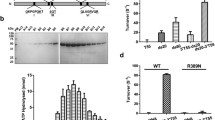
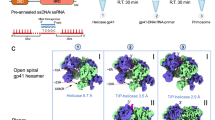
Abstract
Bacteriophage T4 gene 32 protein (gp32) is a prototype for a class of proteins whose fundamental feature includes a very strong affinity for single-stranded nucleic acids. This binding appears to be largely nonspecific with respect to sequence and favors single-stranded over doublestranded nucleic acids. Tight binding to single-stranded nucleic acids is undoubtedly important for the essential role of gp32 in T4 DNA replicaition, repair, and recombination [for a review see 1]. When E. coli, infected with T4 bacteriophage carrying temperature-sensitive mutations in gene 32, is shifted to a nonpermissive temperature, T4 DNA replication ceases [2], and the intracellular T4 DNA is rapidly degraded to small fragments [3]. The unbound concentration of gp32 in vivo is in the range of 2 to 3 µM [4], and it is thus present in “stoichiometric” amounts relative to the number of replication forks present. This finding is consistent with the presumed role of gp32 in T4 DNA replication which is to remove adventitious secondary structures from the single-stranded DNA template just ahead of the advancing replication fork and to protect the resulting ssDNA from nuclease degradation. In vitro DNA replication assays carried out with T4 DNA polymerase (gp43) and three T4 DNA polymerase accessory proteins (gp44, gp45, and gp62) indicate that the addition of gp32 stimulates the rate of DNA synthesis by 100- to 200-fold [5,6].
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Chase, J.W., and Williams, K.R. (1986) Annu. Rev. Biochem. 55, 103–136.
Riva, S., Cascino, A., and Geiduschek, E.P. (1970) J. Mol. Biol. 54, 85–102.
Curtis, M.J., and Alberts, B. (1976) J. Mol. Biol. 102, 793–816.
Von Hippel, P.H., Kowalczykowski, S.C., Lonberg, N., Newport, J.W., Paul, L.S., Stormo, G.D., and Gold, L. (1982) J. Mol. Biol. 162, 795–818.
Nossal, N.G., and Peterlin, B.M. (1979) J. Biol. Chem. 254, 6032–6037.
Alberts, B.M., Barry, J., Bedinger, P., Burke, R.L., Hibner, U., Liu, C.-C., and Sheridan, R. (1980) In Mechanistic Studies on DNA Replication and Genetic Recombination, Vol. 19, 449–471, Alberts, B.M., and Fox, C.F. (eds.). Academic Press, New York.
Formosa, T., Burke, R.L., and Alberts, B.M. (1983) Proc. Natl. Acad. Sci. USA 80, 2442–2446.
Wu, J.-R., and Yeh, Y.-C. (1973) J. Virol. 12, 758–765.
Kodadek, T., Wong, M.L., and Alberts, B.M. (1988) J. Biol. Chem. 263, 9427–9436.
Tomizawa, J.-I., Anraku, N., and Iwama, Y. (1966) J. Mol. Biol. 21, 247–253.
Alberts, B.M., and Frey, L. (1970) Nature 227, 1313–1318.
Berger, H., Warren, A., and Fry, K. (1969) J. Virol. 3, 171–175.
Newport, J.W., Lonberg, N., Kowalczykowski, S.C., and Von Hippel, P. (1981) J. Mol. Biol. 145, 105–121.
Krisch, H.M., Bolle, A., and Epstein, R.H. (1974) J. Mol. Biol. 88, 89–104.
Russel, M., Gold, L., Morrissett, H., and O’Farrell, P. (1976) J. Biol. Chem. 251, 7263–7270.
Lemaire, G., Gold, L., and Yarus, M. (1978) J. Mol. Biol. 126, 73–90.
McPheeters, D.S., Stormo, G.D., and Gold, L. (1988) J. Mol. Biol. 201, 517–535.
Delius, H., Mantell, N.J., and Alberts, B. (1972) J. Mol. Biol. 67, 341–350.
Sheerhagen, M.A., Kuil, M.E., Van Grondelle, R., and Blok, J. (1985) FEBS Lett. 184, 221–225.
Jensen, D.E., Kelly, R.C., and Von Hippel, P.H. (1976) J. Biol. Chem. 251, 7215–7228.
Kowalczykowski, S.C., Lonberg, N., Newport, J.W., and Von Hippel, P.H. (1981) J. Mol. Biol. 145, 75–104.
Scheerhagen, M.A., Bokma, J.T., Vlaanderen, C.A., Blok, J., and Van Grondelle, R. (1986) Biopolymers 25, 1419–1448.
Anderson, R.A., and Coleman, J.E. (1975) Biochemistry 14, 5485–5491.
Spicer, E.K., Williams, K.R., and Konigsberg, W.H. (1979) J. Biol. Chem. 254, 6433–6436.
Prigodich, R.V., Casas-Finet, J., Williams, K.R., Konigsberg, W., and Coleman, J.E. (1984) Biochemistry 23, 522–529.
Kelly, R.C., Jensen, D.E., and Von Hippel, P.H. (1976) J. Biol. Chem. 251, 7240–7250.
Kelly, R.C., and Von Hippel, P.H. (1976) J. Biol. Chem. 251, 7229–7239.
McGhee, J.D., and Von Hippel, P.H. (1974) J. Mol. Biol. 86, 469–489.
Lohman, T.M. (1984) Biochemistry 23, 4656–4665.
Lohman, T.M. (1984) Biochemistry 23, 4665–4675.
Liu, C.C., Burke, R.L., Hibner, U., Barry, J., and Alberts, B. (1978) Cold Spring Harbor Symp. Quant. Biol. 43, 469–487.
Williams, K.R., LoPresti, M.B., Setoguchi, M., and Konigsberg, W. (1980) Proc. Natl. Acad. Sci. USA 77, 4614–4617.
Williams, K.R., LoPresti, M.B., and Setoguchi, M. (1981) J. Biol. Chem. 256, 1754–1762.
Krisch, H.M., and Allet, B. (1982) Proc. Natl. Acad. Sci. USA 79, 4937–4941.
Hosoda, J., and Moise, H. (1978) J. Biol. Chem. 253, 7547–7555.
Giedroc, D., Keating, K., Williams, K., Konigsberg, W., and Coleman, J. (1986) Proc. Natl. Acad. Sci. USA 83, 8452–8456.
Carroll, R.B., Neet, K., and Goldthwait, D.A. (1975) J. Mol. Biol. 91, 275–291.
Hosoda, J., Takacs, B., and Black, C. (1974) FEBS Lett. 47, 338–342.
Moise, H., and Hosoda, J. (1976) Nature 259, 455–458.
Williams, K.R., and Konigsberg, W.H. (1978) J. Biol. Chem. 253, 2463–2470.
Tsugita, A., and Hosoda, J. (1978) J. Mol. Biol. 122, 255–258.
Williams, K.R., Sillerud, L.O., Schafer, D.E., and Konigsberg, W.H. (1979) J. Biol. Chem. 254, 6426–6432.
Lonberg, N., Kowalczykowski, S.C., Paul, L.W., and Von Hippel, P.H. (1981) J. Mol. Biol. 145, 123–138.
Williams, K.R., and Konigsberg, W.H. (1981) In Gene Amplification and Analysis, Vol. 2, 475–508, Chirikjian, J.G., and Papas, T.S. (eds.). Elsevier/North-Holland, Amsterdam.
Burke, R.L., Alberts, B.M., and Hosoda, J. (1980) J. Biol. Chem. 255, 11484–11493.
Doherty, D.H., Gauss, P., and Gold, L. (1982) Mol. Gen. Genet. 188, 77–90.
Prigodich, R.V., Shamoo, Y., Williams, K.R., Chase, J.W., Konigsberg, W.H., and Coleman, J.E. (1985) Biochemistry 25, 3666–3672.
Shamoo, Y., Williams, K.R., and Konigsberg, W. (1988) Proteins Struct. Function Genet. 4, 1–6.
Toulme, J.-J., LeDoan, T., and Helene, C. (1984) Biochemistry 23, 1195–1201.
LeDoan, T., Toulme, J.-J., and Helene, C. (1984) Biochemistry 23, 1202–1207.
Casas-Finet, J., Toulme, J.-J., Cazenave, C., and Santus, R. (1984) Biochemistry 23, 1208–1213.
Keating, K.M., Ghosaini, L.R., Giedroc, D.P., Williams, K.R., Coleman, J.E., and Sturtevant, J.M. (1988) Biochemistry 27, 5240–5245.
Hecht, M.H., Sturtevant, J.M., and Sauer, R.T. (1984) Proc. Natl. Acad. Sci. USA 81, 5685–5689.
Alber, T., Sun, D.P., Wilson, K., Wozniak, J.A., Cook, S.P., and Matthews, B.W. (1987) Nature 330, 41–46.
Howell, E.E., Villafranca, J.E., Warren, M.S., Oatley, S.J., and Kraut, J. (1986) Science 231, 1123–1128.
Greenfeld, N., and Fasman, G.D. (1969) Biochemistry 8, 4108–4116.
Shamoo, Y., Ghosaini, L.R., Keating, K.M., Williams, K.R., Sturtevant, J.M., and Konigsberg, W.H. (1989) Biochemistry, in press.
Giessner-Prettre, C., and Pullman, B. (1971) J. Theor. Biol. 31, 287–294.
Giessner-Prettre, C., and Pullman, B. (1976) Biochem. Biophys. Res. Com-mun. 70, 578–581.
Merrill, B.M., Williams, K.R., Chase, J.W., and Konigsberg, W.H. (1984) J. Biol. Chem. 259, 10850–10856.
Paradiso, P.R., Nakashima, Y., and Konigsberg, W.H. (1979) J. Biol. Chem. 254, 4739–4744.
Paradiso, P.R., and Konigsberg, W.H. (1982) J. Biol. Chem. 257, 1462–1467.
Merrill, B.M., Stone, K.L., Cobianchi, F., Wilson, S.H., and Williams, K.R. (1988) J. Biol. Chem. 263, 3307–3313.
Brayer, G.D., and McPherson, A. (1983) J. Mol. Biol. 169, 565–596.
Chlebowski, J.F., and Mabrey, S. (1977) J. Biol. Chem. 252, 7042–7052.
Engeseth, H.R., and McMillin, D.R. (1986) Biochemistry 25, 2448–2455.
Lepock, J.R., Arnold, L.D., Torrie, B.H., Andrews, B., and Kruuv, J. (1985) Arch. Biochem. Biophys. 241, 243–251.
Giedroc, D.P., Keating, K.M., Williams, K.R., and Coleman, J.E. (1987) Biochemistry 26, 5251–5259.
Keating, K.M., Giedroc, D.P., Harris, L.D., Ghosaini, L.R., Williams, K.R., Sturtevant, J.M., and Coleman, J.E. (1987) In Protein Structure, Folding, and Design 2, 35–44, Oxender, D. (ed.). Alan R. Liss, New York.
Gauss, P., Krassa, K., McPheeters, D.S., Nelson, M.A., and Gold, L. (1987) Proc. Natl. Acad. Sci. USA 84, 8515–8519.
Gamier, J., Osguthorpe, D., and Robson, B. (1978) J. Mol. Biol. 120, 97–120.
Brun, F., Toulme, J., and Helene, C. (1975) Biochemistry 14, 558–563.
O’Connor, T.P., and Coleman, J.E. (1983) Biochemistry 22, 3375–3381.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1989 Springer-Verlag New York Inc.
About this chapter
Cite this chapter
Shamoo, Y., Keating, K.M., Williams, K.R., Konigsberg, W.H. (1989). Structure/Function Relationships in the Bacteriophage T4 Single-Stranded DNA-Binding Protein. In: Adolph, K.W. (eds) Molecular Biology of Chromosome Function. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-3652-8_14
Download citation
DOI: https://doi.org/10.1007/978-1-4612-3652-8_14
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4612-8192-4
Online ISBN: 978-1-4612-3652-8
eBook Packages: Springer Book Archive