Abstract
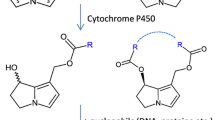
The pyrrolizidine alkaloids (PAs) constitute a large group of hepatotoxic and carcinogenic plant constituents of wide geographic and botanical distribution. These alkaloids are responsible for the death of livestock throughout the world and for occasional human poisonings following the consumption of contaminated foods or the injudicious use of herbal medicines (Bull et al., 1968; Mattocks, 1986; Hirano, 1981; Huxtable, 1980; Peterson and Culvenor, 1983). PAs are relatively nontoxic but are bioactivated in vivo primarily via the liver, through enzymatic dehydrogenation to form highly reactive pyrrole- type metabolites. It is thought that PAs are initially converted to the corresponding dehydropyrrolizidine alkaloids (PA pyrroles) which then can either alkylate protein, DNA or other cellular nucleophiles (Hsu et al. 1975; Reed et al. 1988; Wickramanayake et al., 1985) or be hydrolyzed to the more stable pyrrolic alcohol, such as (R)-6,7-dihydro-7-hydroxy-l-hydroxymethy1-5H-pyrrolizidine (DHP) in the case of retronecine or heliotridine based PAs (Jago et al., 1979; Kedzierski and Buhler, 1985, 1986; Mattocks, 1986; Mattocks and White, 1971). PAs also can be oxidized in vivo to relatively nontoxic PA N-oxides and hydrolyzed to the corresponding amino alcohol (Kedzierski and Buhler, 1986; Mattocks, 1986; Ramsdell et al., 1987).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Buhler, D.R. and Kedzierski, B. (1986). Biological reactive intermediates of pyrrolizidine alkaloids. In Biological Reactive Intermediates ( J.J. Kocsis, D.J. Jollow, C.M. Witmer, J.O. Nelson and R. Snyder, eds.), pp. 611–620, Plenum Publishing Corp., NY.
Bull, L.B., Culvenor, C.C.J. and Dick, A.T. (1968). The Pyrrolizidine Alkaloids, North-Holland, Amsterdam.
Culvenor, C.C.J., Edgar J.A., Smith, L.W. and Tweddale, H.J. (1970). Dihydropyrrolizidines. III. Preparation and reactions of derivatives related to pyrrolizidine alkaloids, Aust. J. Chem. 23, 1853.
Guengerich, F.P. (1977). Separation and purification of multiple forms of microsomal cytochrome P-450. J. Biol. Chem. 252, 3970–3979.
Hirono, I. (1981). Natural carcinogenic products of plant origin. C.R.C. Crit. Rev. Toxicol. 8, 235.
Huxtable, R.J. (1980). Herbal teas and toxins: Novel aspects of pyrrolizidine poisoning in the United States. Persp. Biol. Med. 24, 1.
Jago, M.V., Edgar, J.A., Smith, L.W. and Culvenor, C.C.J. (1979). Metabolic conversion of heliotridine-based pyrrolizidine alkaloids to dehydroheliotridine. Mol. Pharmacol. 6, 402.
Karchesy, J.J. and Deinzer, M.L. (1981). Kinetics of alkylation reactions of pyrrolizidine alkaloid derivatives. Heterocycles, 16, 631.
Kedzierski, B. and Buhler, D.R. (1986). Method for determination of pyrrolizidine alkaloids and their metabolites by high-performance liquid chromatography. Anal. Biochem. 152, 59.
Kedzierski, B. and Buhler, D.R. (1985). Configuration of necine pyrroles-toxic metabolites of pyrrolizidine alkaloids. Toxicol. Lett. 25, 115.
Mattocks, A.R. and White, I.N.H. (1971). The conversion of pyrrolizidine alkaloids to N-oxides and to dihydropyrrolizine derivations by rat-liver microsomes in vitro. Chem.-Biol. Inter. 3, 383.
Mattocks, A.R. (1986). Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, New York, NY. 393 p.
Miranda, C.L., Cheeke, P.R. and Buhler, D.R. (1980). Comparative effects of the pyrrolizidine alkaloids jacobine and monocrotaline on hepatic drug metabolizing enzymes in the rat. Res. Commun. Chem. Pharmcol. 29, 573.
Peterson, J.E. and Culvenor, C.C.J. (1983). Hepatotoxic pyrrolizidine alkaloids. In: Handbook of Natural Toxins. Vol. 1, Plant and Fungal Toxins, R.F. Keeler, K.R. Van Kampen and L.F. James, eds., Academic Press, New York.
Ramsdell, H.S., Kedzierski, B. and Buhler, D.R. (1987). Microsomal metabolism of pyrrolizidine alkaloids from Senecio jacobaea. Isolation and quantification of 6,7-dihydro-7-hydroxy-l-hydroxymethy1–5H-pyrrolizidine and N-oxides by high performance liquid chromatography. Drug Metab. Dispos. 15, 32.
Reed, R.L. and Buhler, D.R. (1988). The synthesis of 3H-putrescine and subsequent biosynthesis of 3H-jacobine, a pyrrolizidine alkaloid from Senecio jacobaea. J. Labelled Cmpds. and Radiopharm. 25, 1041–1047.
Reed, R.L., Ahern, K.G., Pearson, G.D. and Buhler, D.R. (1988). Crosslinking of DNA by dehydroretronecine, a metabolite of pyrrolizidine alkaloids. Carcinogenesis 9, 1355–1361.
Robertson, K.A., Seymour, J.L., Hsia, M.T. and Allen, J.R. (1977). Covalent interaction of dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine alkaloid monocrotaline with cysteine and glutathione. Cancer Res. 37, 3141.
Shu, I.C., Robertson, A.A., Shumaker, R.C. and Allen, J.R. (1975). Binding of tritiated dehydroretrocine to macromolecules. Res. Commmun. Chem. Pathol. Pharmcol. 11, 99–106.
Wickramanayake, P.P., Arbogast, B.L., Buhler, D.R., Deinzer, M.L. and Burlingame, A.L. (1985). Alkylation of nucleosides and nucleotides by dehydroretronecine: Characterization of adducts by liquid secondary ion mass spectrometry. J. Am. Chem. Soc. 107, 2485–2488.
Williams, D.E., Reed, R.L., Kedzierski, B., Guengerich, F.P. and Buhler, D.R. (1989a). Bioaetivation and detoxication of the pyrrolizidine alkaloid senecionine by cytochrome P-450 enzymes in rat liver. Drug Metab. Dispos. 17, 387–392.
Williams, D.E., Reed, R.L., Kedzierski, B., Ziegler, D.M. and Buhler, D.R. (1989b). The role of flavin-containing monooxygenase in the N-oxidation of the pyrrolizidine alkaloid senecionine. Drug. Metab. Dispos. 17, 380–386.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Plenum Press, New York
About this chapter
Cite this chapter
Buhler, D.R., Miranda, C.L., Kedzierski, B., Reed, R.L. (1991). Mechanisms for Pyrrolizidine Alkaloid Activation and Detoxification. In: Witmer, C.M., Snyder, R.R., Jollow, D.J., Kalf, G.F., Kocsis, J.J., Sipes, I.G. (eds) Biological Reactive Intermediates IV. Advances in Experimental Medicine and Biology, vol 283. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-5877-0_75
Download citation
DOI: https://doi.org/10.1007/978-1-4684-5877-0_75
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-5879-4
Online ISBN: 978-1-4684-5877-0
eBook Packages: Springer Book Archive