Abstract
The successful delivery of drug molecules to a desired tissue is contingent upon some degree of direct or indirect identification of that tissue by the drug dosage form. This can be approached from the perspective of engineering the material to be responsive to the environment either at the interface or surrounding the tissue. We discuss the first approach in this chapter and the second approach later in Chap. 7.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Muller, R. H., & Keck, C. M. (2004). Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. Journal of Biotechnology, 113(1), 151–170.
(a) Lincoff, A. M. (1998). Trials of platelet glycoprotein IIb/IIIa receptor antagonists during percutaneous coronary revascularization. The American Journal of Cardiology, 82(8), 36P–42P. (b) Chonn, A., & Cullis, P. R. (1995). Recent advances in liposomal drug–delivery systems. Current Opinion in Biotechnology, 6(6), 698–708.
(a) Harris, J. M., & Chess, R. B. (2003). Effect of pegylation on pharmaceuticals. Nature Reviews Drug Discovery, 2(3), 214–221. (b) Corsi, D. F., Prosperi, D. D., Wang, M., & Thanou, M. (2010). Targeting nanoparticles to cancer. Pharmacological Research, 62(2), 90–99.
Dainty, L. A., Risinger, J. I., Morrison, C., Chandramouli, G. V. R., Bidus, M. A., Zahn, C., et al. (2007). Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecologic Oncology, 105(3), 563–570.
Lopes, J., Santos, G., Barata, P., Oliveira, R., & Lopes, C. M. (2013). Physical and chemical stimuli-responsive drug delivery systems: Targeted delivery and main routes of administration. Current Pharmaceutical Design, 19(41), 7169–7184.
Leon Glass, P. H. (1991). Theory of heart: Biomechanics, biophysics, and nonlinear dynamics of cardiac function. New York: Springer.
Albanese, A., Tang, P. S., & Chan, W. C. W. (2012). The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual Review of Biomedical Engineering, 14, 1–16.
(a) Kensey, K. R., & Cho, Y. I. (2007). The origin of atherosclerosis: What really initiates the inflammatory process (p. 200). Summerville, WA: SegMedica. (b) Ernst, E., Resch, K. L., Matrai, A., & Paulsen, H. F. (1991). Hyperviscosity. An independent risk factor after a survived stroke. Acta Medica Austriaca, 18(Suppl 1), 32–36.
Sloop, G. D. (1996). A unifying theory of atherogenesis. Medical Hypotheses, 47(4), 321–325.
Van den Berg, A. M. J., de Laat, A. W. M., Smith, P. J., Perelaer, J., & Schubert, U. S. (2007). Geometric control of inkjet printed features using a gelating polymer. Journal of Materials Chemistry, 17(7), 677.
Harzallah, O., & Dupuis, D. (2003). Rheological properties of suspensions of TiO2 particles in polymer solutions. 1. Shear viscosity. Rheologica Acta, 42(1–2), 10–19.
Wiese, G., & Healy, T. (1975). Coagulation and electrokinetic behavior of TiO2 and Al2O3 colloidal dispersions. Journal of Colloid and Interface Science, 51(3), 427–433.
Heijman, S. G. J., & Stein, H. N. (1995). Electrostatic and sterical stabilization of TiO2 dispersions. Langmuir, 11(2), 422–427.
Einstein, A. (1905). Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik, 322(8), 549–560.
(a) Hochmuth, R. M., Mohandas, N., & Blackshear, P. L. (1973). Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophysical Journal, 13(8), 747–762. (b) Kolhar, P., Anselmo, A. C., Gupta, V., Pant, K., Prabhakarpandian, B., Ruoslahti, E., & Mitragotri, S. (2013). Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proceedings of the National Academy of Sciences of the United States of America, 110(26), 10753–10758.
Prigogine, I., & Rice, S. A. (Eds.). (1995). Advances in Chemical Physics (Vol. 91). Hoboken, NJ: Wiley.
Kynch, G. J. (1954). The effective viscosity of suspensions. British Journal of Applied Physics, 5(S3), S5–S10.
Elias, H. (2003). Theta solvents. New York: Wiley (Wiley Database of Polymer Properties).
Pauling, L., Corey, R. B., & Branson, H. R. (1951). The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proceedings of the National Academy of Sciences of the United States of America, 37(4), 205–211.
Bicerano, J., Douglas, J. F., & Brune, D. A. (1999). Model for the viscosity of particle dispersions. Journal of Macromolecular Science, Part C: Polymer, 39(4), 561–642.
Helfrich, W. (1973). Elastic properties of lipid bilayers: Theory and possible experiments. Zeitschrift für Naturforschung. Teil C: Biochemie, Biophysik, Biologie, Virologie, 28(11), 693–703.
Johnson, K. L. (1987). Contact mechanics (p. 452). Cambridge, England: Cambridge University Press.
Popov, V. L. (2010). Contact mechanics and friction: Physical principles and applications (p. 378). Berlin, Germany: Springer (Google eBook).
Shadnia, H., Wright, J. S., & Anderson, J. M. (2009). Interaction force diagrams: New insight into ligand–receptor binding. Journal of Computer-Aided Molecular Design, 23(3), 185–194.
Elias, D. R., Poloukhtine, A., Popik, V., & Tsourkas, A. (2013). Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine: Nanotechnology, Biology and Medicine, 9(2), 194–201.
Zhang, S., Li, J., Lykotrafitis, G., Bao, G., & Suresh, S. (2009). Size-dependent endocytosis of nanoparticles. Advanced Materials (Deerfield Beach, Fla.), 21, 419–424.
Davies, M., Brindley, A., Chen, X., Marlow, M., Doughty, S. W., Shrubb, I., & Roberts, C. J. (2005). Characterization of drug particle surface energetics and Young’s modulus by atomic force microscopy and inverse gas chromatography. Pharmaceutical Research, 22(7), 1158–1166.
Rejman, J., Oberle, V., Zuhorn, I. S., & Hoekstra, D. (2004). Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. The Biochemical Journal, 377(Pt 1), 159–169.
(a) Dunn, B. M., & Chaiken, I. M. (1974). Quantitative affinity chromatography. Determination of binding constants by elution with competitive inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 71(6), 2382–2385. (b) Ruoslahti, E., & Pierschbacher, M. (1987). New perspectives in cell adhesion: RGD and integrins. Science, 238(4826), 491–497.
Lauffenburger, D. A., & Linderman, J. J. (1993). Receptors: Models for binding, trafficking, and signaling (p. 376). Oxford, England: Oxford University Press.
Wong, C. C., Cheng, K.-W., & Rigas, B. (2012). Preclinical predictors of anticancer drug efficacy: Critical assessment with emphasis on whether nanomolar potency should be required of candidate agents. The Journal of Pharmacology and Experimental Therapeutics, 341(3), 572–578.
Muzykantov, V., & Torchilin, V. (2002). Biomedical aspects of drug targeting (p. 506). Berlin, Germany: Springer (Google eBook).
Packeu, A., Wennerberg, M., Balendran, A., & Vauquelin, G. (2010). Estimation of the dissociation rate of unlabelled ligand-receptor complexes by a “two-step” competition binding approach. British Journal of Pharmacology, 161(6), 1311–1328.
Gilli, P., Ferretti, V., Gilli, G., & Borea, P. A. (1994). Enthalpy–entropy compensation in drug–receptor binding. The Journal of Physical Chemistry, 98(5), 1515–1518.
Donaruma, L. G. (1986). Surface and interfacial aspects of biomedical polymers. In J. D. Andrade (Ed.), Surface chemistry and physics (Vol. 1, p. 479). New York: Plenum. Journal of Polymer Science Part C: Polymer Letters, 24(8), 427–428. (b) Surfaces of Nanoparticles and Porous Materials (Google eBook) (p. 812). 2002. CRC Press.
Aqvist, J., Medina, C., & Samuelsson, J. E. (1994). A new method for predicting binding affinity in computer-aided drug design. Protein Engineering, 7(3), 385–391.
(a) Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., & Thompson, M. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials, 8(7), 543–557. (b) Batchelor, G. K. (2000). An introduction to fluid dynamics. Cambridge, England: Cambridge University Press.
Serway, R. A. (2012). Physics for scientists and engineers (Vol. 1, 9th ed., p. 784). New Delhi: Cengage Learning.
(a) Norde, W., & Haynes, C. A. (1995). In T. A. Horbett & J. L. Brash (Eds.), Proteins at interfaces II (Vol. 602, pp. 26–40). Washington, DC: American Chemical Society. (b) Yoon, J.-Y., Kim, J.-H., & Kim, W.-S. (1999). The relationship of interaction forces in the protein adsorption onto polymeric microspheres. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 153(1), 413–419.
Lopez, J., Chew, S., Thompson, H., Malter, J., Insler, M., & Beuerman, R. (1992). EGF cell surface receptor quantitation on ocular cells by an immunocytochemical flow cytometry technique. Investigative Ophthalmology & Visual Science, 33(6), 2053–2062.
Heller, H. C., & Hillis, D. M. (2011). Life: The science of biology (p. 1266). New York: W.H. Freeman.
Mehrer, H. (2007). Diffusion in solids: Fundamentals, methods, materials, diffusion-controlled processes (p. 672). Berlin: Springer (Google eBook).
(a) Singh, S. K., Piscitelli, C. L., Yamashita, A., & Gouaux, E. (2008). A competitive inhibitor traps LeuT in an open-to-out conformation. Science, 322(5908), 1655–1661. (b) Rubinow, S. I., & Dembo, M. (1977). The facilitated diffusion of oxygen by hemoglobin and myoglobin. Biophysical Journal, 18(1), 29–42.
Prescott, D. M., & Zeuthen, E. (1953). Comparison of water diffusion and water filtration across cell surfaces. Acta Physiologica Scandinavica, 28(1), 77–94.
Haynie, D. T. (2001). Biological thermodynamics (pp. 130–136). Cambridge, England: Cambridge University Press.
Holdich, R., Kosvintsev, S., Cumming, I., & Zhdanov, S. (2006). Pore design and engineering for filters and membranes. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences, 364(1838), 161–174.
(a) Kuiper, S., Brink, R., Nijdam, W., Krijnen, G. J. M., & Elwenspoek, M. C. (2002). Ceramic microsieves: Influence of perforation shape and distribution on flow resistance and membrane strength. Journal of Membrane Science, 196(2), 149–157. (b) Kuiper, S., van Rijn, C., Nijdam, W., Raspe, O., van Wolferen, H., Krijnen, G., & Elwenspoek, M. (2002). Filtration of lager beer with microsieves: Flux, permeate haze and in-line microscope observations. Journal of Membrane Science, 196(2), 159–170.
Dagan, Z., Weinbaum, S., & Pfeffer, R. (2006). General theory for the creeping motion of a finite sphere along the axis of a circular orifice. Journal of Fluid Mechanics, 117(1), 143.
Kosvintsev, S., Holdich, R. G., Cumming, I. W., & Starov, V. M. (2002). Modelling of dead-end microfiltration with pore blocking and cake formation. Journal of Membrane Science, 208(1), 181–192.
Alberts, B. (2002). Molecular biology of the cell. New York: Garland Science.
Camerino, D. C., Tricarico, D., & Desaphy, J.-F. (2007). Ion channel pharmacology. Neurotherapeutics, 4(2), 184–198.
Wileman, T., Harding, C., & Stahl, P. (1985). Receptor-mediated endocytosis. The Biochemical Journal, 232(1), 1–14.
Kerr, M. C., & Teasdale, R. D. (2009). Defining macropinocytosis. Traffic, 10(4), 364–371.
Campbell, N. A., & Reece, J. B. (2002). Biology (p. 1247). San Francisco: Benjamin-Cummings Publishing Company.
Parham, P. (2005). The immune system, p. 246 (p. 18). New York: Garland Science.
Howe, C. L. (2005). Modeling the signaling endosome hypothesis: Why a drive to the nucleus is better than a (random) walk. Theoretical Biology & Medical Modelling, 2, 43.
Rappoport, J. Z. (2003). Real-time analysis of clathrin-mediated endocytosis during cell migration. Journal of Cell Science, 116(5), 847–855.
Gao, H., Shi, W., & Freund, L. B. (2005). Mechanics of receptor-mediated endocytosis. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9469–9474.
(a) Bredt, D. S., & Nicoll, R. A. (2003). AMPA receptor trafficking at excitatory synapses. Neuron, 40(2), 361–379. (b) Cottrell, J. R., Dubé, G. R., Egles, C., & Liu, G. (2000). Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. Journal of Neurophysiology, 84(3), 1573–1587. (c) Tzlil, S., Deserno, M., Gelbart, W. M., & Ben-Shaul, A. (2004). A statistical-thermodynamic model of viral budding. Biophysical Journal, 86(4), 2037–2048.
Shenoy, V. B., & Freund, L. B. (2005). Growth and shape stability of a biological membrane adhesion complex in the diffusion-mediated regime. Proceedings of the National Academy of Sciences of the United States of America, 102(9), 3213–3218.
Brogioli, D., & Vailati, A. (2001). Diffusive mass transfer by nonequilibrium fluctuations: Fick’s law revisited. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 63(1 Pt 1), 012105.
Freund, L. B., & Lin, Y. (2004). The role of binder mobility in spontaneous adhesive contact and implications for cell adhesion. Journal of the Mechanics and Physics of Solids, 52(11), 2455–2472.
(a) Guseva, K. (2011). Formation and cooperative behaviour of protein complexes on the cell membrane (p. 92). Berlin: Springer. (b) Ohki, S. (1988). Molecular mechanisms of membrane fusion (p. 588). New York: Plenum Press.
(a) Racoosin, E. L., & Swanson, J. A. (1993). Macropinosome maturation and fusion with tubular lysosomes in macrophages. The Journal of Cell Biology, 121(5), 1011–1020. (b) Kerr, M. C., Lindsay, M. R., Luetterforst, R., Hamilton, N., Simpson, F., Parton, R. G., & Teasdale, R. D. (2006). Visualisation of macropinosome maturation by the recruitment of sorting nexins. Journal of Cell Science, 119(Pt 19), 3967–3980.
Schafer, D. A., D’Souza-Schorey, C., & Cooper, J. A. (2000). Actin assembly at membranes controlled by ARF6. Traffic, 1(11), 892–903.
(a) Hackstein, H., Taner, T., Logar, A. J., & Thomson, A. W. (2002). Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood, 100(3), 1084–1087. (b) Hackstein, H., Steinschulte, C., Fiedel, S., Eisele, A., Rathke, V., Stadlbauer, T., et al. (2007). Sanglifehrin A blocks key dendritic cell functions in vivo and promotes long-term allograft survival together with low-dose CsA. American Journal of Transplantation, 7(4), 789–798. (c) Von Delwig, A., Hilkens, C. M. U., Altmann, D. M., Holmdahl, R., Isaacs, J. D., Harding, C. V., et al. (2006). Inhibition of macropinocytosis blocks antigen presentation of type II collagen in vitro and in vivo in HLA-DR1 transgenic mice. Arthritis Research & Therapy, 8(4), R93.
Yoshida, S., Hoppe, A. D., Araki, N., & Swanson, J. A. (2009). Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages. Journal of Cell Science, 122(Pt 18), 3250–3261.
(a) West, M. A., Wallin, R. P. A., Matthews, S. P., Svensson, H. G., Zaru, R., Ljunggren, H.-G., et al. (2004). Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science, 305(5687), 1153–1157. (b) Sallusto, F., Cella, M., Danieli, C., & Lanzavecchia, A. (1995). Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. The Journal of Experimental Medicine, 182(2), 389–400.
Joukov, V., Pajusola, K., Kaipainen, A., Chilov, D., Lahtinen, I., Kukk, E., et al. (1996). A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. The EMBO Journal, 15(2), 290–298.
Park, J., Fan, Z., & Deng, C. X. (2011). Effects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cells. Journal of Biomechanics, 44(1), 164–169.
Gov, N. (2004). Membrane undulations driven by force fluctuations of active proteins. Physical Review Letters, 93(26 Pt 1), 268104.
Lindström, F., Williamson, P. T. F., & Gröbner, G. (2005). Molecular insight into the electrostatic membrane surface potential by 14n/31p MAS NMR spectroscopy: Nociceptin-lipid association. Journal of the American Chemical Society, 127(18), 6610–6616.
Isaacs, L., Ma, Y., Nolte, R. J. M., & Cornelissen, J. J. L. M. (2012). Virus-based nanocarriers for drug delivery. Advanced Drug Delivery Reviews, 64(9), 811–825.
Wender, P. A., Galliher, W. C., Goun, E. A., Jones, L. R., & Pillow, T. H. (2008). The design of guanidinium-rich transporters and their internalization mechanisms. Advanced Drug Delivery Reviews, 60(4–5), 452–472.
Wadia, J. S., & Dowdy, S. F. (2005). Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Advanced Drug Delivery Reviews, 57(4), 579–596.
Wender, P. A., Mitchell, D. J., Pattabiraman, K., Pelkey, E. T., Steinman, L., & Rothbard, J. B. (2000). The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proceedings of the National Academy of Sciences of the United States of America, 97(24), 13003–13008.
(a) Futaki, S. (2005). Membrane-permeable arginine-rich peptides and the translocation mechanisms. Advanced Drug Delivery Reviews, 57(4), 547–558. (b) Mitchell, D. J., Kim, D. T., Steinman, L., Fathman, C. G., & Rothbard, J. B. (2000). Polyarginine enters cells more efficiently than other polycationic homopolymers. The Journal of Peptide Research, 56(5), 318–325.
(a) Tseng, Y.-L., Liu, J.-J., & Hong, R.-L. (2002). Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: A kinetic and efficacy study. Molecular Pharmacology, 62(4), 864–872. (b) Torchilin, V. P., Rammohan, R., Weissig, V., & Levchenko, T. S. (2001). TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8786–8791.
Mishra, A., Gordon, V. D., Yang, L., Coridan, R., & Wong, G. C. L. (2008). HIV TAT forms pores in membranes by inducing saddle-splay curvature: Potential role of bidentate hydrogen bonding. Angewandte Chemie (International Ed. in English), 47(16), 2986–2989.
Mishra, A., Lai, G. H., Schmidt, N. W., Sun, V. Z., Rodriguez, A. R., Tong, R., & Wong, G. C. L. (2011). Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proceedings of the National Academy of Sciences of the United States of America, 108(41), 16883–16888.
Holowka, E. P., Sun, V. Z., Kamei, D. T., & Deming, T. J. (2007). Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nature Materials, 6(1), 52–57.
Berger, N., Sachse, A., Bender, J., Schubert, R., & Brandl, M. (2001). Filter extrusion of liposomes using different devices: Comparison of liposome size, encapsulation efficiency, and process characteristics. International Journal of Pharmaceutics, 223(1–2), 55–68.
Deming, T. J. (1997). Facile synthesis of block copolypeptides of defined architecture. Nature, 390(6658), 386–389.
Kolishetti, N., Dhar, S., Valencia, P. M., Lin, L. Q., Karnik, R., Lippard, S. J., et al. (2010). Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proceedings of the National Academy of Sciences of the United States of America, 107(42), 17939–17944.
Sutton, D., Nasongkla, N., Blanco, E., et al. (2007). Functionalized micellar systems for cancer targeted drug delivery. Pharmaceutical Research, 24, 1029.
Goren, D., Horowitz, A. T., Tzemach, D., et al. (2000). Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clinical Cancer Research, 6, 1949.
Ross, J. F., Chaudhuri, P. K., Ratnam, M., et al. (1994). Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer, 73, 2432.
Yoo, H. S., & Park, T. G. (2004). Folate receptor targeted biodegradable polymeric doxorubicin mi-celles. Journal of Controlled Release, 96, 273.
Park, E. K., Lee, S. B., & Lee, Y. M. (2005). Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials, 26, 1053.
Tucker, G. C. (2003). Alpha V integrin inhibitors and cancer therapy. Current Opinion in Investigational Drugs, 4, 722.
Kessler, H., Diefenbach, B., Finsinger, D., et al. (1995). Design of superactive and selective integrin receptor antagonists containing the RGD sequence. Letters in Peptide Science, 2, 155.
Nasongkla, N., Shuai, X., Ai, H., et al. (2004). cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angewandte Chemie International Edition in English, 43, 6323.
Torchilin, V. P., Lukyanov, A. N., Gao, Z., et al. (2003). Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proceedings of the National Academy of Sciences of the United States of America, 100, 6039.
Kunath, K., Merdan, T., Hegener, O., et al. (2003). Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. The Journal of Gene Medicine, 5, 588.
Author information
Authors and Affiliations
Problems
Problems
-
5.1
We have learned in this chapter that there are implications in solution properties to the change in the geometry of a self-assembly structure. This is apparent when comparing systems consisting of spherical versus tubular shapes, such as micelles, where there is a distinct difference in their respective aspect ratios (A f). If a biomedical engineer is designing an oral drug delivery system where the target tissue is the small intestine, answer the following questions with your knowledge of diffusion in self-assembled systems.
-
(i)
What are the relative viscosities of a spherical micelle (A f = 1) and a tubular micelle (A f = 50)?
-
(ii)
Which geometry micelle would you expect to be a more effective drug delivery system for the oral application described? Why?
-
(iii)
How would the percolation values differ for a spherical system relative to a fiber system?
-
(iv)
If the concentration of both the spheres and tubes is 7 mg/ml, do you think the colloid concentration would be in an acceptable range for this application for each respective shape? Why?
-
(i)
-
5.2
The allergic response involves the aggregation of IgE receptor molecules within glycosphingolipid-cholesterol microdomains, known as lipid RAFTs, at the surface of mast cells to facilitate a process known as degranulation. If each RAFT domain consists of an average surface area of 0.031 μm2 relative and the mast cell is 20 μm in diameter with a cell membrane thickness of 5 nm and a Young’s modulus of 1.2 × 107 Pa, answer the following questions with your knowledge of the interaction surface area between elastic and hard materials.
-
(i)
Calculate the elastic modulus of the mast cell membrane assuming that E* is approximately equal to the bending energy (e bend) within a contact surface area of 0.01 μm2.
-
(ii)
A medical researcher would like to design a functionalized gold nanoparticle system to target a single RAFT domain on a mast cell. What would be the ideal particle radius if the pressure of the interaction between the nanoparticle and cell membrane is 1 × 10–10 Pa?
-
(iii)
What would the ideal nanoparticle radius be if the surface area of the RAFT domain doubled?
-
(i)
-
5.3
A biomedical graduate student has designed a targeted vesicle system and is unsure of the possible mechanism of cellular internalization. If the vesicle system has a 50-nm diameter and is spherical in shape, answer the following questions with your knowledge of active and passive cellular delivery.
-
(i)
Based on the properties of this vesicle system, what mode(s) of cellular delivery would be preferred? Why?
-
(ii)
What is the vesicle size required to avoid lipid membrane fouling (ΔP = 8.75 × 107 ng/nm s2) assuming the pore size is 60 nm in diameter, the dynamic viscosity is 1 with a constant flux of 50 nm/s, and the filter area is represented by the surface area of the cell of 20-μm diameter and 5-nm membrane thickness?
-
(iii)
If you had to operate above the fouling limit, how would you increase the diffusive flux within the membrane?
-
(iv)
How long would it take to envelop the particle when D = 5 × 105 nm2/s and α = 1?
-
(i)
-
5.4
A research lab in biomedical engineering wishes to design a micellar oral drug delivery system that effectively targets the heart. If the micellar system is 20 nm in diameter and charge-neutral, answer the following questions with your knowledge of self-assembled and targeted systems.
-
(i)
Discuss the sequence of physiological constraints in order as the oral drug passes from the mouth to the small intestine.
-
(ii)
Would changing the diameter of the nanoparticle from 20 to 200 nm affect the constraints of the oral delivery system? Why?
-
(iii)
Would changing the surface charge of the nanoparticle to one that is highly cationic affect the constraints of the oral delivery system? Why?
-
(i)
-
5.5
Use the components in the following table and your knowledge of targeted and self-emulsifying drug delivery to determine the desired route of internalization for a vesicle system based on the criteria and physical limitations discussed in this chapter.
-
(i)
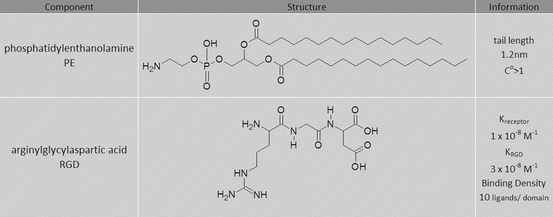
Based on calculations of the thickness, curvature, and size of the self-assembled system, is it likely that it is internalized within the cell through an active or passive mechanism if the bending energy (e bend) is 5 × 106 Pa/m2and Young’s modulus is 1.2 × 107 Pa?
-
(ii)
What is the likely kinetics of binding from your answer to (i) if the rate constant for the binding of RGD receptors to the cell is 1.35 × 10–9 M?
-
(iii)
Do the characteristics of binding suggest that the binding force (F binding = 2 × 10–10 N) of the micelle system with a mass of 1 ng to the target cell type is possible relative to opposing forces (i.e., drag force) if the velocity of the system is 50 mm/s in blood plasma with a viscosity of 4cP, with an acceleration of 5 mm/s2? Why?
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Holowka, E.P., Bhatia, S.K. (2014). Targeted Materials. In: Drug Delivery. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1998-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1998-7_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1997-0
Online ISBN: 978-1-4939-1998-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)


