Abstract
In ALL evaluation of molecular treatment response, assessment of minimal residual disease (MRD) is a substantial independent predictor of outcome, as proven by randomized studies (Conter et al. 2010; Gökbuget et al.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Monitoring MRD in ALL
1.1 Introduction
In ALL evaluation of molecular treatment response, assessment of minimal residual disease (MRD) is a substantial independent predictor of outcome, as proven by randomized studies (Conter et al. 2010; Gökbuget et al. 2012; Bassan and Spinelli 2015). Consequently, MRD is implemented in virtually all clinical protocols in order to supplement or to redefine multifactorial risk stratification with optional customized treatment intensity. The detection of leukemic cells below the limit of classical cytomorphology is feasible either by disease-specific alterations of the immune phenotype or unique genetic features. Several competing and complementing MRD methods have been developed with preference application according to clinical protocols (Van der Velden et al. 2007; van Dongen et al. 2015).
1.2 MRD Assessment by IG/TCR Real-Time PCR
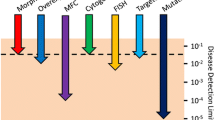
The discontinuous immune receptor genes provide the immune repertoire by somatic recombination of variable (V)-, diversification (D)-, and junction (J)- elements thus forming hypervariable CDR3 (complement determine region 3) regions during lymphocyte maturation. Such rearrangements can serve as clonal index of leukemia blasts originating from lymphoid precursor stages. Additionally, due to a relaxed regulatory control, leukemia blasts can harbor incomplete rearrangements and cross-lineage rearrangements and tend to accumulate simultaneously multiple rearrangements. Quantitative real-time PCR using junction complementary allele-specific oligonucleotides (ASO) frequently reaches a detection limit of 1E-05 with a quantitative range of 1E-04, is applicable to vast majority of cases, and has a high degree of standardization (Van der Velden et al. 2007).
1.3 MRD Assessment by Fusion Gene Transcript
Most frequent recurrent reciprocal translocations are in ALL t(9;22)(q34;q11) (BCR-ABL1), t(12;21)(p13;q23) (ETV6-RUNX1), and t(4;11)(q21;q22) (MLL-AFF1) with age stage associated preponderance in adults, childhood, and infant ALL, respectively. Derived chimeric fusion transcripts are validated marker for MRD detection by real-time PCR with an achievable detection limit of 1E-06. The methodology has been standardized by the Europe Against Cancer (EAC) program (Gabert et al. 2003).
1.4 NGS (Next-Generation Sequencing)
High-throughput sequencing (HTS) of immune receptor genes by next-generation sequencing (NGS) is a novel option for MRD. This methodology provides comprehensive qualitative and quantitative information regarding clonal consistence of the diagnostic sample and shares one protocol for index determination and MRD assessment without need of individual reagents. Potential sub- and new emerging leukemic clones also are covered. PCR steps during library construction can introduce bias effecting results internal controls and normalization calculations are necessary the generated data volume is high and data interpretation demand biostatistics expertise. Due to high sample capacity, NGS favors a centralized concept and service is available to commercial providers by academic centers (Kotrova et al. 2015).
1.5 Flow Cytometry
MRD by multicomponent flow cytometry (MFC) distinguishes leukemia-associated immune phenotypes (LAIP) and regular cells. LAIP consists of cell lineage maturation stage-specific (backbone) markers in combination with illegitimate markers. The standard four to six color approaches have been developed simultaneously by several centers. Therefore, the applied marker panels depend on study protocol. The consistently achieved detection limit is 1E-04. Recently, increase of specificity and sensitivity was enabled by high-throughput procedures demanding eight or ten color equipment. Here the options for targeted and visualized antigens allow simultaneous visualization of all developmental lymphocyte stages serving as background to distinguish leukemic cells. The EuroFlow Consortium validated available antibody panels and controls which can be applied in a standardized way, including automated gating with supportive software, data storage and comparison, accurate quantitative result, and option for IVD development. Similar to the NGS approach, the generated data volume is high and data interpretation demands biostatistics expertise; nevertheless, the concept allows decentralized data acquisition (Pedreira et al. 2013).
1.6 Limitations of MRD Assessment
The determined level of MRD always is a result of complex interrelation of baseline characteristics of tumor and patient, time point of MRD evaluation, therapeutic agents, course of clearance, and degree of therapy resistance. Several measurements therefore are mandatory. Adverse circumstances for MRD assessment are clonal selection and clonal evolution, since the associated index might be missed. Potentially impacted are leukemia with initial oligoclonality as observed in approximately 15% of B-ALL and up to 1000 subclones have been reported (Wu et al. 2016). Phenotypic plasticity under treatment and massive lymphocyte regeneration can cause false negativity or positivity, a solvable problem by applying mentioned high-throughput methodologies. Achievable detection limit is correlated with cell count of sample, and aplastic samples are challenging. Finally, all methodologies use different sample preparations, and analyses refer to different units, a circumstance which interferes result comparison.
1.7 MRD in the Setting of HSCT
As all adult patients with ALL who relapse after initial chemotherapy have an absolute indication for allo-HSCT, pediatric patients are stratified into different treatment groups. Main prognostic determinants in these patients are the blast immune phenotype, time to relapse, and site of relapse. High-risk patients who experienced early isolated BM relapse, early relapse involving BM, and any BM relapse of T-lineage ALL have clear indication for HSCT. Intermediate-risk patients experienced early or late combined BM relapse and a late isolated BM relapse of a B-cell precursor (BCP).
ALL and very early and early isolated extramedullary relapse of either BCP-ALL or T-ALL have indication for HSCT if post induction MRD exceeds a threshold of 1E-03 (Eckert et al. 2013).
During the past decades, it could be clearly shown by several studies that the level of MRD immediately prior to transplant does have a clear prognostic impact on post-HSCT outcome (Knechtli et al. 1998). Retrospective studies in children with relapsed ALL revealed an important cutoff for post-HSCT outcome. Patients who received transplantation with an MRD load of ≥10 to 4 leukemic cells had a by far inferior prognosis than patients with lower MRD loads before transplant (Bader et al. 2009). Based on these findings, several studies are now underway investigating strategies to improve outcome in these ultrahigh-risk patients. Adaption of transplant approaches might allow successful transplantation (Leung et al. 2012).
Spinelli et al. showed that almost half of the patients with high levels of MRD before transplantation achieved molecular remission by day +100 (Spinelli et al. 2007). This finding indicates that MRD detection post transplant provides additional value to the MRD assessment prior to transplantation. It could be demonstrated in prospective clinical studies that the close monitoring of MRD by different approaches allows the prediction of relapse and may therefore form the basis of different intervention strategies making use of leukemia-specific targeted therapy (Bader et al. 2015; Balduzzi et al. 2014). Future perspectives will focus on MRD-guided intervention to prevent overt relapse (Rettinger et al. 2017).
2 Monitoring MRD in AML
2.1 Introduction
The possibility of defining residual disease far below the level of 5% leukemic cells is changing the landscape of risk classification. This so-called measurable/MRD approach at present establishes the presence of leukemia cells down to levels of 1:103 to 1:106 white blood cells, compared to 1:20 for morphology. Recently the ELN proposed a new response criterium: CR without minimal residual disease (CRMRD-) is defined as CR with negativity for a genetic marker by RT-qPCR or CR with negativity by multicolor flow cytometry (MFC) (Döhner et al. 2017).
The reasons to apply MRD assessment in AML are (1) to provide an objective establishment of remission status; (2) to better predict outcome and guide post-remission treatment; (3) to identify early relapse as a robust post transplant surveillance, in order to enable early intervention; and (4) to be used as a surrogate endpoint to fasten drug testing and approval.
A recent ELN MRD consensus document was published with the aim to identify key clinical and scientific issues in the measurement and application of MRD in AML and to provide guidelines for the current and future use of MRD in clinical practice (Schuurhuis et al. 2018).
2.2 Methods for MRD Detection
2.2.1 MRD Detection by PCR
Real-time quantitative PCR (RT-qPCR) allows MRD detection in cases with chimeric fusion genes generated by balanced chromosomal rearrangements (Grimwade and Freeman 2014). Other genetic alterations can also be used for MRD detection including insertions/duplications, point mutations and gene overexpression. Apart from t(15;17) and RUNX1–RUNX1T1 and CBFB–MYH11, currently NPM1 is the best-validated molecular marker for MRD assessment. PCR assessment of MRD is in about 50% of patients in principle possible. The methodology has been standardized for several molecular markers for clinical implementation in the Europe Against Cancer (EAC) program (Gabert et al. 2003).
2.2.2 Immune MRD by Multicolor Flow Cytometry
The basic principle is to identify at diagnosis leukemia-associated immune phenotypes (LAIP). These LAIPs consist of normally occurring markers, present in aberrant combinations in AML but in very low frequencies in normal and regenerating BM. The background levels of LAIP in normal and regenerating BM levels, in particular, although low, prevent specific detection of aberrancies with sensitivities higher than 1:10,000.
If no diagnosis sample is present, one can make use of “different from normal” approach which uses a standard fixed antibody panel to recognize leukemic cells based on their difference with normal hematopoietic cells (Loken et al. 2012).
Currently, immune MRD aberrancies may be detected in over 90% of AML cases at diagnosis.
2.3 MRD in Clinical Studies
Despite a multitude of prognostic factors at diagnosis, the outcome of patients is still highly variable and not individually predictable. It thus seems that prognosticators at diagnosis will not enable clinicians to reach the ultimate goal of truly individualized risk assessment. Treatment parameters may be more useful (Ossenkoppele and Schuurhuis 2013).
Two large, prospective, multicenter studies have identified flow cytometry-based MRD as an independent prognostic indicator in adults with AML (Freeman et al. 2013; Terwijn et al. 2013). Flow cytometry-based MRD was assessed in a multicenter, multinational study in adults with AML between 18 and 60 years of age by HOVON/SAKK investigators (Terwijn et al. 2013). Patients were treated according to protocol, without knowledge of MRD-related data. In this study, lower levels of MRD were associated with better outcomes than higher levels, and MRD levels >0.1% of white blood cells after the second cycle of chemotherapy were associated with higher risk of relapse in multivariate analysis. The UK NCRI group assessed MRD using flow cytometry in 427 patients older than 60 years of age (Freeman et al. 2013). MRD negativity after the first cycle of chemotherapy conferred significantly better 3-year survival after CR. MRD-positive patients had increased relapses and higher risk of early relapse (median time to relapse, 8.5 v 17.1 months, respectively).
An example indicative for the usage of molecular MRD was recently published by Ivey et al. (2016) who showed in a large study by NCRI that the presence of MRD, assessed by Q-PCR of NPM1-mutated transcripts, provided powerful prognostic information independent of other risk factors. Persistence of NPM1-mutated transcripts in blood was present in 15% of the patients after the second chemotherapy cycle and was associated with a greater risk of relapse after 3 years of follow-up than was an absence of such transcripts (82% vs. 30%; hazard ratio, 4.80) and a lower rate of survival (24% vs. 75%; hazard ratio for death, 4.38).
Many other studies point in the same direction that MRD status after two cycles of chemotherapy is highly predictive independently from other prognostic factors for outcome (Hourigan et al. 2017). However surrogacy for survival has not been proven yet (Ossenkoppele and Schuurhuis 2016).
2.4 Pretransplant MRD
Evidence is accumulating that the presence of MRD assessed by multicolor flow cytometry immediately prior to allogeneic HCT is a strong, independent predictor of post transplant outcomes in AML (Buckley et al. 2017; Walter et al. 2015). In a recent update, Araki et al. showed that in 359 adults, the 3-year relapse rate was 67% in MRD-positive patients, compared to 22% in MRD-negative patients, resulting in OS of 26% vs. 73%, respectively (Araki et al. 2016). This applies for the myeloablative as well as for the non-myeloablative transplant setting.
Also molecular MRD as measured by RT-PCR in NPM1-mutated AML has a significant impact on outcome after allo-HSCT (Balsat et al. 2017). Performing an allo-HSCT in case of a suboptimal reduction (<4 log10) of NPM1 levels after chemotherapy resulted in improved overall survival. In patients with optimal (≥4 log10) reduction of NPM1 levels after chemotherapy, allo-HSCT had no significant effect on survival. However, no prospective studies using MRD to guide post-remission therapy are available at the time of this publication. Regardless, it is clear that novel treatment strategies before, during, and after transplant are urgently needed to improve outcomes in AML. Thereby depth of response prior to transplant, as measured by level of MRD, has emerged as one of the most important predictors of transplant outcome. Randomized trials are warranted to determine if MRD-guided preemptive therapy is associated with improved outcome.
Currently no clinical trial including transplantation trials should be performed without including MRD assessment.
2.5 Future Developments
New technologies are emerging to assess MRD. Quantifying leukemic stem cells is such a promising approach (Terwijn et al. 2014; Zeijlemaker et al. 2016). Next-generation sequencing for MRD assessment can, theoretically, be applied to all leukemia-specific genetic aberrations. In a recent HOVON study, it was shown that persistence of gene mutations in CR appeared to be a highly significant independent prognostic value for relapse and overall survival (Jongen-Lavrencic et al. 2018).
Key Points
-
MRD should be included in the definition of CR.
-
MRD positivity is an independent predictor of relapse after chemotherapy in AML patients and a negative predictor for ALL patients.
-
Pretransplant MRD positivity is highly indicative for relapse.
-
MRD assessment should be implemented in every clinical trial.
References
Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34:329–36.
Bader P, Kreyenberg H, Henze G, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–84.
Bader P, Kreyenberg H, Stackelberg A, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol. 2015;33:1275–84.
Balduzzi A, Di Maio L, Silvestri D, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol. 2014;164:396–408.
Balsat M, Renneville A, Thomas X, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French Association Group. J Clin Oncol. 2017;35:185–93.
Bassan R, Spinelli O. Minimal residual disease monitoring in adult ALL to determine therapy. Curr Hematol Malig Rep. 2015;10:86–95.
Buckley SA, Wood BL, Othus M, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102:865–73.
Conter V, Bartram C, Valsecchi M, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–14.
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Eckert C, von Stackelberg A, Seeger K, et al. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia—long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346–55.
Freeman SD, Virgo P, Couzens S, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31:4123–31.
Gabert J, Beillard E, van der Velden V, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer Program. Leukemia. 2003;17:2318–57.
Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–76.
Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for prime time? Blood. 2014;124:3345–55.
Hourigan CS, Gale RP, Gormley NJ, et al. Measurable residual disease testing in acute myeloid leukaemia. Leukemia. 2017;31:1482–90.
Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–33.
Jongen-Lavrencic M, Grob T, Kavelaars F, et al. Comprehensive molecular residual disease detection of clinical value in acute myeloid leukemia. N Engl J Med. 2018;378:1189–99.
Knechtli C, Goulden N, Hancock J, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072–9.
Kotrova M, Muzikova K, Mejstrikova E, et al. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood. 2015;126:1045–7.
Leung W, Pui C-H, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468–72.
Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood. 2012;120:1581–8.
Ossenkoppele G, Schuurhuis GJ. MRD in AML: time for redefinition of CR? Blood. 2013;121:2166–8.
Ossenkoppele G, Schuurhuis GJ. MRD in AML: does it already guide therapy decision-making? Hematology Am Soc Hematol Educ Program. 2016;2016:356–65.
Pedreira C, Costa E, Lecrevisse Q. Overview of clinical flow cytometry data analysis: recent advances and future challenges. Trends Biotechnol. 2013;31:415–25.
Rettinger E, Merker M, Salzmann-Manrique E, et al. Pre-emptive immunotherapy for clearance of molecular disease in childhood acute lymphoblastic leukemia after transplantation. Biol Blood Marrow Transplant. 2017;23:87–95.
Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: consensus document from ELN MRD Working Party. Blood. 2018;131(12):1275–91. https://doi.org/10.1182/blood-2017-09-801498.
Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92:612–8.
Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31:3889.
Terwijn M, Zeijlemaker W, Kelder A, et al. Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia. PLoS One. 2014;9(9):e107587.
van der Velden V, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11.
van Dongen J, van der Velden V, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125:3996–4009.
Walter RB, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–44.
Wu J, Jia S, Wang C, et al. Minimal residual disease detection and evolved IGH clones analysis in acute B lymphoblastic leukemia using IGH deep sequencing. Front Immunol. 2016;7:403.
Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJ, et al. A simple one-tube assay for immunophenotypical quantification of leukemic stem cells in acute myeloid leukemia. Leukemia. 2016;30:439–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 EBMT and the Author(s)
About this chapter
Cite this chapter
Bader, P., Kreyenberg, H., Ossenkoppele, G. (2019). Monitoring Minimal Residual Disease in ALL and AML. In: Carreras, E., Dufour, C., Mohty, M., Kröger, N. (eds) The EBMT Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-02278-5_57
Download citation
DOI: https://doi.org/10.1007/978-3-030-02278-5_57
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-02277-8
Online ISBN: 978-3-030-02278-5
eBook Packages: MedicineMedicine (R0)