidative damage has been confirmed by observations that, in transgenic mice without such activity, sperm stored in the cauda epididymis have a high level of DNA oxidation. In addition, when transgenic males were mated with WT female mice, there was an increase in miscarriages, embryonic, and congenital malformations that were attributed to the oxidized state of paternal DNA and probably to the oocyte’s inability to successfully repair such damage (Chabory et al. 2009; Aitken et al. 2009). In addition to the secreted caput GPx, the mouse cauda epididymis also secretes a plasma-type GPX that contributes to ROS recycling events in the sperm storage compartment. Although this epididymal protection of sperm in transit provided by GPx has been found in several mammalian species, it does not fully apply to the human model which appears to rely more on peroxiredoxins (PRDX) than GPx (Fernandez and O’Flaherty 2018). Regardless of the type of ROS recycling enzyme used, it should be noted that maintaining an optimal ROS balance in the testis and particularly in the epididymis is of great importance. This protection is all the more important in the epididymis because at this stage in the life of sperm cells, they are transcriptionally and translationally silent and have no way of countering the harmful actions of ROS. One of the few responses that a sperm cell can mount in response to oxidative damage is apoptosis. Sperm cells retain the possibility of committing suicide through an almost classic apoptotic process that will also generate ROS (Koppers et al. 2011). In addition to the enzymatic recycling activities of ROS, which also include catalase and SOD2, epididymal and seminal fluids provide many nonenzymatic antioxidant molecules against toxic oxygen-derived molecules. Conventional hydrophilic and lipophilic scavengers, including vitamins, polyamines, carnitine, taurine, and trace elements such as selenium, are present in epididymal and seminal fluids and contribute to the protection of sperm cells.
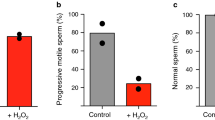
Whether or not it is relevant to enhance the antioxidant capacity of these fluids through antioxidant supplementation has been the subject of a debate that has stirred (and continues to stir) the scientific and clinical community (for a recent review see: Aitken et al. 2019). The rationale is logical, as it is clear that infertile men often have high levels of ROS and/or reduced antioxidant capacity in their seminal plasma and spermatozoa. Several oral antioxidant supplementation trials have been conducted over the years, but we are still waiting for a rigorous, carefully designed, large-scale, multicenter, double-blind, placebo-controlled, and randomized trial using rigorous selection criteria for both male and female patients. In addition, the dose of antioxidants, the choice of molecules to be used alone or in combination, and the duration of treatment are all aspects to be taken into serious consideration. A meta-analysis conducted in 2011 by Gharagozloo and Aitken, in which 20 clinical studies were rigorously evaluated, showed with little doubt that antioxidant supplementation reduces oxidative stress in semen and increases mobility in asthenozoosperm patients (Gharagozloo and Aitken 2011).
It remains to be seen how this translates into an improvement in the pregnancy rate. Indeed, because the latter is influenced by so many different factors, such confirmation may be a long time coming since it would require a very large and expensive trial to determine whether oral antioxidant supplements can significantly influence fertility. It may be more realistic to focus on whether such supplements are able to reverse biochemical markers of oxidative stress (lipid aldehyde formation, oxidative DNA damage, etc.…) in a sustained and reliable manner. We might be optimistic about the potential success of such trials because a carefully designed oral antioxidant supplementation has recently been reported to protect rodent sperm DNA from the harmful effects of acutely induced or chronic ROS and to result in improved reproductive success (Gharagozloo et al. 2016).
Despite the appeal of oral antioxidant supplementation to reduce oxidative damage to sperm DNA, caution should be exercised in developing these therapeutic approaches. If oxidative stress to the DNA of the sperm cell is a problem, so too is reductive stress (which may be the result of excess antioxidant) an undesirable situation (Cohen-Bacrie et al. 2009, Menezo et al. 2010; Gharagozloo and Aitken 2011). This is easily understandable considering that epididymal sperm maturation partly uses oxidative events (disulfide bridging of protamines) to stabilize the sperm nucleus and lock it into an optimally condensed state. If sperm cells are exposed to an excess of antioxidant, the risk is to promote decondensation of the nucleus which potentially has an impact on its sensitivity to other damage and also on the motility of the cell (if the sperm head is larger). Therefore, it is of paramount importance before any attempt at exogenous antioxidant treatment to assess the level of oxidation of the DNA in the patient’s sperm cell. We recently reported the optimization of a test using flow cytometry and an antibody against the oxidized guanine residue 8-OHdG that allowed us to determine a pathological threshold for the oxidation of human spermatozoa DNA in the clinic (Vorilhon et al. 2018). The systematic use of such a test in combination or not with a test designed to assess sperm DNA fragmentation would allow the selection of patients who would be best able to benefit from antioxidant supplementation.
Conclusion
Evidence from a wide variety of species over several decades has clearly demonstrated that the male reproductive system is very vulnerable to oxidative stress. When the ROS attack is severe, we may see a disruption of spermatogenesis or a suppression of sperm fertilizing potential, depending on where in the process of sperm production and maturation the oxidative stress occurs. Because sperm DNA is more vulnerable to oxidative stress than the cellular machinery controlling fertilization (Aitken et al. 1998a, b), it is also possible to arrive at a situation with lower levels of oxidative stress, wherein sperm production and fertilizing potential are not affected but the integrity of sperm DNA and RNA is seriously compromised. Under these circumstances, fertilization may be achieved with DNA/RNA damaged spermatozoa, with possible consequences for the developmental normality of the embryo and long-term health trajectory of the offspring. This is a particularly important concern in relation to the safety of assisted conception procedures, especially when ICSI is used as the insemination strategy (Aitken et al. 2018). The data from animal models are incontrovertible in demonstrating that oxidative damage to the male germ line can have detrimental impacts on embryonic development and offspring health as a consequence of such mechanisms; there is now an urgent need to address this question in our own species.
References
Aitken RJ (1997) Molecular mechanisms regulating human sperm function. Mol Hum Reprod 3(3):169–173
Aitken RJ (2011) The capacitation-apoptosis highway: oxysterols and mammalian sperm function. Biol Reprod 85(1):9–12. https://doi.org/10.1095/biolreprod.111.092528
Aitken RJ (2016) Oxidative stress and the ethiology of male infertility. J Assist Reprod Genet 33(12):1691–1692
Aitken RJ (2018) Not every sperm is sacred; a perspective on male infertility. Mol Hum Reprod 24(6):287–298
Aitken RJ, Curry BJ (2011) Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal 14(3):367–381. https://doi.org/10.1089/ars.2010.3186
Aitken RJ, Paterson M, Fisher H et al (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108:2017–2025
Aitken RJ, Gordon E, Harkiss D et al (1998a) Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 59(5):1037–1046
Aitken RJ, Harkiss D, Knox W et al (1998b) A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 111:645–656
Aitken RJ, De Iuliis GN, McLachlan RI (2009) Biological and clinical significance of DNA damage in the male germ line. Int J Androl 32(1):46–56. https://doi.org/10.1111/j.1365-2605.2008.00943.x
Aitken RJ, Jones KT, Robertson SA (2012) Reactive oxygen species and sperm function in sickness and in health. J Androl 33(6):1096–1106. https://doi.org/10.2164/jandrol.112.016535
Aitken RJ, Smith TB, Jobling MS et al (2014) Oxidative stress and male reproductive health. Asian J Androl 16(1):31–38. https://doi.org/10.4103/1008-682X
Aitken RJ, De Iuliis G, Drevet JR (2019) Role of oxidative stress in the etiology of male infertility and the potential therapeutic value of antioxidants. In: Henkel R, Samanta L, Agarwal A (eds) Oxidants, antioxidants, and impact of the oxidative stress in male reproduction. Academic Press-Elsevier, Oxford, UK, pp 90–100
Awda BJ, Buhr MM (2010) Extracellular signal-regulated kinases (ERKs) pathway and reactive oxygen species regulate tyrosine phosphorylation in capacitating boar spermatozoa. Biol Reprod 83(5):750–758. https://doi.org/10.1095/biolreprod.109.082008
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:1. https://doi.org/10.1155/2014/360438
Bennetts LE, Aitken RJ (2005) A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev 71(1):77–87
Björndahl L, Kvist U (2010) Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod 16(1):23–29. https://doi.org/10.1093/molehr/gap099
Brouwers JF, Boerke A, Silva PF et al (2011) Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol Repord 85(1):128–136. https://doi.org/10.1095/biolreprod.111.091207
Brütsch SH, Wang CC, Li L, Stender H et al (2015) Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the Alox15 gene does not rescue such knock-in mice. Antioxid Redox Signal 22(4):281–293. https://doi.org/10.1089/ars.2014.5967
Chabory E, Damon C, Lenoir A et al (2009) Epididylis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 119(7):2074–2085. https://doi.org/10.1172/JCI38940
Champroux A, Damon-Soubeyrand C, Goubely C et al (2018a) Nuclear integrity but not Topology of mouse sperm chromosome is affected by oxidative DNA damage. Genes (Basel);9(10). pii: E501. https://doi.org/10.3390/genes9100501
Champroux A, Cocquet J, Henry-Berger J et al (2018b) A decade of exploring the mammalian sperm Epigenome: paternal epigenetic and transgenerational inheritance. Front Cell Dev Biol 6:50. https://doi.org/10.3389/fcell.2018.00050
Chen Q, Yan M, Cao Z et al (2016) Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351(6271):397–400. https://doi.org/10.1126/science.aad7977
Cohen-Bacrie P, Belloc S, Ménézo YJ et al (2009) Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril 91(5):1801–1805. https://doi.org/10.1016/j.fertnstert.2008.01.086
Cooper TG, Noonan E, von Eckardstein S et al (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16(3):231–245. https://doi.org/10.1093/humupd/dmp048
De Iuliis GN, Thomson LK, Mitchell LA et al (2009) DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod 81(3):517–524. https://doi.org/10.1095/biolreprod.109.076836
Donà G, Fiore C, Andrisani A et al (2011) Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum Reprod 26(12):3264–3273. https://doi.org/10.1093/humrep/der321
Drevet JR (2006) The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol 250(1–2):70–79
Fernandez MC, O’Flaherty C (2018) Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum Reprod 33:1394. https://doi.org/10.1093/humrep/dey221
Gharagozloo P, Aitken RJ (2011) The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 26:1628–1640
Gharagozloo P, Gutiérrez-Adán A, Champroux A et al (2016) A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum Reprod 31(2):252–262. https://doi.org/10.1093/humrep/dev302
Giwercman A, Lindstedt L, Larsson M et al (2010) Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl 33(1):e221–e227. https://doi.org/10.1111/j.1365-2605.2009.00995
Henkel RR (2011) Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl 13(1):43–52. https://doi.org/10.1038/aja.2010.76
Houston BJ, Nixon B, King BV et al (2016) The effects of radiofrequency electromagnetic radiation on sperm function. Reproduction 152(6):R263–R276
Houston BJ, Nixon B, Martin JH et al (2018) Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol Reprod 98(4):593–606. https://doi.org/10.1093/biolre/ioy009
Johnson GD, Lalancette C, Linnemann AK et al (2011) The sperm nucleus: chromatin, RNA, and the nuclear matrix. Reproduction 141(1):21–36. https://doi.org/10.1530/REP-10-0322
Jones R, Mann T, Sherins RJ (1978) Adverse effects of peroxidized lipid on human spermatozoa. Proc R Soc Lond B Biol Sci 201(1145):413–417
Jones R, Mann T, Sherins R (1979) Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril 31(5):531–537
Kocer A, Henry-Berger J, Noblanc A et al (2015) Oxidative DNA damage in mouse sperm chromosomes: Size matters. Free Radic Biol Med 89:993–1002. https://doi.org/10.1016/j.freeradbiomed
Koppers AJ, Mitchell LA, Wang P et al (2011) Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J 436(3):687–698. https://doi.org/10.1042/BJ20110114
Leclerc P, de Lamirande E, Gagnon C (1997) Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med 22(4):643–656
Lee KM, Ward MH, Han S et al (2009) Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res 33:250–258
Lenzi A, Picardo M, Gandini L et al (1996) Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update 2(3):246–256
Lewis B, Aitken RJ (2001) A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J Androl 22(4):611–622
Lewis SE, Boyle PM, McKinney KA et al (1995) Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril 64(4):868–870
Liang R, Senturker S, Shi X et al (1999) Effects of Ni(II) and Cu(II) on DNA interaction with the N-terminal sequence of human protamine P2: enhancement of binding and mediation of oxidative DNA strand scission and base damage. Carcinogenesis 20(5):893–898
McLay DW, Clarke HJ (2003) Remodelling the paternal chromatin at fertilization in mammals. Reproduction 125(5):625–633
Ménézo Y, Dale B, Cohen M (2010) DNA damage and repair in human oocytes and embryos: a review. Zygote 18(4):357–365. https://doi.org/10.1017/S0967199410000286
Moazamian R, Polhemus A, Connaughton H et al (2015) Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod 21(6):502–515. https://doi.org/10.1093/molehr/gav014
Noblanc A, Damon-Soubeyrand C, Karrich B et al (2013) DNA oxidative damage in mammalian spermatozoa: where and why is the male nucleus affected? Free Radic Biol Med 65:719–723. https://doi.org/10.1016/j.freeradbiomed.2013.07.044
O’Flaherty C, de Lamirande E, Gagnon C (2006) Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med 41(4):528–540
Ohno M, Sakumi K, Fukumura R et al (2014) 8-oxoguanine causes spontaneous de novo germline mutations in mice. Sci Rep 4:4689. https://doi.org/10.1038/srep04689
Sawyer DE, Mercer BG, Wiklendt AM et al (2003) Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat Res 529(1–2):21–34
Sharma U, Conine CC, Shea JM et al (2016) Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351(6271):391–396. https://doi.org/10.1126/science.aad6780
Shoeb M, Ansari NH, Srivastava SK et al (2014) 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem 21(2):230–237
Smith TB, De Iuliis GN, Lord T et al (2013a) The senescence-accelerated mouse prone 8 as a model for oxidative stress and impaired DNA repair in the male germ line. Reproduction 146(3):253–262. https://doi.org/10.1530/REP-13-0186
Smith TB, Dun MD, Smith ND et al (2013b) The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J Cell Sci 126(Pt 6):1488–1497. https://doi.org/10.1242/jcs.121657
Vorilhon S, Brugnon F, Kocer A et al (2018) Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum Reprod 33(4):553–562. https://doi.org/10.1093/humrep/dey038
Wachsman JT (1997) DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutat Res 375(1):1–8
Walters JLH, De Iuliis GN, Dun MD (2018) Pharmacological inhibition of arachidonate 15-lipoxygenase protects human spermatozoa against oxidative stress. Biol Reprod 98(6):784–794. https://doi.org/10.1093/biolre/ioy058
Wu Q, Ni X (2015) ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr Drug Targets 16(1):13–19
Wu X, Zhang Y (2017) TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 18(9):517–534. https://doi.org/10.1038/nrg.2017.33
Zhao J, Zhang Q, Wang Y et al (2014) Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 102:998–1005
Authors’ Roles
J.R.D. drafted the article, which was then edited and revised by R.J.A. Both authors approved the final version of this article.
Conflict of Interest
J.R.D. and R.J.A. are scientific advisors of a US-based biotech company (Celloxess LLC, New Jersey, USA) which has a commercial interest in the detection and treatment of oxidative stress.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Drevet, J.R., Aitken, R.J. (2019). Oxidative Damage to Sperm DNA: Attack and Defense. In: Baldi, E., Muratori, M. (eds) Genetic Damage in Human Spermatozoa. Advances in Experimental Medicine and Biology, vol 1166. Springer, Cham. https://doi.org/10.1007/978-3-030-21664-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-21664-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21663-4
Online ISBN: 978-3-030-21664-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)