Abstract
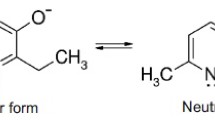
Papaverine hydrochloride (papaver) is an isoquinoline alkaloid widely used as a smooth muscle relaxant agent. It is metabolized mainly in the liver by the P-450 system (O-demethylation) to yield several phenolic metabolites (4’-,6-, 7-, 3’-, and 4’,6- demethylated phenols) (Belpaire et al., 1975). Papaver induces hepatotoxicity by an idiosyncratic immune effect (Kiaer et al., 1974) or a direct metabolite-related hepatocellular toxic effect (Acosta et al., 1980; Davila et al., 1989). At the present time, however, there is no direct evidence which suggests that toxic metabolites derived from papaver are responsible for liver cell injury. We had demonstrated previously that a time lag of 6 hr was necessary before papaver injured cultured hepatocytes and that the papaver-phenolic metabolites were considerably less toxic than the parent compound. They were ranked in toxicity as follows: papaver>60H>4’0H>3’OH (Acosta et al., 1980; Davila et al., 1989). According to these results, it seems that the metabolite(s) is (are) not the major cause of liver injury. It is interesting to note, however, that certain principal metabolites of papaver are nearly as toxic as the parent compound, while others clearly represent detoxification pathways; therefore, we can not rule out the possibility that papaver-derived intermediate metabolite(s) is (are) involved in the toxic insult. Some mechanistic alternatives are suggested: 1) papaver itself may be directly responsible for cellular toxicity or be metabolized to toxic products by a pathway not involving O-dealkylation; 2) phenol formation (e.g. at 4’-position) may require an additional O-dealkylation or aromatic hydroxylation to yield a catechol which may undergo oxidation to a reactive ortho-quinone; 3) quinone-methides may be formed following O-dealkylation (Figure 1); and 4) the time lag of 6 hr may represent the time required for bioconversion and accumulation of sufficient quantities of toxic metabolites and/or saturate and deplete intracellular glutathione stores.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Belpaire, F.M., Bogaert, M.G., and Rossel, M.T. (1975). Metabolism of papaverine I. Identification of metabolites in rat liver. Xenobiotica 5, 413–420.
Kiaer, H.W., Olsen, S., and Ronnov-Jenssen, V. (1974). Hepatotoxicity of papaverine. Arch. Pathos. 98, 292–296.
Acosta, D., Anuforo, D.C., and Smith, R.V. (1980). Cytotoxicity of acetaminophen and papaverine in primary cultures of rat hepatocytes. Toxicol. Appl. Pharmacol., 53, 306–314.
Davila, J.C., Hsieh, G.C., Reddy, C.G., Acosta, D., and Davis, P.J. (1989). Toxicity assessment of papaverine hydrochloride and papaverine-derived metabolites in vitro. The Pharmacologist 31, 179.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Plenum Press, New York
About this chapter
Cite this chapter
Davila, J.C., Acosta, D., Davis, P.J. (1991). The Possible Role of Glutathione on the Hepatotoxic Effect of Papaverine Hydrochloride in Vitro . In: Witmer, C.M., Snyder, R.R., Jollow, D.J., Kalf, G.F., Kocsis, J.J., Sipes, I.G. (eds) Biological Reactive Intermediates IV. Advances in Experimental Medicine and Biology, vol 283. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-5877-0_91
Download citation
DOI: https://doi.org/10.1007/978-1-4684-5877-0_91
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-5879-4
Online ISBN: 978-1-4684-5877-0
eBook Packages: Springer Book Archive