Abstract
Antimicrobial peptides are the backbone of first-line defense against various microorganisms in the animal kingdom. Thus, not surprisingly, they are gaining attention in the science and medical fields as a rich repository of new pro-drugs. Below, we focus our attention on the Brevinin family of anuran peptides. While most of them show strong antibacterial activities, some, e.g. Brevinin-2R, appear to be promising anticancer molecules, exhibiting better a therapeutic window than widely-use anticancer drugs like doxorubicin. We briefly introduce the field, followed by highlighting the promising therapeutic properties of Brevinins. Next, we provide information about the cloning and phylogenetic aspects of Brevinin genes. In the final paragraphs of this chapter, we discuss possible large-scale production methods of Brevinins, giving examples of some systems that are already in use. Towards the end, we discuss various means of modification of biologic properties of Brevinins, either by chemical modifications or by amino acid substitution and sequence rearrangements. In this context, also other unique properties of Brevinins are briefly mentioned. Finally, we discuss the future of the Brevinin field, particularly highlighting yet to be answered biologic questions, like for example presumed anti-viral and antitumor activities of Brevinin family members.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Amidophosphoribosyltransferase
- Bionanomaterials
- Brevinin
- Bufodienolides
- Bufogenines
- Cathepsin
- Esculentin
- Hypoglycemia
- Japonicin
- Magainins
- Nanocarrier
- Nigrocin
- Palustrin
- Peptidomimetica
- Phosphatidylserines
- Rana box
- Ranacyclin
- Ranalexin
- Ranateurin
- Ranid frogs
- Temporin
1 Introduction
The discovery of new therapeutic tools is one of the priority areas of biomedical scientific research. Billions of US dollars are spent on the search and development of new medicines every year. Pharmaceutical companies spend an average of $4 billion on the placing of one new drug unit in the market. In some cases, the cost reaches $11 billion (Sources: InnoThink Center for Research in Biomedical Innovation and Thomson Reuters Fundamentals via FactSet Research Systems).
Protection mechanisms of multicellular organisms from an aggressive environment, including bacterial and viral pathogens, have been evolving and improving for millions of years. That is why animal- and plant-derived materials have remained the main sources of leads for new drug development. Plants are the most popular source for searching of new biologically active compounds. Thus, the animal kingdom is actively “mined” for new generations of potentially more effective therapeutics [1].
Members of the Ranidae family of amphibians reside in a wide range of habitats (from tropical to subarctic regions) [2]. The ability of amphibians to survive in such different conditions may be attributed to the evolution of many different morphological, physiological, biochemical and behavioral adaptations. Like for other species, skin is one of the most important organs for amphibians, as it fulfills many functions such as i) respiration, (ii) water regulation and (iii) defense (barrier). Glands are major functional components of the skin of amphibians. Three types of glands are widely distributed in amphibians’ skin: mucus glands, granular glands and the tubulosaccular or alveolar glands [1]. Mucus glands help to maintain a moisture and slippery skin surface. Granular glands are the place of a wide range of chemical compounds synthesis. Their secretions have a protective function against bacterial and fungal infection as well as predators [3]. Main types of amphibian skin biologically active compounds are: biogenic amines, bufodienolides (bufogenines and bufotoxins = steroids), alkaloids, peptides and proteins [4, 5].
Amphibian skin, especially granular gland secretions, is a rich source of novel therapeutic agents such as antimicrobial peptides (AMPs), polypeptides and proteins. An important event in this area was the discovery of magainins peptides isolated from the skin of Xenopus laevis [6]. Magainins exhibit broad-spectrum antimicrobial activity, inhibiting the growth of both Gram positive and Gram negative bacteria, Candida albicans, Cryptococcus neoformansand, Saccharomyces cerevisiae and also demonstrated to induce lysis in several protozoan species [6]. Those discoveries stimulated great interest in amphibian skin peptides [7, 8]. These peptides are stored in granular glands, which are localized mostly in the skin of dorsal area and are surrounded by myocytes and innervated by sympathetic fibers [9, 10]. Adrenergic stimulation of myocytes leads to compression of serous cells, which discharge their contents by a holocrine-like mechanism. As a result, secretions contain not only antimicrobial peptides and other biologically-active agents, but also cytosolic components and cells’ genetic material [11]. The main advantage of using amphibian skin as the object of investigation is the use of gentle methods (e.g. skin stimulation by norepinephrine [12] or gentle electrical stimulation [13] for sample preparation. Hence, there is no need to harm or kill animals for this work.
The ranid frogs synthesize and secrete multiple active components. Skin secretions of the R. palustris contain at least 22 antimicrobial peptides [14]. On the basis of amino acid sequence similarity, antimicrobial peptides from ranid frogs may be divided into 14 families: Brevinin-1, Brevinin-2, Esculentin-1, Esculentin-2, Japonicin-1, Japonicin-2, Nigrocin-2, Palustrin-1, Palustrin-2, Ranacyclin, Ranalexin, Ranateurin-1, Ranateurin-2, and Temporin [15]. In this chapter, we use the modified Simmaco nomenclature [10]. Peptides belonging to a species are named by the initial letter in capitals (or more than one letter in case of uncertainty) of the species to indicate their origin. Lower case letters are used to designate isoforms, e.g. Brevinin-1Ea and Brevinin-1Eb from R. e sculenta [15]. In 2012, a new nomenclature for amphibian skin peptides was proposed [16].
Brevinins are among the most ubiquitous antibacterial peptides, which consist of two families: Brevinin-1 (approximately length of 24 residues) and Brevinin-2 (approximately length of 33–34 residues). The first members (and protoplasts) of the Brevinin superfamily peptides were discovered in 1992. They were isolated from Rana brevipoda porsa and called Brevinin-1 (FLPVLAGIAAKVVPALFCKITKKC) and Brevinin-2 (GLLDSLKGFAATAGKGVLQSLLSTASCKLAKTC), respectively. These proteins demonstrated microbicidal activity against a wide range of Gram-positive, Gram-negative bacteria and strains of pathogenic fungi [17]. Since that time about 350 types of Brevinins have been discovered (according to DADP database [18]. Skin secretions of the marsh frog Rana ridibunda exhibited significant healing effects on wound treatment process [19]. Furthermore, the antibacterial properties of two peptides named Temporin-Ra and Temporin-Rb isolated from the aforementioned frog species have justified the potential therapeutic application of AMPs [20].
Specimens of the Brevinin superfamily share some common features. These peptides are linear, amphipathic and cationic. Most of them have a C-terminal disulfide-bridged cyclic heptapeptide (Cys18-(Xaa)4-Lys-Cys24), also called «Rana box» [21]. This sequence was thought to play a crucial role for antibacterial activity of those peptides. However, this hypothesis was refuted after discovery of C-terminal truncated Brevinin-1 and Brevinin-2 family peptides from Rana okinavana [22] and R. septentrionalis, respectively [23]. Those peptides did not have the characteristic C-terminal cyclic heptapeptide domain, but instead contained a C-terminally-amidated residue [22, 23].
The amino acid sequence of Brevinin-1 is poorly conserved across species and has four invariant residues (Ala9, Cys18, Lys23, Cys24) [24]. Pro14 is often present in Brevinin-1 peptides, and it was shown that this residue produces a stable kink in the molecule [25]. Functional activities of antibacterial peptides are largely determined by their structural features. The presence of cationic amino acids facilitates the interaction of Brevinins with the anionic phospholipids of the bacterial membranes and with negatively charged eukaryotic cell membranes (cancer cells, erythrocytes). In aqueous solution, Brevinin-1 exists predominantly as a random coil but adopts an amphipathic α-helical structure in hydrophobic membrane-mimetic environment such as 50 % trifluoroethanol [26]. It has been postulated that the α-helical structure in such an environment leads to perturbation of the phospholipid bilayer of targeted membranes. Such membrane function disturbances lead to growth inhibition or death of the targeted microorganisms. This hypothesis correlates with experimental results performed with synthetic D-amino acids peptides [27]. Biological activity of such analogs were similar to their corresponding native peptides, so a mechanism based on an interaction with chiral binding sites of receptors, enzymes, or other membrane proteins can be ruled out. The number and distribution of positive charges could be the cause of selectivity for some of these peptides to bacterial membranes [9]. Two main mechanisms of amphipathica-helical peptides interactions with membranes were suggested: the “barrel-stave” (Fig. 10.1a) and the “carpet-like” models (Fig. 10.1b) [28, 29]. The primary structure of Brevinin-2 is also poorly conserved with the invariant amino acid residues in the peptide being Lys7, Cys27, Lys28, Cys33 [23].
Three basic models of Brevinins’ antimicrobial and antibacterial activity, based on their mode of interaction with cellular membranes. The channel (barrel-stave) model (a) suggests that antimicrobial peptides form a typical pore. Inner/channel side of such pores would be made of polar residues (blue) of the peptides, whereas the hydrophobic ones (yellow) are in contact with the membrane phospholipids. The “carpet-like” model (b), predicts that peptides accumulate massively at the membrane interphase. Such sequestration of the membrane would lead to the disruption of the membrane integrity. The “two-states” (toroidal) model (c) could be interpreted as a variant of the “carpet-like” model, however with a different final outcome. The massive peptide accumulation creates mechanical tension. To relieve that tension, some peptides are forced to adopt a transmembrane orientation, forming a mixed phospholipid-peptide pore spanning the membrane. In a further step, the pore undergoes a stochastic disruption (loses its wall-integrity,with relocation of the monomers at both sides of the membrane), and thus membrane destabilization leading to the loss of its integrity
2 Gene Organization and cDNA Cloning
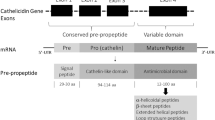
A similar structural organization is being observed in biosynthetic precursors of AMPs including a signal peptide strongly conserved among different AMP families, an intervening region enriched with aspartic and glutamic residues, and an AMP region at the C-terminus [30]. While being well conserved among the members of an AMP family in different frog species, the intervening sequence regions represent considerable variation among peptides of different AMP families. Sequence hyper-variability is observed in the C-terminal AMP coding regions not only in peptides of different families but also in peptides belonging to the same AMP family [31].
Studies on frog skin secretion revealed a huge structural diversity of the antimicrobial peptides from ranid frogs. Using neighbor joining method it was found that peptides from the closely related species segregate together, forming different clades. This suggests that these peptides formed as a consequence of relatively recent gene duplication events after the species diverged from each other. For example, it was found that R. sphenocephala was morphologically and genetically classified as being a close relative of R. pipens. Brevinin-1Sc is found in the R. pipiens clade, but other Brevinin-1Sa and-1Sb are found in the R. berlandieri clade, which suggested unknown phylogenetical relationships [24].
Nowadays, by the use of cDNA cloning technology, the precursors of several AMPs belonging to the Brevinin family have been studied. Protein sequence analysis of cloned cDNA led to the identification of the peptides. On the other hand, the deduced amino acid sequences can then be used as a guide for reverse-phase-chromatography purification of the individual peptides and their amino acid sequences can be uncovered by mass-spectrometry.
Through ‘shotgun’ cloning, AMPs such as Brevinin-1P, Brevinin-1S and Brevinin-1V have been identified from three species of Chinese frogs, including Odorrana schmackeri, Odorrana versabilis and Pelophylax plancyi fukienensis [32]. Wang and colleagues reported the deduced sequences of Brevinin-1LT1 and Brevinin-1LT2 from the skin of Hylarana latouchii using molecular cloning technique. Precursors of Brevinin-1RTa, Brevinin-1RTb, Brevinin-1RTc, Brevinin-2RTa, and Brevinin-2RTb have been identified from the skin-derived cDNA library of Amolops ricketti [33]. After isolating AMPs from skin secretion/extract of amphibians, a number of investigators have been interested in analyzing the expression of dermal peptides using semi-quantitative RT-PCR system. Ohnuma and Conlon have investigated the differential expression of some AMPs such as Preprobrevinin-2 in developing larvae and adult tissues of Rana ornativentris, which highlighted that the expression of amphibian AMP genes is correlated with metamorphosis but is subjected to differential regulation [34].
3 The Recombinant Expression of AMPs
Large quantities of AMPs are needed to meet the requirement for studies in basic science as well as clinical trials. Procuring the peptides from natural sources and chemical syntheses are not cost-effective. The most attractive tool for large-scale production of antimicrobial peptides is the recombinant approach.
Various AMPs belonging to different families and their cDNAs have been cloned. A prokaryotic expression system such as Escherichia coli is commonly applied. AMPs are expressed in E. coli as fusion proteins to protect the bacterial host from the lethal effect of AMP and the peptide from proteolytic degradation. Several major fusion-protein systems for the expression of AMPs in E. coli have been reported which are summarized here:
-
Thioredoxin as a low-molecular weight protein (~12 kDa) has been frequently exploited as carrier protein of antimicrobial peptides. This protein exhibits a chaperon activity that can promote the expression of recombinant peptides in E. coli.
-
GST (glutathione S-transferase) is a commonly used carrier protein for fusion expression of antimicrobial peptides in E. coli. GST fusion proteins can be quickly purified from crude lysate by glutathione-affinity chromatography. The commercial GST-fusion plasmids usually contain a specific protease recognition site releasing the desired peptide from the fusion protein. Due to the relatively large size (~26 kDa) of GST, the efficiency of the system decreases and makes the fusion highly susceptible to proteolytic degradation as well.
-
PurF fusion, the protein fragment containing the N-terminal 152 amino acids of PurF (amidophosphoribosyltransferase) is widely used as a carrier for the expression of antimicrobial peptides. Insoluble expression of AMP PurF fusions can not only protect the host cell from the peptides’ intrinsic toxic effects but also effectively protect the peptides from proteolytic digestion. The inclusion bodies of PurF fusions can be easily removed from the cell lysate by centrifugation.
-
Inteins chitin-binding domain: upon applying intein system, the usage of exogenous proteases or chemicals is eliminated to remove the carrier protein. Consequently, the downstream process of expression is simplified, and the target protein can be obtained at high purity in a one-step purification employing a single column.
-
Npro fusion technology, which benefits from autoproteolytic function of N-terminal autoprotease, while Npro is originally extracted from classical swine fever virus (CSFV). The target protein/peptide is fused to the C-terminus of Npro and is expressed in inclusion bodies in E. coli. The expressed fusion protein must be dissolved under chaotropic condition. Upon switching to cosmotropic in vitro refolding conditions, the fused partner with an authentic N-terminus is released from the C-terminal end of the autoprotease by self-cleavage. A special Npro mutant called EDDIE has been designed for preparative application, which possesses a better solubility and cleavage rates [35–38].
A few peptides of the Brevinin family have been purified through the thioredoxin fusion system so far. The synthetic gene of Brevinin-2R has been also cloned into the pET32a (+) vector to allow the expression of Brevinin-2R as a Trx fusion protein in E. coli [39]. Brevinin-2GU, an antimicrobial peptide isolated from skin secretion of the Asian frog Hylarana guntheri possesses insulin-releasing activity. The coding sequence of Brevinin-2GU gene has been expressed using pET32a (+) vector as a Trx fusion protein in E. coli to produce over a 45 % yield of the total cell proteins. After purifying the soluble fusion protein by Ni2+-chelating chromatography, the fusion partner was cleaved by Factor Xa protease to release mature Brevinin-2GU [40].
4 Anti-pathogen Activity of Brevinins
All peptides belonging to the Brevinin superfamily show high potency against a wide range of Gram-positive and Gram-negative bacteria, and against strains of pathogenic fungi (Table 10.1). Also, it was found that a carboxamidomethylated linearized derivative of Brevinin-1 (CAM-Brevinin) displayed antiviral activity against HSV-1M (35.0 ± 2.8 % protection; ID50 could not be determined; c = 100 mg/ml) and against HSV-2G (71.6 ± 1.8 % protection; ID50 = 75 mg/ml) [41].
Unfortunately most Brevinins, perhaps with the exception of Brevinin-2R, have strong hemolytic properties that impede their application as antimicrobial agents. However, some experiments indicate that this negative effect could be decreased by certain structural modifications. It was shown, for example, that transposition of brevinin-1E (FLPLLAGLAANFLPKIFCKITRKC), which was isolated from the skin secretion of Rana Esculenta, C-terminal sequence CKITRKC to central position (FLPLLAGLCKITRKCAANFLPKIF) leads to considerable reduction of its hemolytic activity without loss of antibacterial activity [42]. Replacement of Leu18 to Lys in the Brevinin-2-related peptide GIWDTIKSMGKVFAGKILQNL-NH2 from Lithobates septentrionalis resulted in a higher level of erythrocyte integrity. It was also shown that the analogs of GIWDTIKSMGKVFAGKILQNL-NH2: (Lys4, Lys18) and (Lys4, Ala16, Lys18) retained activity against Acinetobacter baumannii (MIC = 3–6 μM) and had very low hemolytic activity (LC50 > 200 μM) [43]. Structure–activity studies also revealed that a linear acetamidomethylcysteinyl analog of Brevinin-1E had appreciably less hemolytic activity in comparison with the native peptide [26].
5 Effect of Brevinins on Cytokine Release
Activation of innate immunity system results in the stimulation pro-inflammatory cytokines release, including interferon-γ (IFN-γ), tumor necrosis factor-alpha (TNF), and interleukin (IL)-8 by mononuclear cells via Toll-like receptor-2 (TLR-2) pathway [44]. The effect of two AMPs belonging to the Brevinin family (Brevinin-2GU, and B2RP-ERa) on the release of pro-inflammatory and anti-inflammatory cytokines from peripheral blood mononuclear cells (PBM) have been assessed in the presence of 1 and 20 μg/ml of AMPs. Brevinin-2GU, and B2RP-ERa significantly reduced release of TNF from concanavalin A (ConA)-stimulated PBM cells while Brevinin-2GU reduced IFN-γ release from unstimulated PBM cells [44]. On the other hand, secretion of the anti-inflammatory cytokines including TGF-β, IL-4, and IL-10 from both control- and ConA-treated PBM cells was significantly increased by B2RP-ERa [44]. The potent activities of AMPs in the regulation of anti-inflammatory cytokines release suggest a possible therapeutic role of these peptides.
6 Anticancer Activity of Brevinins
A unique peptide Brevinin-2R (KLKNFAKGVAQSLLNKASCKLSGQC) was isolated from Rana ridibunda. This peptide consists of 25 amino acids and has strong homology with Brevinin-2Ej and -2Ee. The antimicrobial spectrum of Brevinin-2R displayed activities against: S. aureus, M. luteus, Bacillus spKR-8104, E. coli, S. typhimurium, P. aeruginosa, K. pneumonidae and fungi, such as C. albicans and C. tropicalis. The most important property of Brevinin-2R peptide is low hemolytic activity (no more than 2.5 % of dead cells at up to 200 μg/ml of the peptide) [45]. This fact allowed the researchers to consider Brevinin-2R as a new potential therapeutic agent. Brevinin-2R kills different tumor cells (Jurkat, BJAB, MCF-7, L929, A549) at 1–10 μg/ml concentration, and exerts higher cytotoxicity in comparison with commercial doxorubicin and cisplatin drugs (P < 0.0001). In experiments with normal cell lines (CD3+ T cells from human donor and lung fibroblast), the level of cytotoxicity was approximately two times lower [45]. Brevinin-2 kills cells in a caspase-independent manner, implying cell death mechanisms other than classical apoptosis. After treatment with Brevinin-2R, a decrease of both the mitochondrial membrane potential (ΔΨm) and the ATP level was observed [45].
The main mechanism of its anticancer action is most likely the same as for pathogens namely the modification of membrane properties, especially membrane permeability. Brevinins preferentially interact with cancer cells because the outer membrane surface of these cells has an additional negative charge due to the presence of higher levels of O-glycosylated mucines [46], negatively charged phosphatidylserines [47] or higher number of microvilli, which leads to increasing of membrane surface area [48]. Also it was found that Brevinin-2R interacts with the lysosomal compartment, initiating lysosomal damage and cathepsin leakage into the cytosol, which leads to cell damage. These data suggest that Brevinin-2R-induced cell death also involves autophagy processes [45].
7 Other Activities of Brevinins
Experiments carried out on the rat BRIN-BD11 clonal β-cell line revealed a novel activity of several Brevinins: the stimulation of insulin release. This new function may give an additional protection for frogs by stimulating insulin secretion and causing hypoglycemia in attacking predators [13]. Examples of such insulin-releasing peptides belonging to the Brevinin family include: Brevinin-2GUb from Hylarana güntheri [49], Brevinin-2-related peptide (B2RP) from Lithobates septentrionalis [50], Brevinin-1 peptides from Lithobates palustris [13], Pelophylax saharicus [51] and Glandirana emeljanovi frog (insulin releasing stimulatory effect was shown on RINm5F insulinoma derived cells) [52].
Brevinin-1CBb (FLPFIARLAAKVFPSIICSVTKKC) provided a significant (p < 0.05) stimulation of insulin release (269 % of basal rate at a concentration of 1 μM with a maximum response of 285 % of basal rate at a concentration of 3 μM) from BRIN-BD11 clonal β-cells [12]. At the same condition, B2RP (Brevinin-2-related peptide GIWDTIKSMGKVFAGKILQNL-NH2) showed 148 % of basal rate at a concentration of 1 μM with a maximum response of 222 % of basal rate at a concentration of 3 μM [50]. These values were comparable to those produced by insulinotropic peptides, GLP-1 and GIP (under the same experimental conditions). Unfortunately, however, the peptides were cytotoxic at the tested concentrations [53]. Also, it was shown that increasing the cationicity of B2RP (Asp4 → Lys) enhanced the insulin-releasing potency (137 % of basal rate at a concentration of 0.3 μM; p < 0.05), while increasing amphipathicity and hydrophobicity showed reduced insulin-releasing potency of analog [50]. Those proteins might represent promising candidates for the development of therapeutically valuable agents for the treatment of patients with type 2 diabetes. Most investigators assumed that stimulation of insulin release was caused not only by the capacity of Brevinins to destabilize cell membranes but rather via other, as yet unidentified, mechanisms.
About 2,000 biologically active anuran peptides have been found and characterized (according to DADP database [18]). Due to the high sequence variability and a wide range of functional activities, these proteins constituted a strong basis for theoretical and experimental research leading to the design of new biologically active peptides and peptidomimetica.
8 Functionalization of Nanostructures with Peptides
Functionalization of nanostructures with various biomolecules including DNA, Herceptin, carbohydrates, lipids, peptides and proteins has multiple potential applications in biomedical imaging, clinical diagnosis, antimicrobial therapy, drug delivery and cancer treatment [54]. Several researches have been developing/discovering novel effective antimicrobial reagents to fight the increase of antibiotic-resistant in microorganisms [55]. Liu and colleagues introduced core-shell nanoparticles formed by self-assembly of amphiphilic peptide with potential antimicrobial activity against a broad spectrum of pathogens including bacteria and fungi [56]. Peptides, particularly cationic peptides, belonging to the Brevinin family represented antimicrobial effect against several multi-drug resistant microorganisms [45]. Recent reports clearly demonstrated that peptide-functionalized nanoparticles can considerably enhance the antibacterial activity of biomolecules [54]. Thus, a Brevinin functionalized nanostructure would be of great importance from the objective of developing advanced functional bionanomaterials with antimicrobial properties. Researchers reported the functionalization of a novel gold-based nanocarrier with a therapeutic application (PMI (p12)) as well as a receptor-targeted (CRGDK) peptide to investigate the biological and medicinal effects of conjugated gold nanoparticles on breast cancer cells [57]. Lia et al. have developed AuNPs (gold nanoparticles) to make not only hybrid model system for selective target binding along with cancer therapeutic effects but also sensitive probes for sensing/imaging various analytes/targets such as ions and molecules [58].
Biomedical imaging is another field of application of peptide-functionalized nanoparticles. Synthesis of water-soluble gold nanoparticles functionalized with a Tat protein-derived peptide sequence facilitates the transfer of nanoparticles across the cell membrane, and therefore simplify the visualization of cellular or tissue components as well as nuclear targeting by electron microscopy [59]. The combined results of these studies have implications for functionalizing or decorating Brevinin-2R as an antimicrobial peptide onto nanostructure surfaces to create a hybrid model system for biological purposes.
9 Closing Remarks
Anuran bioactive peptides show great medical potential and will undoubtedly enter the clinic in a not so distant future. Major challenges to their large scale production, and also to research in this area as a whole, are fixed secondary structures achieved by those peptides, when secreted naturally. These secondary structures (i.e. cyclization) are often difficult to mimic, when peptides are produced in procaryotic expression system. These problems are, however, possible to overcome using current biochemical methods.
While most AMPs exhibit strong antibacterial activities, few of them (i.e. Brevinin-2R) show anticancer properties and low hemolytic activity, thus making them potentially compatible with an in vivo use. Noticeable hemolytic activity of most AMPs may be overcome either by structural modifications or simply by applying such drugs externally, directly on the site of infection, thus minimizing systemic load.
We have summarized the typical antibacterial activities of various AMPs (see Table 10.1). Interestingly, virtually no research has so far been done on antiviral activity of AMPs. With the growing demand for effective antiviral drugs (i.e. SARS, HIV, West Nile Virus, Ebola-virus), lipid-membrane-directed activities of AMPs may prove an effective antiviral drugs. Thus, the authors predict strong scientific and commercial interest in antiviral testing of AMPS.
Abbreviations
- AMPs:
-
Antimicrobial peptides
- ConA:
-
Concanavalin A
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- PBM:
-
Peripheral blood mononuclear cells
- TLR-2:
-
Toll-like receptor-2
- TNF:
-
Tumor necrosis factor-alpha
References
Clarke BT (1997) The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol Rev Camb Philos Soc 72(3):365–379
Duellman WE, Trueb L (1994) Biology of amphibians. Johns Hopkins University Press, Baltimore
Preusser HJ, Habermehl G, Sablofski M, Schmall-Haury D (1975) Antimicrobial activity of alkaloids from amphibian venoms and effects on the ultrastructure of yeast cells. Toxicon: Off J Int Soc Toxinol 13(4):285–289
Daly JW, Myers CW, Whittaker N (1987) Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxicon: Off J Int Soc Toxinol 25(10):1023–1095
Habermehl GG (1981) Venomous animals and their toxins. Springer, Berlin
Zasloff M (1987) Magainins, a class of antimicrobial peptides from xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A 84(15):5449–5453
Bevins CL, Zasloff M (1990) Peptides from frog skin. Annu Rev Biochem 59:395–414. doi:10.1146/annurev.bi.59.070190.002143
Rafferty MJ, Bradford AM, Bowie JH, Wallace JC, Tyler MJ (1993) Peptides from Australian frogs. The structure of the dynastins from the Banjo frogs Limnodynastes interioris, Limnodynastes dumerilii and Limndodynastes terraereginae. Aust J Chem 46:833–842
Simmaco M, Mignogna G, Barra D (1998) Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47(6):435–450. doi:10.1002/(SICI)1097-0282, (1998)47:6 < 435::AID-BIP3 > 3.0.CO;2-8
Simmaco M, Mignogna G, Barra D, Bossa F (1994) Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J Biol Chem 269(16):11956–11961
Chen T, Farragher S, Bjourson AJ, Orr DF, Rao P, Shaw C (2003) Granular gland transcriptomes in stimulated amphibian skin secretions. Biochem J 371(Pt 1):125–130. doi:10.1042/BJ20021343
Mechkarska M, Ojo OO, Meetani MA, Coquet L, Jouenne T, Abdel-Wahab YH, Flatt PR, King JD, Conlon JM (2011) Peptidomic analysis of skin secretions from the bullfrog Lithobates catesbeianus (Ranidae) identifies multiple peptides with potent insulin-releasing activity. Peptides 32(2):203–208. doi:10.1016/j.peptides.2010.11.002
Marenah L, Flatt PR, Orr DF, McClean S, Shaw C, Abdel-Wahab YH (2004) Brevinin-1 and multiple insulin-releasing peptides in the skin of the frog Rana palustris. J Endocrinol 181(2):347–354
Basir YJ, Knoop FC, Dulka J, Conlon JM (2000) Multiple antimicrobial peptides and peptides related to bradykinin and neuromedin N isolated from skin secretions of the pickerel frog, Rana palustris. Biochim Biophys Acta 1543(1):95–105
Conlon JM (2008) Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 29(10):1815–1819. doi:10.1016/j.peptides.2008.05.029
Thomas P, Kumar TV, Reshmy V, Kumar KS, George S (2012) A mini review on the antimicrobial peptides isolated from the genus Hylarana (Amphibia: Anura) with a proposed nomenclature for amphibian skin peptides. Mol Biol Rep 39(6):6943–6947. doi:10.1007/s11033-012-1521-3
Morikawa N, Hagiwara K, Nakajima T (1992) Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun 189(1):184–190
Novkovic M, Simunic J, Bojovic V, Tossi A, Juretic D (2012) DADP: the database of anuran defense peptides. Bioinformatics 28(10):1406–1407. doi:10.1093/bioinformatics/bts141
Mashreghi M, Rezazade Bazaz M, Mahdavi Shahri N, Asoodeh A, Mashreghi M, Behnam Rassouli M, Golmohammadzadeh S (2013) Topical effects of frog “Rana ridibunda” skin secretions on wound healing and reduction of wound microbial load. J Ethnopharmacol 145(3):793–797. doi:10.1016/j.jep.2012.12.016
Asoodeh A, Zardini HZ, Chamani J (2012) Identification and characterization of two novel antimicrobial peptides, temporin-Ra and temporin-Rb, from skin secretions of the marsh frog (Rana ridibunda). J Pept Sci: Off Publ Eur Pept Soc 18(1):10–16. doi:10.1002/psc.1409
Clark DP, Durell S, Maloy WL, Zasloff M (1994) Ranalexin. A novel antimicrobial peptide from bullfrog (Rana catesbeiana) skin, structurally related to the bacterial antibiotic, polymyxin. J Biol Chem 269(14):10849–10855
Conlon JM, Sonnevend A, Jouenne T, Coquet L, Cosquer D, Vaudry H, Iwamuro S (2005) A family of acyclic brevinin-1 peptides from the skin of the Ryukyu brown frog Rana okinavana. Peptides 26(2):185–190. doi:10.1016/j.peptides.2004.08.008
Conlon JM, Abraham B, Sonnevend A, Jouenne T, Cosette P, Leprince J, Vaudry H, Bevier CR (2005) Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virgatipes (Ranidae, Aquarana). Regul Pept 131(1–3):38–45. doi:10.1016/j.regpep.2005.06.003
Conlon JM, Kolodziejek J, Nowotny N (2004) Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta 1696(1):1–14
Suh JY, Lee KH, Chi SW, Hong SY, Choi BW, Moon HM, Choi BS (1996) Unusually stable helical kink in the antimicrobial peptide–a derivative of gaegurin. FEBS Lett 392(3):309–312
Kwon MY, Hong SY, Lee KH (1998) Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim Biophys Acta 1387(1–2):239–248
Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB (1990) All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A 87(12):4761–4765
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta 1758(9):1184–1202. doi:10.1016/j.bbamem.2006.04.006
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462(1–2):55–70
Nicolas P, Vanhoye D, Amiche M (2003) Molecular strategies in biological evolution of antimicrobial peptides. Peptides 24(11):1669–1680. doi:10.1016/j.peptides.2003.08.017
Tazato S, Conlon JM, Iwamuro S (2010) Cloning and expression of genes enocoding antimicrobial peptides and bradykinin from the skin and brain of Oki Tago’s brown frog, Rana tagoi okiensis. Peptides 31(8):1480–1487. doi:10.1016/j.peptides.2010.04.031
Chen T, Li L, Zhou M, Rao P, Walker B, Shaw C (2006) Amphibian skin peptides and their corresponding cDNAs from single lyophilized secretion samples: identification of novel brevinins from three species of Chinese frogs. Peptides 27(1):42–48. doi:10.1016/j.peptides.2005.06.024
Wang H, Ran R, Yu H, Yu Z, Hu Y, Zheng H, Wang D, Yang F, Liu R, Liu J (2012) Identification and characterization of antimicrobial peptides from skin of Amolops ricketti (Anura: Ranidae). Peptides 33(1):27–34. doi:10.1016/j.peptides.2011.10.030
Ohnuma A, Conlon JM, Iwamuro S (2010) Differential expression of genes encoding preprobrevinin-2, prepropalustrin-2, and preproranatuerin-2 in developing larvae and adult tissues of the mountain brown frog Rana ornativentris. Comp Biochem Physiol Toxicol Pharmacol CBP 151(1):122–130. doi:10.1016/j.cbpc.2009.09.004
Li Y (2009) Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol Appl Biochem 54(1):1–9. doi:10.1042/BA20090087
Ueberbacher R, Rodler A, Hahn R, Jungbauer A (2010) Hydrophobic interaction chromatography of proteins: thermodynamic analysis of conformational changes. J Chromatogr A 1217(2):184–190. doi:10.1016/j.chroma.2009.05.033
Cheng X, Lu W, Zhang S, Cao P (2010) Expression and purification of antimicrobial peptide CM4 by Npro fusion technology in E. coli. Amino Acids 39(5):1545–1552. doi:10.1007/s00726-010-0625-0
Ke T, Liang S, Huang J, Mao H, Chen J, Dong C, Huang J, Liu S, Kang J, Liu D, Ma X (2012) A novel PCR-based method for high throughput prokaryotic expression of antimicrobial peptide genes. BMC Biotechnol 12:10. doi:10.1186/1472-6750-12-10
Mehrnejad F, Naderi-Manesh H, Ranjbar B, Maroufi B, Asoodeh A, Doustdar F (2008) PCR-based gene synthesis, molecular cloning, high level expression, purification, and characterization of novel antimicrobial peptide, brevinin-2R, in Escherichia coli. Appl Biochem Biotechnol 149(2):109–118. doi:10.1007/s12010-007-8024-z
Zhou QF, Li MY, Li CW (2009) Cloning and expression of a novel insulin-releasing peptide, brevinin-2GU from Escherichia coli. J Biosci Bioeng 107(4):460–463. doi:10.1016/j.jbiosc.2008.12.011
Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA (2000) Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis 19(3):187–194
Kumari VK, Nagaraj R (2001) Structure-function studies on the amphibian peptide brevinin 1E: translocating the cationic segment from the C-terminal end to a central position favors selective antibacterial activity. J Pept Res: Off J Am Pept Soc 58(5):433–441
Conlon JM, Ahmed E, Condamine E (2009) Antimicrobial properties of brevinin-2-related peptide and its analogs: efficacy against multidrug-resistant Acinetobacter baumannii. Chem Biol Drug Des 74(5):488–493. doi:10.1111/j.1747-0285.2009.00882.x
Popovic S, Urban E, Lukic M, Conlon JM (2012) Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides 34(2):275–282. doi:10.1016/j.peptides.2012.02.010
Ghavami S, Asoodeh A, Klonisch T, Halayko AJ, Kadkhoda K, Kroczak TJ, Gibson SB, Booy EP, Naderi-Manesh H, Los M (2008) Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med 12(3):1005–1022. doi:10.1111/j.1582-4934.2008.00129.x
Papo N, Shai Y (2005) Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci CMLS 62(7–8):784–790. doi:10.1007/s00018-005-4560-2
Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res 51(11):3062–3066
Zwaal RF, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89(4):1121–1132
Conlon JM, Power GJ, Abdel-Wahab YH, Flatt PR, Jiansheng H, Coquet L, Leprince J, Jouenne T, Vaudry H (2008) A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura:Ranidae). Regul Pept 151(1–3):153–159. doi:10.1016/j.regpep.2008.04.002
Abdel-Wahab YH, Patterson S, Flatt PR, Conlon JM (2010) Brevinin-2-related peptide and its [D4K] analogue stimulate insulin release in vitro and improve glucose tolerance in mice fed a high fat diet. Horm Metab Res 42(9):652–656. doi:10.1055/s-0030-1254126
Marenah L, Flatt PR, Orr DF, Shaw C, Abdel-Wahab YH (2006) Skin secretions of Rana saharica frogs reveal antimicrobial peptides esculentins-1 and -1B and brevinins-1E and -2EC with novel insulin releasing activity. J Endocrinol 188(1):1–9. doi:10.1677/joe.1.06293
Kim JH, Lee JO, Jung JH, Lee SK, You GY, Park SH, Kim HS (2010) Gaegurin-6 stimulates insulin secretion through calcium influx in pancreatic beta Rin5mf cells. Regul Pept 159(1–3):123–128. doi:10.1016/j.regpep.2009.07.014
Abdel-Wahab YH, Power GJ, Flatt PR, Woodhams DC, Rollins-Smith LA, Conlon JM (2008) A peptide of the phylloseptin family from the skin of the frog Hylomantis lemur (Phyllomedusinae) with potent in vitro and in vivo insulin-releasing activity. Peptides 29(12):2136–2143. doi:10.1016/j.peptides.2008.09.006
Veerapandian M, Yun K (2011) Functionalization of biomolecules on nanoparticles: specialized for antibacterial applications. Appl Microbiol Biotechnol 90(5):1655–1667. doi:10.1007/s00253-011-3291-6
Mohan R, Shanmugharaj AM, Sung Hun R (2011) An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J Biomed Mater Res B Appl Biomater 96(1):119–126. doi:10.1002/jbm.b.31747
Liu L, Xu K, Wang H, Tan PK, Fan W, Venkatraman SS, Li L, Yang YY (2009) Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol 4(7):457–463. doi:10.1038/nnano.2009.153
Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, Liang XJ (2012) Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 33(4):1180–1189. doi:10.1016/j.biomaterials.2011.10.058
Lia T, Heab X, Wang Z (2012) The application of peptide functionalized gold nanoparticles. In: Hepel M, Zhong C (eds) Functional nanoparticles for bioanalysis, nanomedicine, and bioelectronic devices, vol 2. American Chemical Society, Washington, DC, pp 55–68. doi:10.1021/bk-2012-1113
de la Fuente JM, Berry CC (2005) Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug Chem 16(5):1176–1180. doi:10.1021/bc050033+
Goraya J, Wang Y, Li Z, O’Flaherty M, Knoop FC, Platz JE, Conlon JM (2000) Peptides with antimicrobial activity from four different families isolated from the skins of the North American frogs Rana luteiventris, Rana berlandieri and Rana pipiens. Eur J Biochem/ FEBS 267(3):894–900
Conlon JM, Sonnevend A, Patel M, Al-Dhaheri K, Nielsen PF, Kolodziejek J, Nowotny N, Iwamuro S, Pal T (2004) A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica. Regul Pept 118(3):135–141. doi:10.1016/j.regpep.2003.12.003
Acknowledgments
SG was supported by Parker B Francis fellowship in Respiratory Disease. MJL kindly acknowledge the core/startup support from Linköping University, from Integrative Regenerative Medicine Center (IGEN), from Cancerfonden (CAN 2013/391), and from VR-NanoVision (K2012-99X -22325-01-5).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Savelyeva, A., Ghavami, S., Davoodpour, P., Asoodeh, A., Łos, M.J. (2014). An Overview of Brevinin Superfamily: Structure, Function and Clinical Perspectives. In: Grimm, S. (eds) Anticancer Genes. Advances in Experimental Medicine and Biology, vol 818. Springer, London. https://doi.org/10.1007/978-1-4471-6458-6_10
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6458-6_10
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6457-9
Online ISBN: 978-1-4471-6458-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)