Abstract
Numerous studies have demonstrated a contributory role of iron in the pathogenesis of various inherited and chemically-induced disorders of porphyrin metabolism (porphyrias) (De Matteis et al, 1988, Smith and Francis, 1987, Lambrecht et al, 1988). However, the precise mechanism by which iron leads to excess tissue porphyrin accumulation and excretion in these disorders remains to be determined. Recent studies from these laboratories (Woods and Calas, 1989) have demonstrated that reduced porphyrins (porphyrinogens) are readily oxidized in vitro by reactive oxidizing species, such as superoxide (O −2 ) and hydroxyl (OH·) radicals, and that this effect is dramatically stimulated by the presence of iron compounds in the reaction mixture. Inasmuch as mitochondria are a principal locus both of porphyrin metabolism (e.g. Tait, 1978) as well as of the generation of reduced oxygen species (O −2 ), H2O2) (Forman and Boveris, 1982) in cells, it is reasonable to postulate that, in the presence of excess iron, reduced porphyrins could be oxidized by reactive oxidants derived from the mitochondrial electron transport chain, contributing to the accumulation and excretion of oxidized porphyrins observed in iron-exacerbated porphyrinopathies. The present studies were conducted to test the hypothesis that reduced porphyrins are oxidized by reactive oxidants derived from the mitochondrial electron transport chain in the presence of iron.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
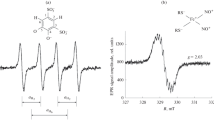
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Borg, D.C. and Schaich, K.M. (1987). Iron and iron-derived radicals, In: Oxygen Radicals and Tissue Injury. Proceedings of an Upjohn Symposium. ( Ed. Halliwell, B ), pp. 20–26.
Boveris, A. and Chance, B. (1973). The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 134, 707–716.
Bucher, J.R., Tein, M. and Aust, S.D. (1983). The requirement for ferric in the initiation of lipid peroxidation by chelated ferrous iron. Biochem. Biophys. Res. Commun. 111, 777–784.
Burkitt, M.J. and Gilbert, B.C. (1989). The control of iron-induced oxidative damage in isolated rat liver mitochondria by respiration state and ascorbate. Free Rad. Res. Commun. 5, 333–344.
De Matteis, F., Harvey, C., Reed, C. and Hempenius, R. (1988). Increased oxidation of uroporphyrinogen by an inducible liver microsomal system. Biochem. J. 250, 161–169.
Forman, H.J. and Boveris, A. (1982). Superoxide radical and hydrogen peroxide in mitochondria. In: Free Radicals in Biology, Vol 5 (Ed. Pryor, WA ), pp. 65–90, Academic Press, New York.
Halliwell, B. and Guttridge, J.M.C. (1984). Oxygen toxicity, oxygen radicals, transistion metals and disease. Biochem. J. 219, 1–4.
Johnson, D. and Lardy, H. (1967). Isolation of liver and kidney mitochondria. Methods Enzymol. 10, 94–96.
Lambrecht, R.W., Sinclair, P.R., Bement, W.J., Sinclair, J.F., Carpenter, H.M., Buhler, D.R., Urquhart, A.J. and Elder, E.H. (1988). Hepatic uroporphyrin accumulation and uroporphyrinogen decarboxylase activity in cultured chick-embyyo hepatocytes and in Japanese quail and mice treated with polyhalogenated and aromatic compounds. Biochem. J. 253, 131–138.
Loschen, G.L., Flohe, L. and Chance, B. (1971). Respiratory chain linked H202 production in pigeon heart mitochondria. FEBS. Lett. 18, 261–264.
Smith A.G. and Francis, J.E. (1987). Chemically-induced formation of an inhibitor of hepatic uroporphyrinogen decarboxylase in inbred mice with iron overload. Biochem. J. 246, 221–226.
Smith, P.K., Krohn, R.I., Hermanson, G.T. (1985). Measurement of protein using bicinchoninic acid. Analytic. Biochem. 150, 76–85.
Tait, G.H. (1978). The biosynthesis and degradation of heme. In: Herne and Hemoproteins (Eds. De Matteis, F. and Aldridge, W.N. ), pp. 1–48, Springer-Verlag, Berlin.
Woods, J.S. (1988). Attenuation of porphyrinogen oxidation by glutathione in vitro and reversal by porphyrinogenic trace metals. Biochem. Biophys. Res. Commun. 152, 1428–1434.
Woods, J.S. amd Calas, C.A. (1989). Iron stimulation of free radical-mediated porphyrinogen oxidation by hepatic and renal mitochondria. Biochem. Biophys. Res. Commun. 160, 101–08.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Plenum Press, New York
About this chapter
Cite this chapter
Woods, J.S., Sommer, K.M. (1991). Oxidation of Reduced Porphyrins by the Mitochondrial Electron Transport Chain: Stimulation by Iron and Potential Role of Reactive Oxygen Species. In: Witmer, C.M., Snyder, R.R., Jollow, D.J., Kalf, G.F., Kocsis, J.J., Sipes, I.G. (eds) Biological Reactive Intermediates IV. Advances in Experimental Medicine and Biology, vol 283. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-5877-0_121
Download citation
DOI: https://doi.org/10.1007/978-1-4684-5877-0_121
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-5879-4
Online ISBN: 978-1-4684-5877-0
eBook Packages: Springer Book Archive